Abstract
Metabolism is dynamic and must function in context-specific ways to adjust to changes in the surrounding cellular and ecological environment. When isotopic tracers are used, metabolite flow (i.e., metabolic flux) can be quantified through biochemical networks to assess metabolic pathway operation. The cellular activities considered across multiple tissues and organs result in the observed phenotype and can be analyzed to discover emergent, whole-system properties of biology and elucidate misconceptions about network operation. However, temporal and spatial challenges remain significant hurdles and require novel approaches and creative solutions. We survey current investigations in higher plant and animal systems focused on dynamic isotope labeling experiments, spatially resolved measurement strategies, and observations from re-analysis of our own studies that suggest prospects for future work. Related discoveries will be necessary to push the frontier of our understanding of metabolism to suggest novel solutions to cure disease and feed a growing future world population.
Graphical Abstract
Introduction
Ensuring global health and nutrition for future generations will require transformative gains in agricultural productivity [3–5] and improved strategies to combat disease [6,7]. Spatial and temporal complexity are at the forefront of challenges that limit success in metabolic engineering and, more generally, restrict our understanding of metabolism. Eukaryotic metabolism is segregated between organs, cells, and subcellular organelles, operating over circadian and developmental time regimes, and must continuously adjust to environmental perturbations that vary in duration and intensity. Unfortunately, most experimental efforts are limited to analysis of isolated components, steady-state conditions, and simplified systems that cannot fully recapitulate the spatial and temporal dynamics that occur in vivo. Isotopic tracers (i.e., stable isotopes such as 13C, 15N, 2H, 18O, etc. or radioisotopes such as 14C or 3H) are the primary tools used by researchers to quantitatively track the dynamics of metabolism. Thus, their importance to metabolic studies cannot be overstated, and recent advances in mass spectrometry (MS) technologies (including wide availability of high-resolution and tandem MS instruments) offer a burgeoning opportunity to further extend the scope of isotope tracers to upgrade the information content of metabolomics datasets. Here we examine recent applications of isotopic labeling experiments to assess spatial and temporal aspects of metabolism that would be otherwise inaccessible to measurement.
Assessing temporal dynamics and metabolite turnover
When plants see the light—Temporal dynamics that balance light harvesting and carbon assimilation
One of the most crucial functions for a plant is to maximize photosynthesis for growth and metabolism; however incident light fluctuates with seasonal daylength, cloud cover, sun flecks, and canopy shading. The changes in light intensity are dynamic and unpredictable such that plant response is not optimized for agricultural productivity [13], and daily starch production must account for unforeseen cloud cover. Recent 13C and 14C labeling studies have indicated starch breakdown occurs during the latter part of photoperiods [15], potentially explaining how plants adjust to the unknown cumulative light levels per day. 13C tracer studies have described the systemic changes that occur when canopies shade leaves, including induced senescence to maximize resource allocation for growth and survival [16]. At the cellular level, metabolic adaptations to accommodate altered light levels can include seemingly counterproductive paths and futile cycles such as the oxidative pentose phosphate (OPP) pathway. Sharkey and coworkers [10–12] indicate that the oxidative portion of pentose phosphate metabolism (i.e., G6P shunt) could play an important role in stabilizing photosynthesis and would therefore account for a portion of total day-time respiration in leaves, however details about the spatial origin of the G6P supply for the shunt and other aspects of operation remain to be elucidated and might benefit from labeling-based flux analyses.
Identification of futile cycles with isotope tracers
Isotope labeling studies offer tremendous potential for deciphering the operation of metabolic networks [17] and discovering hidden metabolic functions [18]. In particular, the operation of futile cycles can be very difficult to detect without isotope tracers [19]. A futile cycle is a ‘wasteful’ process in which two metabolic pathways run simultaneously in opposite directions but have no overall effect other than to dissipate energy. Prior studies have applied isotope labeling experiments to quantify rates of futile cycling in E. coli [20], B. subtilis [21], and S. cerevisiae [22]. In plants, recent 14C/13C studies suggest that futile cycles help maintain nutrient status under altered environments [23,24], a process that is particularly relevant to future agriculture with finite phosphorus resources and intensive nitrogen fertilizer production. Coordination of glycolytic metabolism with sugar sensing and sucrose cycling is thought to consume large amounts of ATP necessary to balance resource allocation in plants [25], and similar processes to ensure homeostatic regulation of glucose metabolism are also well-studied in mammalian physiology. For example, various futile cycles involving PEP [26], pyruvate [27,28], and glucose [29] have been hypothesized to play important roles in regulating glucose-stimulated insulin secretion by pancreatic islet cells. Direct examination of flux through these cyclic pathways was only possible through the development of novel stable isotope technologies adapted for cultured islets.
Sometimes, new futile cycles can emerge as a by-product of metabolic engineering. Liu et al. [30] applied pulse-labeling experiments with [U-13C6]glucose to discover an energy-dissipating phosphorylation/dephosphorylation cycle that became highly active when B. subtilis was engineered to overproduce N-acetylglucosamine (GlcNAc). Identification and removal of the responsible kinase enzyme led to a doubling of GlcNAc productivity due to restored healthy growth of the overproducing strain. Dynamic 13CO2 labeling experiments have been similarly used to quantify ATP-consuming cycles involving photorespiration [14] and pyruvate cycling [2] in photosynthetic species. While these pathways are not futile cycles per se, they are responsible for losses in photosynthetic efficiencies that can range up to 50% or more [31]. The description of these substrate cycles as ‘futile’ may become outdated, as perceived ‘wasteful’ steps likely evolved to serve a meaningful purpose (e.g., as in the case of photorespiration) and possibly to accommodate environmental fluctuations that are not studied in controlled lab experiments aimed at minimizing variability. With increased emphasis now being placed on reducing metabolic burden in recombinant host organisms by eliminating inefficient pathways, it is likely that systematic identification and evaluation of futile cycles will play an ever more important role in metabolic engineering [32].
Dynamic labeling experiments can be used to cluster metabolites based on their turnover rate
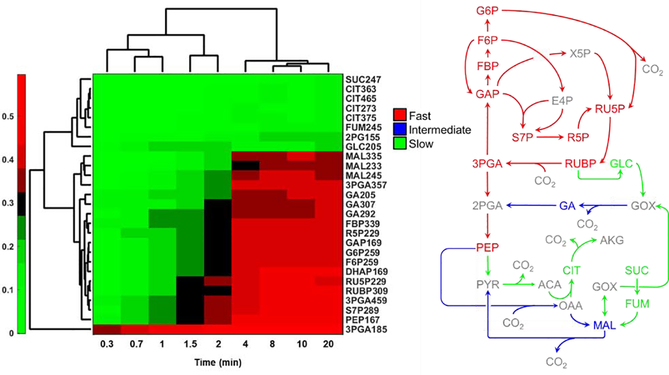
A variety of algorithms have been developed to infer network associations and identify co-regulated metabolic modules based on correlations between measured metabolite abundances [33–35]. The mummichog algorithm [36] provided a significant advance by grouping peaks into modules using statistical techniques that are robust to missing or incorrect peak annotations. While many of these methods have proven useful in extracting biologically meaningful information from high-dimensional metabolomics datasets, few if any have attempted to leverage the additional information that is potentially available from isotope labeling studies to reconstruct networks de novo. To test whether metabolic interactions can be inferred from correlation-based analysis of isotope labeling, we investigated a previous dataset where photosynthetic cyanobacteria were labeled with 13CO2 over 20 minutes [2]. Targeted measurements of 13C enrichment were obtained using a combination of GC-MS and LC-MS/MS analysis of metabolite extracts. Hierarchical clustering of the atom percent enrichments (APEs) for each target ion (Fig. 1) showed clear discrimination between metabolites that were rapidly labeled (mainly in the Calvin-Benson cycle) versus those that were slowly labeled (in photorespiration and TCA pathways). A few metabolites that lie on the boundary between these two network regions (e.g., malate and glycerate) exhibited intermediate rates of enrichment. This example illustrates how clustering based on dynamic labeling trajectories can be used to effectively group metabolites into local modules that reflect network proximity and/or pathway activity.
Fig. 1. Hierarchical clustering analysis (HCA) of dynamic 13C labeling in cyanobacterial metaboltes.
Target ions were clustered based on APE (left; JD Young unpublished). Pathways and metabolites were color-coded in the network diagram according to the HCA outputs (right). Abbreviations can be found in [2].
Extending temporal descriptions to proteins and lipid
Stable isotope approaches like those used to profile turnover rates of small molecules in central metabolism can also be applied to quantify network dynamics in lipid and protein metabolism. For example, the differences in the unlabeled fraction could be used to group amino acids and distinguish auto-, mixo- and heterotrophic metabolism in duckweed [37]. Stable isotopes are now frequently paired with MS, often high-resolution MS (HRMS), to assess protein turnover more comprehensively across the proteome [38,39]. In plants, inorganic 15N [40,41] or 13C [42] have become preferred tracers for studying protein biosynthesis because of: i) the associated challenges with differential amino acid uptake, ii) interference with feedback regulation, iii) amino acid influences on protein turnover rates, and iv) use of amino acids for non-protein purposes [42]. In animals and humans, administration of deuterated water (2H2O) has been used for several decades as a non-invasive approach to assess protein and lipid turnover rates in vivo. Shankaran et al. [43] recently applied 2H2O labeling combined with sensitive tandem MS analysis to perform proteome-wide monitoring of skeletal muscle protein synthesis rates over several weeks from blood samples.
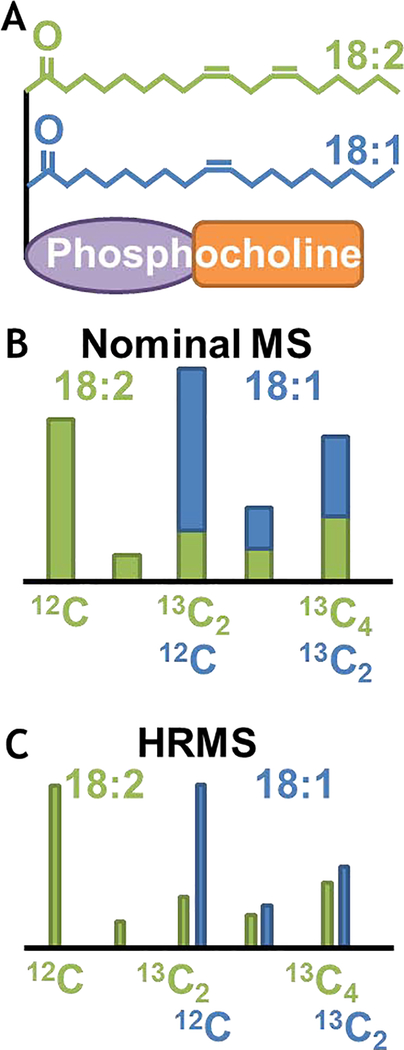
The technical considerations are slightly different for lipid molecular species that number in the hundreds of thousands and comprise many near-overlapping masses between 500–1000 amu, which challenge instrument resolution. Isotope labeling investigations of lipids [44,45] have mostly involved 14C [46–50]; however, soft electrospray ionization MS methods maintain intact lipid structures [51] and offer new opportunities for stable isotopes [52]. One of the challenges specifically to quantification of stable isotope labeling is that the m/z ranges of different lipid species can overlap due to varying degrees of unsaturation. A current frontier [53] is to use HRMS to resolve these species based on their mass defects (Fig. 2) and eventually build quantitative flux maps for lipid metabolism [54]. In addition, multi-tracer studies (Fig. 3) can be designed to simultaneously probe multiple intersecting metabolic pathways with a single isotope labeling experiment [55]. The capacity to elucidate incomplete metabolic networks and to quantify macromolecule synthesis and turnover (not just accumulation) that contributes to signaling, regulation and metabolic response remains an opportunity for the use of isotope labeling strategies.
Fig. 2. Nominal & HRMS of lipids.
A) Example of phosphatidylcholine (PC) structure. B) Isotopologue distribution of fatty acids at nominal mass resolution. C) HRMS-derived isotopologue distribution.
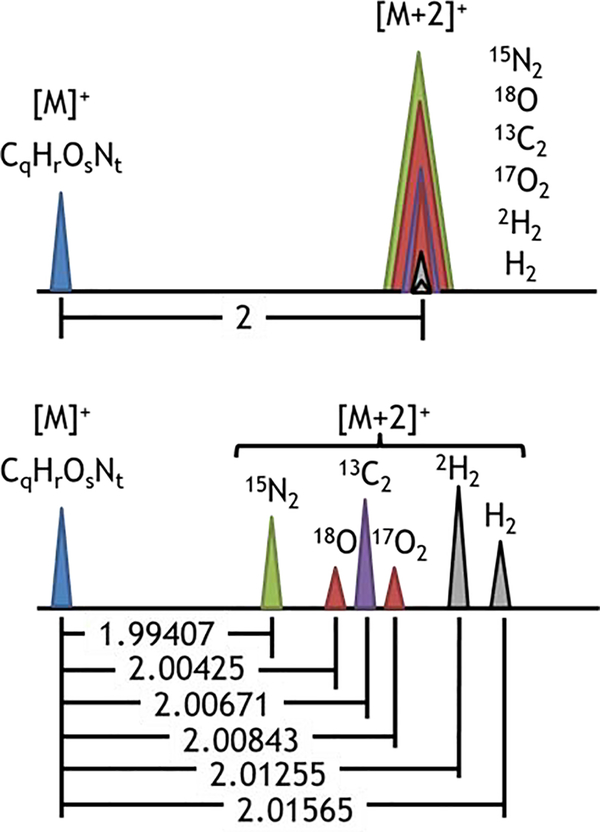
Fig. 3. Identification of isotopologue peaks.
Change from a molecular mass [M]+ to a mass of [M+2]+ can be the result of substitution of various isotopes (i.e., 15N for 14N, 13C for 12C, 18O for 16O, 17O for 16O, or 2H for 1H) or can result from the addition of hydrogens to the molecule as in the case of changing the degree of unsaturation in a lipid. Here the differences in mass from substitution of the indicated isotope are shown. The change in mass from addition of two hydrogens (+H2) is also presented.
Profiling spatial segregation of metabolic functions, fluxes and pool sizes
Subcellular compartmentation – when location matters
Metabolism in eukaryotes is organized at the cellular and subcellular levels and can include significant metabolite channeling described elsewhere [56,57]. Subcellular compartmentalization presents a unique challenge for isotope labeling studies due to the existence of the same metabolic pathways in different organelles, creating compartment-specific 13C labeling patterns that are difficult to resolve [58–60]. In such systems, knowledge of major metabolic pathways within subcellular compartments and inter-compartmental transporters is essential [61]. Methods to address organelle compartmentalization involve measuring organelle-specific metabolites as readouts of the metabolism in each compartment [62–67], subcellular fractionation of organelles [68,69], or technologies to image metabolites at the cellular level [70,71]. In some cases, careful selection of target analytes from different cell/tissue types can be used to resolve compartment-specific fluxes [72,73] that reflect cellular or subcellular metabolic heterogeneity. For example, metabolic flux ratio (METAFoR) analysis was used to indirectly calculate compartment-specific labeling patterns of pyruvate and oxaloacetate from measurements of PEP (assumed cytosolic) and oxoglutarate (assumed mitochondrial) [74]. This model subsequently enabled the application of 13C flux analysis to several species of yeast including Saccharomyces cerevisiae [75,76]. Further generalizations on this approach (i.e., SUMOFLUX) are based on incorporation of machine learning [77], and through the years a number of compartmentalized metabolic flux maps have been generated as reviewed elsewhere [59,60].
Shlomi et al. [78] recently described a spatial-fluxomics approach for quantifying metabolic fluxes specifically in mitochondria and cytosol of mammalian cell cultures, performing isotope tracing in intact cells followed by rapid subcellular fractionation and quenching of metabolism within 25 seconds, followed by LC/MS-based metabolomics analysis. The method was applied to uncover the surprising result that reductive glutamine metabolism was, in fact, the major producer of cytosolic citrate (rather than glucose oxidation) to support fatty acid biosynthesis under standard normoxic conditions in HeLa cells, and that succinate dehydrogenase-deficient tumor cells reverse the citrate synthase flux to produce oxaloacetate in mitochondria [78]. Reversibility of isocitrate dehydrogenase has been previously described in other lipid-producing systems including brown adipocytes [79], melanomas [80] and developing canola embryos [81] and may reflect conserved principles for carbon use efficiency. Other insights into mitochondrial metabolism have been the result of selective permeabilization of the cytosolic membrane with digitonin, as developed by Nonnenmacher et al. [82], or regression-based methods to indirectly infer differences between cytosolic and mitochondrial metabolism from isotope labeling data. As an example of the latter, Christen and coworkers [83] used a regression approach to estimate the fractional distribution of pyruvate between compartments by assuming that lactate is produced from cytosolic pyruvate and alanine is produced from mitochondrial pyruvate.
Multi-cellular interactions between different tissues and the involvement of metabolically inactive pools
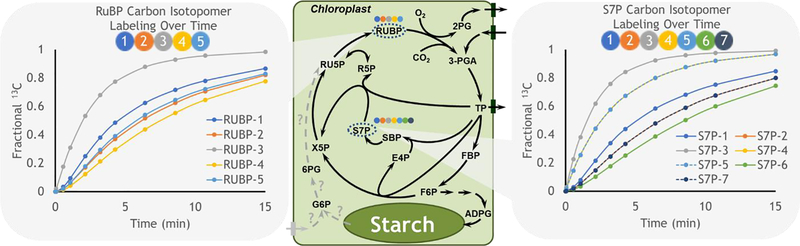
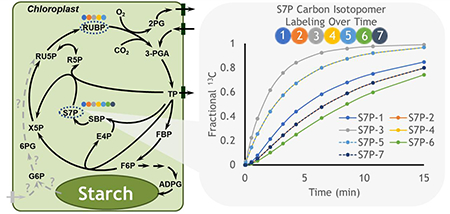
The distribution of pyruvate across multiple locations is an indication of the central role of this metabolite. In plants it is involved in cycles including C4 photosynthetic metabolism which relies on fine spatial architecture to implement a carbon concentrating mechanism that enhances carbon assimilation. Labeling studies in C4 plants have described: i) the dynamic and spatial complexity of photosynthetic metabolism that takes place across bundle sheath and mesophyll cells [84,85], ii) gradients of intermediates consistent with diffusion driven movement between cells with the possible exception of pyruvate [84], iii) the role of alanine and pyruvate in shuttling nitrogen between cell types [84,85] and v) the capacity for aspartate to be converted to malate for decarboxylation in the bundle sheath [85]. The rapid labeling in the C4 transfer metabolite malate approaches a stable level quickly that is less than 20% of total 13CO2 enrichment because malate (and to a lesser extent other metabolites) exist as pools in multiple locations, some of which are not involved in the C4 mechanism. Unlabeled pools are termed ‘inactive’ and are also observed in C3 metabolism [14,86], indicating that the spatial separation of active metabolism could reflect subcellular features in addition to differing cell types. However interestingly, sedoheptulose 7-phosphate (S7P), that is integral to Calvin-Benson cycle metabolism shows a complete depletion of the unlabeled (M0) fraction within 15 min of 13CO2 pulse labeling [14] and other Calvin-Benson intermediates become fully labeled within 60 min [84]. The decrease in M0 to near zero levels in S7P and other metabolites suggests that these pools are either not present in spatially segregated locations or that there is rapid movement allowing for near equilibrium labeling descriptions between compartments. The latter, which would include other metabolic pathways such as the reversible steps of the oxidative pentose phosphate pathway (OPPP) thought to jointly occur in the cytosol in some tissues [87], is less likely in leaves because precursors such as glucose 6-phosphate are not labeled quickly and would result in slower overall labeling in S7P. The depletion of M0 indicates that the S7P pool is completely turned over and that none of it is inactive within the time frame of the labeling experiment. Further, in silico modeling (Fig. 4) indicates that some carbon positions take many iterations of the Calvin-Benson cycle to label completely because of fluxes through asymmetric bond breaking and forming reactions with multiple precursor pools. Indeed, the property of asymmetry in labeling provides the mechanistic underpinning to distinguish metabolic steps and to quantify pathway fluxes including the Calvin-Benson cycle [8,9].
Fig. 4. Carbon isotopomers for Calvin-Benson Cycle intermediates.
Isotopic labeling for ribulose 1,5-bisphosphate (RUBP) and sedoheptulose 7-phosphate (S7P) were derived through in silico modeling (DK Allen unpublished) using the INCA framework [1] and assuming no photorespiration. Positional carbon enrichments are indicated following the color scheme of the circles that represent individual carbon atoms. S7P carbon atoms 2 and 7 (orange and dark blue) are identical, as are atoms 4 and 5 (yellow and light blue). Qualitative enrichment patterns are consistent with well-known pathway bond rearrangements [8,9] but can vary with flux descriptions. Dotted gray lines and question marks within the metabolic network describe one of several hypothetical versions of the G6P shunt [10–12] which is not included in the metabolite simulations. Abbreviations are based on [14] and canonical textbook descriptions.
Conclusions/Perspectives
As flux analyses continue to progress and take advantage of new instrumentation or labeling experiment design, there remains a need to increase throughput and examine new organs, tissues, and multi-species consortia. In plants, attempts to close this gap emphasize labeling tissue disks [88], cell suspensions [89,90], hairy roots [91], whole plants [92], petioles or hypocotyls [93], stems [94], or shoot tips [95]. In microbial systems, techniques are progressing from studies of axenic cell cultures to co-culture systems [96] and microbiomes [97] studied under conditions that mimic natural environments. Finally, studies of mammalian metabolism are advancing from cell lines to primary cells [29] to in vivo labeling experiments in whole animals [98,99] or human subjects [100,101]. However, there remains a large disconnect between genetic and metabolic investigations where the former emphasizes phenotyping through easily obtained measurements to select desirable qualities that can then be studied in depth through the latter. There also are few studies that link metabolism across developmental time or the spatial attributes of the entire living plant, animal, or microbial system. Thus, significant hurdles that impede our understanding of metabolism in living systems relate directly to spatial and temporal aspects that would benefit from expanded use of isotope tracers. Development of new methods and enhanced instrumentation will enable more intricate studies to reveal novel aspects of plants, animals, and microbial consortia under continually changing biological conditions, which are most relevant to understanding the natural complexity of metabolism.
Highlights.
Metabolism is segregated at the subcellular and cellular levels and responds in a context-specific manner to environmental and developmental changes that occur over time
The dynamics of metabolism can be assessed and quantified as metabolic fluxes using stable and radio-isotopes
Advances in instrumentation and novel experimental designs with isotopes are leading to new insights that will be important to solve global challenges in food security and disease
Acknowledgements
The authors acknowledge support from the United States Department of Agriculture, including the Agricultural Research Service and National Institute of Food and Agriculture (2017-67013-26156; 2016-67013-24585), the National Institutes of Health (U01 CA235508), and the National Science Foundation (DBI 1616820). This work was also supported by the U.S. Department of Energy, Office of Science, Biological and Environmental Research Division, under award number LANLF59T.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young JD: INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 2014, 30:1333–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young JD, Shastri AA, Stephanopoulos G, Morgan JA: Mapping photoautotrophic metabolism with isotopically nonstationary (13)C flux analysis. Metab Eng 2011, 13:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, LongSP, et al. : Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci U S A 2015, 112:8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long SP, Marshall-Colon A, Zhu XG: Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161:56–66. [DOI] [PubMed] [Google Scholar]
- 5.Simkin AJ, Lopez-Calcagno PE, Raines CA: Feeding the world: improving photosynthetic efficiency for sustainable crop production. J Exp Bot 2019, 70:1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wishart DS: Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 2016, 15:473–484. [DOI] [PubMed] [Google Scholar]
- 7.Newgard CB: Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab 2017, 25:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassham JA, Benson AA, Kay LD, Harris AZ, Wilson AT, Calvin M: The Path of Carbon in Photosynthesis. XXI. The Cyclic Regeneration of Carbon Dioxide Acceptor1. Journal of the American Chemical Society 1954, 76:1760–1770. [Google Scholar]
- 9.Sharkey TD: Discovery of the canonical Calvin-Benson cycle. Photosynth Res 2019, 140:235–252. [DOI] [PubMed] [Google Scholar]
- 10.Preiser AL, Fisher N, Banerjee A, Sharkey TD: Plastidic glucose-6-phosphate dehydrogenases are regulated to maintain activity in the light. Biochem J 2019, 476:1539–1551.** Preiser et al. monitored G6PDH during the day and found the enzyme activity can be up to ~50% of maximum even at high light conditions. They postulate that the oxidative steps in OPPP, referred to as the G6P shunt, may help maintain the balance in redox and energy between light harvesting and carbon metabolic reactions in the chloroplast across dynamically changing environments.
- 11.Li J, Weraduwage SM, Preiser AL, Tietz S, Weise SE, Strand DD, Froehlich JE, Kramer DM, Hu J,Sharkey TD: A Cytosolic Bypass and G6P Shunt in Plants Lacking Peroxisomal Hydroxypyruvate Reductase. Plant Physiol 2019, 180:783–792.** The authors utilize a peroxisomal hydroxypyruvate reductase mutant to study the impact of altered photorespiration. Changes in photorespiration may induce a cytosolic bypass with export of GAP to avoid triose phosphate isomerase inhibition in the chloroplast and import of G6P. G6P would stimulate the G6P shunt and the consumption of ATP that could be linked to the observed enhancement of photosynthetic cyclic electron flow for ATP production.
- 12.Weise SE, Liu T, Childs KL, Preiser AL, Katulski HM, Perrin-Porzondek C, Sharkey TD: Transcriptional Regulation of the Glucose-6-Phosphate/Phosphate Translocator 2 Is Related to Carbon Exchange Across the Chloroplast Envelope. Front Plant Sci 2019, 10:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kromdijk J, Glowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP: Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354:857–861. [DOI] [PubMed] [Google Scholar]
- 14.Ma F, Jazmin LJ, Young JD, Allen DK: Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proc Natl Acad Sci U S A 2014, 111:16967–16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez O, Ishihara H, George GM, Mengin V, Flis A, Sumner D, Arrivault S, Feil R, Lunn JE, Zeeman SC, et al. : Leaf Starch Turnover Occurs in Long Days and in Falling Light at the End of the Day. Plant Physiol 2017, 174:2199–2212.** Through a series of 13C/14C experiments the authors describe starch plateaus and turnover during the day, that could be part of a strategy to survive and grow diurnally when the amount of daily light received for photosynthesis cannot be predicted by a plant a priori.
- 16.Law SR, Chrobok D, Juvany M, Delhomme N, Linden P, Brouwer B, Ahad A, Moritz T, Jansson S,Gardestrom P, et al. : Darkened Leaves Use Different Metabolic Strategies for Senescence and Survival. Plant Physiol 2018, 177:132–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauer U: Metabolic networks in motion: 13C-based flux analysis. Mol Syst Biol 2006, 2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen AE, Dupont CL, Obornik M, Horak A, Nunes-Nesi A, McCrow JP, Zheng H, Johnson DA, Hu H,Fernie AR, et al. : Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 2011, 473:203–207. [DOI] [PubMed] [Google Scholar]
- 19.Christiansen T, Christensen B, Nielsen J: Metabolic network analysis of Bacillus clausii on minimal and semirich medium using C-13-Labeled glucose. Metabolic Engineering 2002, 4:159–169. [DOI] [PubMed] [Google Scholar]
- 20.Sauer U, Lasko DR, Fiaux J, Hochuli M, Glaser R, Szyperski T, Wuthrich K, Bailey JE: Metabolic flux ratio analysis of genetic and environmental modulations of Escherichia coli central carbon metabolism. Journal of Bacteriology 1999, 181:6679–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhl M, Zamboni N, Sauer U: Dynamic flux responses in riboflavin overproducing Bacillus subtilis to increasing glucose limitation in fed-batch culture. Biotechnol Bioeng 2010, 105:795–804. [DOI] [PubMed] [Google Scholar]
- 22.Feng XY, Zhao HM: Investigating xylose metabolism in recombinant Saccharomyces cerevisiae via C-13 metabolic flux analysis. Microbial Cell Factories 2013, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lattanzi FA, Ostler U, Wild M, Morvan-Bertrand A, Decau ML, Lehmeier CA, Meuriot F, Prud’homme MP, Schaufele R, Schnyder H: Fluxes in central carbohydrate metabolism of source leaves in a fructan-storing C3 grass: rapid turnover and futile cycling of sucrose in continuous light under contrasted nitrogen nutrition status. J Exp Bot 2012, 63:2363–2375. [DOI] [PubMed] [Google Scholar]
- 24.He JZ, Dorion S, Lacroix M, Rivoal J: Sustained substrate cycles between hexose phosphates and free sugars in phosphate-deficient potato (Solanum tuberosum) cell cultures. Planta 2019, 249:1319–1336.* 14C glucose was used to assess changes in metabolism resulting from a phosphorus deficiency. The authors speculate that futile cycling between free sugars and sugar phosphates may be an important component of the metabolic adaptation and homeostasis in phosphorus-deficient conditions.
- 25.Claeyssen E, Dorion S, Clendenning A, He JZ, Wally O, Chen J, Auslender EL, Moisan MC, Jolicoeur M,Rivoal J: The futile cycling of hexose phosphates could account for the fact that hexokinase exerts a high control on glucose phosphorylation but not on glycolytic rate in transgenic potato (Solanum tuberosum) roots. PLoS One 2013, 8:e53898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, Shulman GI, Kibbey RG: Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem 2009, 284:26578–26590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pongratz RL, Kibbey RG, Shulman GI, Cline GW: Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 2007, 282:200–207. [DOI] [PubMed] [Google Scholar]
- 28.Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD: 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci U S A 2002, 99:2708–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall ML, Pound LD, Trenary I, O’Brien RM, Young JD: Novel stable isotope analyses demonstrate significant rates of glucose cycling in mouse pancreatic islets. Diabetes 2015, 64:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Link H, Liu L, Du G, Chen J, Sauer U: A dynamic pathway analysis approach reveals a limiting futile cycle in N-acetylglucosamine overproducing Bacillus subtilis. Nat Commun 2016, 7:11933.** Liu et al. developed a generally applicable approach to reveal limiting steps within a synthetic pathway by monitoring metabolite dynamics and simplified kinetic modelling. Stable isotope labeling experiments predicted an energy-dissipating futile cycle, and deletion of the responsible glucokinase increased GlcNAc productivity by more than two-fold.
- 31.Zhu XG, Long SP, Ort DR: What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 2008, 19:153–159. [DOI] [PubMed] [Google Scholar]
- 32.Wu G, Yan Q, Jones JA, Tang YJ, Fong SS, Koffas MA: Metabolic Burden: Cornerstones in Synthetic Biology and Metabolic Engineering Applications. Trends Biotechnol 2016. [DOI] [PubMed] [Google Scholar]
- 33.Steuer R, Kurths J, Fiehn O, Weckwerth W: Observing and interpreting correlations in metabolomic networks. Bioinformatics 2003, 19:1019–1026. [DOI] [PubMed] [Google Scholar]
- 34.Kotze HL, Armitage EG, Sharkey KJ, Allwood JW, Dunn WB, Williams KJ, Goodacre R: A novel untargeted metabolomics correlation-based network analysis incorporating human metabolic reconstructions. BMC Syst Biol 2013, 7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirhaji L, Milani P, Leidl M, Curran T, Avila-Pacheco J, Clish CB, White FM, Saghatelian A, Fraenkel E: Revealing disease-associated pathways by network integration of untargeted metabolomics. Nat Methods 2016, 13:770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B: Predicting network activity from high throughput metabolomics. PLoS Comput Biol 2013, 9:e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans EM, Freund DM, Sondervan VM, Cohen JD, Hegeman AD: Metabolic Patterns in Spirodela polyrhiza Revealed by (15)N Stable Isotope Labeling of Amino Acids in Photoautotrophic, Heterotrophic, and Mixotrophic Growth Conditions. Front Chem 2018, 6:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Nelson CJ, Trosch J, Castleden I, Huang S, Millar AH: Protein Degradation Rate in Arabidopsis thaliana Leaf Growth and Development. Plant Cell 2017, 29:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson CJ, Alexova R, Jacoby RP, Millar AH: Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol 2014, 166:91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan KT, Rendahl AK, Chen WP, Freund DM, Gray WM, Cohen JD, Hegeman AD: Proteome Scale-Protein Turnover Analysis Using High Resolution Mass Spectrometric Data from Stable-Isotope Labeled Plants. J Proteome Res 2016, 15:851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauer MA, Xu B, Sutton F: Metabolic labeling with stable isotope nitrogen (15N) to follow amino acid and protein turnover of three plastid proteins in Chlamydomonas reinhardtii. Proteome Sci 2014, 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishihara H, Obata T, Sulpice R, Fernie AR, Stitt M: Quantifying protein synthesis and degradation in Arabidopsis by dynamic 13CO2 labeling and analysis of enrichment in individual amino acids in their free pools and in protein. Plant Physiol 2015, 168:74–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankaran M, King CL, Angel TE, Holmes WE, Li KW, Colangelo M, Price JC, Turner SM, Bell C,Hamilton KL, et al. : Circulating protein synthesis rates reveal skeletal muscle proteome dynamics. J Clin Invest 2016, 126:288–302.* The authors introduced a strategy for monitoring skeletal muscle protein synthesis rates in rodents and humans over days or weeks by monitoring label incorporation from 2H2O into circulating proteins secreted into the blood.
- 44.Roughan PG, Slack CR: Cellular organization of glycerolipid metabolism. Annu Rev Plant Physiol 1982, 33:97–132. [Google Scholar]
- 45.Allen DK, Bates PD, Tjellstrom H: Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: Past, present and future. Prog Lipid Res 2015, 58:97–120. [DOI] [PubMed] [Google Scholar]
- 46.Shockey J, Regmi A, Cotton K, Adhikari N, Browse J, Bates PD: Identification of Arabidopsis GPAT9 (At5g60620) as an Essential Gene Involved in Triacylglycerol Biosynthesis. Plant Physiol 2016, 170:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, Wang G, Li J, Bates PD, Wang X, Allen DK: Phospholipase Dzeta Enhances Diacylglycerol Flux into Triacylglycerol. Plant Physiol 2017, 174:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shockey J, Lager I, Stymne S, Kotapati HK, Sheffield J, Mason C, Bates PD: Specialized lysophosphatidic acid acyltransferases contribute to unusual fatty acid accumulation in exotic Euphorbiaceae seed oils. Planta 2019, 249:1285–1299. [DOI] [PubMed] [Google Scholar]
- 49.Fan J, Zhou C, Yu L, Li P, Shanklin J, Xu C: Diversion of Carbon Flux from Sugars to Lipids Improves the Growth of an Arabidopsis Starchless Mutant. Plants (Basel) 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurlock AK, Wang K, Takeuchi T, Horn PJ, Benning C: In vivo lipid ‘tag and track’ approach shows acyl editing of plastid lipids and chloroplast import of phosphatidylglycerol precursors in Arabidopsis thaliana. Plant J 2018, 95:1129–1139. [DOI] [PubMed] [Google Scholar]
- 51.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM: Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246:64–71. [DOI] [PubMed] [Google Scholar]
- 52.Pollard M, Delamarter D, Martin TM, Shachar-Hill Y: Lipid labeling from acetate or glycerol in cultured embryos of Camelina sativa seeds: A tale of two substrates. Phytochemistry 2015, 118:192–203. [DOI] [PubMed] [Google Scholar]
- 53.Ecker J, Liebisch G: Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog Lipid Res 2014, 54:14–31. [DOI] [PubMed] [Google Scholar]
- 54.Allen DK: Assessing compartmentalized flux in lipid metabolism with isotopes. Biochim Biophys Acta 2016, 1861:1226–1242. [DOI] [PubMed] [Google Scholar]
- 55.Trotzmuller M, Triebl A, Ajsic A, Hartler J, Kofeler H, Regittnig W: Determination of the Isotopic Enrichment of (13)C- and (2)H-Labeled Tracers of Glucose Using High-Resolution Mass Spectrometry: Application to Dual- and Triple-Tracer Studies. Anal Chem 2017, 89:12252–12260.* A high-resolution mass spectrometry (HRMS) method is presented for tracer enrichment determination in blood plasma for several triple combinations of 13C- and 2H-labeled glucose tracers. Ions arising from 2H-labeled tracers were completely differentiated from those arising from 13C-labeled tracers, thereby allowing the enrichment of each tracer to be simply calculated.
- 56.Zhang Y, Beard KFM, Swart C, Bergmann S, Krahnert I, Nikoloski Z, Graf A, Ratcliffe RG, Sweetlove LJ,Fernie AR, et al. : Protein-protein interactions and metabolite channelling in the plant tricarboxylic acid cycle. Nat Commun 2017, 8:15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sweetlove LJ, Fernie AR: The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat Commun 2018, 9:2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamboni N: C-13 metabolic flux analysis in complex systems. Current Opinion in Biotechnology 2011, 22:103–108. [DOI] [PubMed] [Google Scholar]
- 59.Moreira TB, Lima JM, Coca GC, Williams TCR: Insights into the spatial and temporal organisation of plant metabolism from network flux analysis. Theoretical and Experimental Plant Physiology 2019, 31:215–226. [Google Scholar]
- 60.Allen DK: Quantifying plant phenotypes with isotopic labeling & metabolic flux analysis. Curr Opin Biotechnol 2016, 37:45–52. [DOI] [PubMed] [Google Scholar]
- 61.Wahrheit J, Nicolae A, Heinzle E: Eukaryotic metabolism: Measuring compartment fluxes. Biotechnology Journal 2011, 6:1071–1085. [DOI] [PubMed] [Google Scholar]
- 62.Allen DK, Shachar-Hill Y, Ohlrogge JB: Compartment-specific labeling information in 13C metabolic flux analysis of plants. Phytochemistry 2007, 68:2197–2210. [DOI] [PubMed] [Google Scholar]
- 63.Allen DK, Laclair RW, Ohlrogge JB, Shachar-Hill Y: Isotope labelling of Rubisco subunits provides in vivo information on subcellular biosynthesis and exchange of amino acids between compartments. Plant Cell Environ 2012, 35:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen DK, Evans BS, Libourel IGL: Analysis of isotopic labeling in peptide fragments by tandem mass spectrometry. PLOS ONE 2014, 9:e91537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen DK, Goldford J, Gierse JK, Mandy D, Diepenbrock C, Libourel IGL: Quantification of peptide m/z distributions from 13C-labeled cultures with high-resolution mass spectrometry. Anal Chem 2014, 86:1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lonien J, Schwender J: Analysis of metabolic flux phenotypes for two arabidopsis mutants with severe impairment in seed storage lipid synthesis. Plant Physiol 2009, 151:1617–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyle NR, Sengupta N, Morgan JA: Metabolic flux analysis of heterotrophic growth in Chlamydomonas reinhardtii. PLoS One 2017, 12:e0177292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furtauer L, Weckwerth W, Nagele T: A Benchtop Fractionation Procedure for Subcellular Analysis of the Plant Metabolome. Front Plant Sci 2016, 7:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arrivault S, Guenther M, Florian A, Encke B, Feil R, Vosloh D, Lunn JE, Sulpice R, Fernie AR, Stitt M, et al. : Dissecting the subcellular compartmentation of proteins and metabolites in arabidopsis leaves using non-aqueous fractionation. Mol Cell Proteomics 2014, 13:2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasdekis AE, Alanazi H, Silverman AM, Williams CJ, Canul AJ, Cliff JB, Dohnalkova AC, StephanopoulosG: Eliciting the impacts of cellular noise on metabolic trade-offs by quantitative mass imaging. Nat Commun 2019, 10:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu R, Pan N, Zhu Y, Yang Z: T-Probe: An Integrated Microscale Device for Online In Situ Single Cell Analysis and Metabolic Profiling Using Mass Spectrometry. Anal Chem 2018, 90:11078–11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mandy DE, Goldford JE, Yang H, Allen DK, Libourel IG: Metabolic flux analysis using (1)(3)C peptide label measurements. Plant J 2014, 77:476–486. [DOI] [PubMed] [Google Scholar]
- 73.Rossi MT, Kalde M, Srisakvarakul C, Kruger NJ, Ratcliffe RG: Cell-Type Specific Metabolic Flux Analysis: A Challenge for Metabolic Phenotyping and a Potential Solution in Plants. Metabolites 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blank LM, Sauer U: TCA cycle activity in Saccharomyces cerevisiae is a function of the environmentally determined specific growth and glucose uptake rates. Microbiology-Sgm 2004, 150:1085–1093. [DOI] [PubMed] [Google Scholar]
- 75.Blank LM, Kuepfer L, Sauer U: Large-scale 13C-flux analysis reveals mechanistic principles of metabolic network robustness to null mutations in yeast. Genome Biol 2005, 6:R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christen S, Sauer U: Intracellular characterization of aerobic glucose metabolism in seven yeast species by 13C flux analysis and metabolomics. Fems Yeast Research 2011, 11:263–272. [DOI] [PubMed] [Google Scholar]
- 77.Kogadeeva M, Zamboni N: SUMOFLUX: A Generalized Method for Targeted 13C Metabolic Flux Ratio Analysis. PLoS Comput Biol 2016, 12:e1005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee WD, Mukha D, Aizenshtein E, Shlomi T: Spatial-fluxomics provides a subcellular-compartmentalized view of reductive glutamine metabolism in cancer cells. Nat Commun 2019, 10:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK: Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J Biol Chem 2008, 283:20621–20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filipp FV, Scott DA, Ronai ZA, Osterman AL, Smith JW: Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res 2012, 25:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwender J, Shachar-Hill Y, Ohlrogge JB: Mitochondrial metabolism in developing embryos of Brassica napus. J Biol Chem 2006, 281:34040–34047. [DOI] [PubMed] [Google Scholar]
- 82.Nonnenmacher Y, Palorini R, d’Herouel AF, Kramer L, Neumann-Schaal M, Chiaradonna F, Skupin A,Wegner A, Hiller K: Analysis of mitochondrial metabolism in situ: Combining stable isotope labeling with selective permeabilization. Metab Eng 2017, 43:147–155. [DOI] [PubMed] [Google Scholar]
- 83.Christen S, Lorendeau D, Schmieder R, Broekaert D, Metzger K, Veys K, Elia I, Buescher JM, Orth MF,Davidson SM, et al. : Breast Cancer-Derived Lung Metastases Show Increased Pyruvate Carboxylase-Dependent Anaplerosis. Cell Rep 2016, 17:837–848. [DOI] [PubMed] [Google Scholar]
- 84.Arrivault S, Obata T, Szecowka M, Mengin V, Guenther M, Hoehne M, Fernie AR, Stitt M: Metabolite pools and carbon flow during C4 photosynthesis in maize: 13CO2 labeling kinetics and cell type fractionation. J Exp Bot 2017, 68:283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weissmann S, Ma F, Furuyama K, Gierse J, Berg H, Shao Y, Taniguchi M, Allen DK, Brutnell TP: Interactions of C4 Subtype Metabolic Activities and Transport in Maize Are Revealed through the Characterization of DCT2 Mutants. Plant Cell 2016, 28:466–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szecowka M, Heise R, Tohge T, Nunes-Nesi A, Vosloh D, Huege J, Feil R, Lunn J, Nikoloski Z, Stitt M, et al. : Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell 2013, 25:694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kruger NJ, von Schaewen A: The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol 2003, 6:236–246. [DOI] [PubMed] [Google Scholar]
- 88.Beshir WF, Mbong VBM, Hertog M, Geeraerd AH, Van den Ende W, Nicolai BM: Dynamic Labeling Reveals Temporal Changes in Carbon Re-Allocation within the Central Metabolism of Developing Apple Fruit. Front Plant Sci 2017, 8:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X, Misra A, Nargund S, Coleman GD, Sriram G: Concurrent isotope-assisted metabolic flux analysis and transcriptome profiling reveal responses of poplar cells to altered nitrogen and carbon supply. Plant J 2018, 93:472–488. [DOI] [PubMed] [Google Scholar]
- 90.Masakapalli SK, Bryant FM, Kruger NJ, Ratcliffe RG: The metabolic flux phenotype of heterotrophic Arabidopsis cells reveals a flexible balance between the cytosolic and plastidic contributions to carbohydrate oxidation in response to phosphate limitation. Plant J 2014, 78:964–977. [DOI] [PubMed] [Google Scholar]
- 91.Masakapalli SK, Ritala A, Dong L, van der Krol AR, Oksman-Caldentey KM, Ratcliffe RG, Sweetlove LJ: Metabolic flux phenotype of tobacco hairy roots engineered for increased geraniol production. Phytochemistry 2014, 99:73–85. [DOI] [PubMed] [Google Scholar]
- 92.Dersch LM, Beckers V, Rasch D, Melzer G, Bolten C, Kiep K, Becker H, Blasing OE, Fuchs R, Ehrhardt T,et al. : Novel Approach for High-Throughput Metabolic Screening of Whole Plants by Stable Isotopes. Plant Physiol 2016, 171:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dethloff F, Orf I, Kopka J: Rapid in situ (13)C tracing of sucrose utilization in Arabidopsis sink and source leaves. Plant Methods 2017, 13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang P, Guo L, Jaini R, Klempien A, McCoy RM, Morgan JA, Dudareva N, Chapple C: A (13)C isotope labeling method for the measurement of lignin metabolic flux in Arabidopsis stems. Plant Methods 2018, 14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koley S, Raorane ML, Junker BH: Shoot tip culture: a step towards 13C metabolite flux analysis of sink leaf metabolism. Plant Methods 2019, 15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gebreselassie NA, Antoniewicz MR: (13)C-metabolic flux analysis of co-cultures: A novel approach. Metab Eng 2015, 31:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorka S, Dietrich M, Mayerhofer W, Gabriel R, Wiesenbauer J, Martin V, Zheng Q, Imai B, Prommer J, Weidinger M, et al. : Rapid Transfer of Plant Photosynthates to Soil Bacteria via Ectomycorrhizal Hyphae and Its Interaction With Nitrogen Availability. Front Microbiol 2019, 10:168.** 13CO2 and 15NH4 labeling studies were applied to investigate the short-term flow of plant-assimilated C and fungal-obtained soil N through an in situ ectomycorrhiza system for the first time.
- 98.Hasenour CM, Wall ML, Ridley DE, Hughey CC, James FD, Wasserman DH, Young JD: Mass spectrometry-based microassay of 2H and 13C plasma glucose labeling to quantify liver metabolic fluxes in vivo. Am J Physiol Endocrinol Metab 2015, 309:E191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J,et al. : Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al. : Lactate Metabolism in Human Lung Tumors. Cell 2017, 171:358–371 e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sunny NE, Parks EJ, Browning JD, Burgess SC: Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 2011, 14:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]