Abstract
Background
Alterations in the levels of gamma-aminobutyric acid (GABA) and glutamate+glutamine (Glx), which are major inhibitory and excitatory neurotransmitters, respectively, are frequently associated with insomnia. Previous reports also suggested the involvement of the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) in insomnia and shorter sleep duration. In the current study, we investigated whether the GABA and Glx levels were altered in the ACC/mPFC in subclinical insomnia while focusing on the sleep duration.
Methods
We examined levels of GABA and Glx in the ACC/mPFC of the brain with magnetic resonance spectroscopy in 166 individuals with subjective sleep complaints but without a diagnosis of insomnia. Participants were divided into two groups according to sleep duration (≥ 6 hours/night: n=79 vs. < 6 hours/night: n=74), which was measured using a wrist-worn actigraphy. Working memory function and overall subjective sleep quality were assessed with a computerized neuropsychological test and self-report questionnaire, respectively.
Results
GABA levels in the ACC/mPFC were lower in the shorter sleep duration group relative to the longer sleep duration group (t=−2.21, p=0.03). Glx levels did not differ between the two groups (t=−0.20, p=0.84). Lower GABA levels were associated with lower spatial working memory performance in the shorter sleep duration group (β=−0.21, p=0.03), but not the longer sleep duration group (β=0.04, p=0.72).
Conclusion
Shorter sleep duration was associated with lower GABA levels in the ACC/mPFC. These findings may provide insight into the underlying mechanisms of impaired working memory function related to insomnia and sleep loss.
Keywords: sleep duration, gamma-aminobutyric acid, anterior cingulate cortex, medial prefrontal cortex, magnetic resonance spectroscopy, working memory function
1. INTRODUCTION
Short sleep duration is steadily on the rise, with more than 30% of adults in the US reported sleeping less than 6 hours a day [1]. Although sleep duration may vary among individuals, 7–9 hours of sleep per night was recommended by the National Sleep Foundation for optimal health outcomes including cognitive functions [2]. Short sleep duration has also been reported as an index of insomnia severity since the shorter sleep duration was associated with higher risks for neurobiological and neuropsychological deficits [3, 4] as well as structural [5, 6] and functional [7] brain changes.
Neuroimaging findings from the total or partial sleep deprivation, which is an extreme form of short sleep duration, implies that shorter sleep duration may cause an alteration in metabolism and function of the prefrontal regions including anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) [8, 9]. Since the ACC/mPFC is one of the main components of the default mode network (DMN) that is involved in wakefulness and insomnia, specific vulnerability and involvement of the ACC/mPFC has also been reported in association with sleep duration [10, 11]. Increased cerebral metabolism in the ACC, both during sleep and while awake, was in association with sleep complaints [12, 13]. Reduced gamma-aminobutyric acid (GABA) levels were found in the ACC for insomnia patients, in which lower GABA levels were associated with shorter sleep duration [14]. Based on previous studies that indicate the role of ACC and mPFC in insomnia, we focused on the neurotransmitter levels in the ACC and mPFC in relation to short sleep duration.
Several lines of evidence suggest that GABA, the main inhibitory neurotransmitter in the brain, plays a crucial role in sleep initiation and maintenance. Benzodiazepine receptor agonists are commonly used to promote sleep in insomnia treatment via enhanced receptor affinity for GABA [15]. GABAergic activation increased during sleep onset and continues to rise during sleep to inhibit cells involved in arousal [16]. In alignment with these findings, reduced GABA levels in various brain regions were reported in insomnia patients [14, 17]. A reduction in inhibitory GABAergic neurotransmission may be related to the hyperarousal that is characterized in insomnia [18].
Milder forms of short sleep duration in individuals with sleep complaints have less been investigated despite its high prevalence, partly due to the complex nature of the determinants involved in sleep duration, including genetic, environmental, and cultural factors [19]. In the present study, we examined GABA and glutamate+glutamine (Glx) levels in the ACC/mPFC in individuals with subjective sleep complaints but without insomnia diagnosis. We hypothesized that participants with a shorter sleep duration might have reduced GABA levels. In addition, we examined whether reduced GABA levels are associated with decreased working memory function.
2. METHODS
2.1. Participants
One hundred sixty-six (male: 84, female: 82) individuals who had subjective sleep complaints that lasted for at least one month without a diagnosis of sleep disorders were recruited. Subjective sleep-related complaints included difficulty falling asleep, not staying asleep, waking up during the night or too early in the morning, or waking up feeling exhausted in the morning. Participants were excluded if they had 1) current or a history of primary sleep disorders by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) administered in the interview; 2) major medical, neurological or psychiatric disorders; 3) current use of sleep medications; or 4) contraindications to MRI.
Participants were divided into the following two groups according to actigraphy-measured total sleep time acquired the night before the MRI scan: 1) shorter sleep duration (less than 6 hours of sleep/previous night) and 2) longer sleep duration (more than 6 hours of sleep/previous night). As the recommended sleeping hours are 7–9 hours/night for adults [1], shorter sleep duration was defined as sleeping less than 6 hours [20], which was also associated with cognitive deficits [21, 22]. The study protocol was approved by the Institutional Review Board of Ewha W. University, and all participants provided written informed consent prior to enrollment.
2.2. Measurement of sleep characteristics
Participants were provided with a wrist-worn actigraphy (Actigraph GT3X+, Actigraph®, Pensacola, FL, USA) and were instructed to wear it for the previous night before the MRI scan session. Actigraphy-recorded sleep-related parameters included total sleep time, sleep efficiency, and wake time after sleep onset (WASO). Sleep efficiency indicates the ratio of total sleep time (or total amount of time spent asleep) compared to the total amount of time spent in bed. WASO is an indicator of sleep fragmentation since it is defined as the amount of time spent awake after sleep onset. Actigraphy data were processed using the Actilife 6-software (Version 6.13.1, ActiGraph).
Subjective sleep quality was evaluated with the Pittsburgh Sleep Quality Index (PSQI) [23]. Question 4 of the PSQI provided information about self-reported habitual sleep duration: “During the past month, how many hours of actual sleep did you get at night?”
2.3. Measurement of working memory function
Spatial working memory (SWM) function was measured using the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB) on the same day of MRI scan. We focused on the working memory since a shorter duration of sleep has been reported to affect working memory [24, 25]. Participants are instructed to find and collect tokens inside the boxes presented on the screen. During a single trial, tokens do not appear inside the boxes that have already been selected. Thus, opening a box that already yielded a token or was previously found to be empty was counted as an error. Strategy score reflects whether the participant follows a predetermined sequence of starting the search at a certain box and returning to that box to start a new search, once the token has been found [26]. The number of total errors and strategy scores calculated from the CANTAB software were used as SWM outcomes. Fewer errors and a lower strategy score indicated better performance.
2.4. MRI data acquisition and processing
Magnetic resonance spectroscopy (MRS) and structural MRI data were obtained using a 3.0 Tesla Philips Achieva MR scanner (Philips Medical System, Netherlands) equipped with a 32-channel head coil. High-resolution three-dimensional T1-weighted images were acquired using a magnetization-prepared rapid gradient echo imaging sequence with the following acquisition parameters: repetition time (TR) = 7.4 ms, echo time (TE) = 3.4 ms, field of view = 220 × 220 mm2, voxel size = 1 × 1 × 1 mm3, slice thickness=1 mm, 180 contiguous sagittal slices, flip angle = 8°, number of excitation = 1. The volumetric T1-weighted images were utilized for spectroscopic voxel localization, tissue segmentation, and registration.
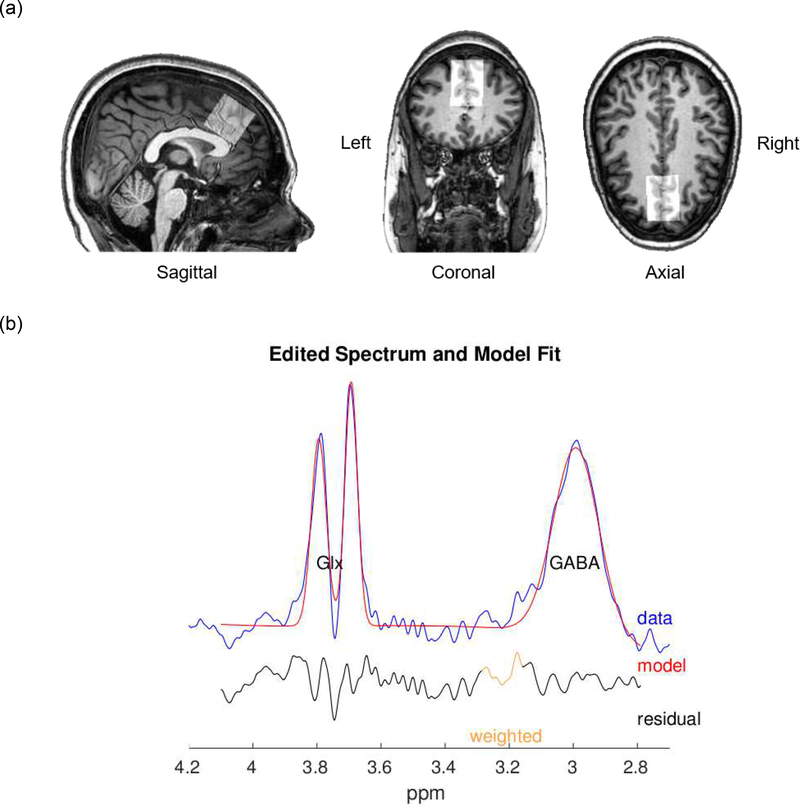
MRS spectra were acquired to measure GABA and glutamate+glutamine (Glx) levels within the voxel of interest (VOI), located in the medial prefrontal and ACC region (3 × 3 × 3 cm ). Specifically, the VOI was aligned with the interhemispheric fissure in the transverse and coronal planes, while placed anterior to the genu of the corpus callosum in the sagittal plane to mainly include the ACC and mPFC while avoiding the skull and lateral ventricles (Figure 1). We used the Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) sequence with the following parameters: TR = 2,000 ms, TE = 80 ms, pulse duration = 20 ms, 160 dynamic scans with 16-phase cycles, scan duration = 11 min, water suppression method = multiple optimizations insensitive suppression train (MOIST), second-order pencil beam shimming. The water suppression band is applied at a frequency of 4.7 ppm. Gaussian editing pulses are alternatively applied at 1.9 ppm (Edit-on) and 1.5 ppm (Edit-off) in even- and odd-numbered acquisitions, respectively, to assess macromolecule-suppressed GABA.
Fig. 1.
(a) Voxel position in the anterior cingulater cortex and medial prefrontal cortex overlaid on a sagittal, coronal, and axial T1-weighted image. The voxel-of-interest was aligned with the interhemispheric fissure in the transverse and coronal planes, while placed anterior to the genu of the corpus callosum in the sagittal plane to mainly include the anterior cingulate cortex. (b) Edited spectrum and model fit of the Glx peak at 3.75ppm and GABA peak at 3ppm using Gannet 3.0. Images were obtained from a representative study participant. GABA, gamma-aminobutyric acid; Glx, glutamate+glutamine
Quantification of GABA and Glx signals was performed using Gannet 3.0, a Matlab-based quantitative batch-processing tool for analyzing GABA-edited MRS spectra [27]. Unsuppressed water and spectroscopy data from the scanner were extracted and filtered with an exponential line broadening of 3 Hz to process the time-domain data into a frequency-domain edited spectrum. Frequency and phase correction was performed on individual frequency domain spectra with spectral registration after a Fourier transformation to maximize the quality of the edited spectrum [28]. The edited spectrum contains a single GABA peak at 3 ppm and a pseudo-doublet Glx peak at 3.75 ppm, fitted using a single Gaussian peak with a five-parameter Gaussian model [27]. The unsuppressed water signal was modeled using a Lorentzian-Gaussian function. Estimates of GABA and Glx levels in “institutional units” relative to the unsuppressed water signal are derived. As the GABA signal ratio is assumed to be 2:1 for gray and white matter, tissue-correction was performed [29]. Spectra with a normalized residual fitting error below 10% were included in the final analysis to control for measurement quality.
2.5. Statistical analyses
Demographic and sleep-related characteristics were compared using independent t-tests and chi-square tests for continuous and categorical variables, respectively. Group differences in tissue-corrected GABA and Glx levels, as well as the scores of spatial working memory tasks, were assessed using multivariate regression models including age, sex, and PSQI total score as covariates. Sex and age were included as covariates since previous literature reported the effects of sex and age on GABA and Glx levels [30, 31]. We included PSQI total score that represents subjective sleep quality as a covariate. All statistical analyses were performed using STATA software package, version 13.0 (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Demographic and sleep-related characteristics
Data from 153 participants are included in the analyses. Demographic and sleep-related characteristics of the shorter sleep duration group (n = 74) and longer sleep duration group (n = 79) are presented in Table 1. Six participants without actigraphy data, and seven participants without available MRS data were excluded. There was no significant difference in age (t = −0.03, p = 0.97) and years of education (t = −1.25, p = 0.21) between the shorter sleep duration group and the longer sleep duration group. However, a significant difference in sex proportion (χ2 = 7.11, p = 0.01) was found in the shorter sleep duration group (male: 45; female: 29) and longer sleep duration group (male: 31; female: 48). Therefore, sex was included as a covariate in all subsequent analyses.
Table 1.
Demographic and sleep-related characteristics of participants
| Longer sleep duration (n = 79) | Shorter sleep duration (n = 74) | t | p | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 37.6 ± 11.9 | 37.6 ± 12.0 | −0.03 | 0.97 |
| Sex (No. of male/female) | 31/48 | 45/29 | 0.01* | |
| Education (years) | 15.5 ± 2.5 | 16.0± 2.0 | −1.25 | 0.21 |
| Actigraphy measures | ||||
| Total sleep time (min) | 423.13± 53.7 | 295.0± 54.5 | 14.7 | <0.001 |
| Sleep efficiency (%) | 88.5 ± 4.7 | 83.0 ± 7.4 | 5.55 | <0.001 |
| Wake time after sleep onset (min) | 48.6 ± 25.5 | 53.9 ± 29.1 | −1.19 | 0.23 |
| Self-reported sleep measures | ||||
| PSQI, total (score) | 6.1 ± 2.1 | 7.1 ± 2.2 | −3.04 | 0.003 |
| PSQI, habitual sleep duration (min) | 393.5 ± 66.2 | 360.5 ± 61.2 | 3.21 | 0.002 |
Data are presented as mean ± standard deviation unless specified otherwise. Based on actigraphy-measured sleep duration acquired the night before the MRI scan, participants were divided into the following two groups: 1) shorter sleep duration (< 6 hours) and 2) longer sleep duration (≥ 6 hours).
p value from the chi-square test
PSQI, Pittsburgh sleep quality index.
Shorter sleep duration group demonstrated lower total sleep time (t = 14.7, p < 0.001) and sleep efficiency (t = 5.55, p < 0.001), measured using the actigraphy the day before the MRI, compared to the longer sleep duration group (Table 1). Shorter sleep duration group also had a significantly lower PSQI total score (t = −3.04, p = 0.003) as well as self-reported habitual sleep duration (t = 3.21, p = 0.002)(Table 1). Actigraphy-measured total sleep time was well correlated with self-reported habitual sleep duration during the past month (β = 0.22, p < 0.001)(Supplementary Figure).
3.2. MRS data quality and tissue segmentation
Edited spectra of GABA were excluded if fitting errors > 10%. There were no group differences in fitting errors of the GABA peak (t = 0.03, p = 0.97) and Glx peak (t = 1.69, p = 0.09), indicating that overall MRS data quality was comparable between the two groups (Supplementary Table). There were no significant group differences in the tissue fractions of cerebral spinal fluid (t = 0.27, p = 0.79), gray matter (t = −0.47, p = 0.64) and white matter (t = 0.20, p = 0.84) in the VOI (Supplementary Table).
3.3. Group difference in GABA and Glx levels and working memory function
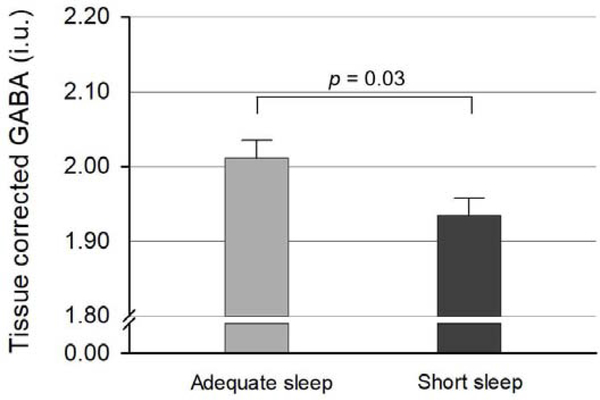
Tissue-corrected GABA levels in the ACC and medial prefrontal VOI were significantly lower in the shorter sleep duration group compared to the longer sleep duration group (t = −2.21, p = 0.03; Table 2 and Figure 2), after controlling for age, sex, and PSQI total score. There was no significant difference in tissue-corrected Glx levels between the two groups (t = −0.20, p = 0.84; Table 2), after controlling for age, sex, and PSQI total score. In addition, there was no significant group difference in working memory performance (total errors: t = 0.71, p = 0.48; strategy score: t = 0.71, p = 0.48; Table 2).
Table 2.
Comparison of neurotransmitter levels and working memory functions
| Longer sleep duration (n = 79) | Shorter sleep duration (n = 74) | t | p | |
|---|---|---|---|---|
| Neurometabolite levels | ||||
| GABA (i.u.) | 2.012 ± 0.212 | 1.932 ± 0.206 | −2.21 | 0.03 |
| Glx (i.u.) | 4.748 ± 0.600 | 4.746 ± 0.472 | −0.20 | 0.84 |
| Neuropsychological measures | ||||
| SWM, total errors (score) | 53.3 ± 61.3 | 57.4 ± 63.5 | 0.71 | 0.48 |
| SWM, strategy (score) | 37.0 ± 25.9 | 39.4 ± 27.1 | 0.71 | 0.48 |
Data are presented as mean ± standard deviation, which are available in 153 participants.
Neurotrasmitter levels are tissue-corrected values.
The t and p values are derived from the multivariate regression model including the following variables: GABA, Glx levels, SWM total error and strategy scores as dependent variables; sleep duration group as an independent variable; age, sex, and total PSQI score as covariates.
GABA, gamma-aminobutyric acid; Glx, glutamate+glutamine; SWM, Spatial working memory task.
Fig. 2.
Tissue corrected GABA levles in the anterior cingulate cortex/medial prefrontal cortex were significantly lower in the shorter sleep duration group compared to the longer sleep duration group, after controlling for age, sex, and self-reported sleep quality (t = −2.21, p = 0.03). Error bars indicate standard error of means. GABA, gamma-aminobutyric acid; i.u., institutional unit
3.4. Correlations between GABA levels and working memory function
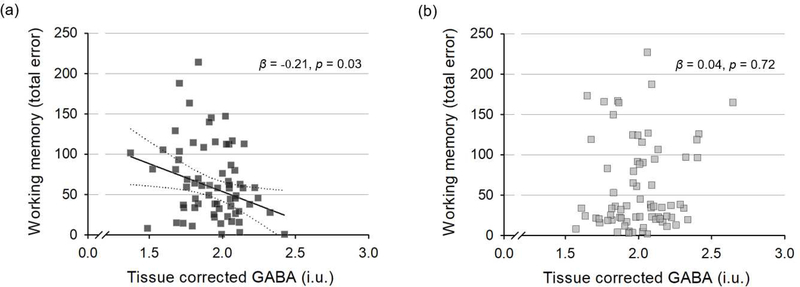
Lower tissue-corrected GABA levels in the ACC and medial prefrontal cortex were associated with worse performance in working memory within the shorter sleep duration group. Specifically, tissue-corrected GABA levels showed a significant negative association with SWM total errors (β = −0.21, p = 0.03; Figure 3), after controlling for age, sex, and PSQI total score. The association between tissue-corrected GABA levels and SWM total errors was not significant for the longer sleep duration group (β = 0.04, p = 0.72; Figure 3). Tissue-corrected GABA levels did not show significant associations with SWM strategy scores for both groups.
Fig. 3.
Scatter plots with the line of best fit are shown between tissue-corrected GABA and working memory outcome. (a) In the shorter sleep duration group, GABA levels showed a negative correlation with number of total errors (β = −0.21, p = 0.03), after controlling for age, sex, and self-reported sleep quality. (b) In the longer sleep duration group, there was no significant correlation between GABA concentration and number of total errors in the working memory tests (β = 0.04, p = 0.72). GABA, gamma-aminobutyric acid; i.u., institutional unit.
4. DISCUSSION
Shorter sleep duration was associated with lower GABA levels in the ACC/mPFC among individuals with subjective sleep complaints. It is notable that the study participants were not experimentally deprived of sleep, but naturally experienced a shorter sleep duration relative to the recommended hours. These results may suggest that GABA levels in the ACC/mPFC may be sensitive markers altered in shorter sleep duration. In addition, lower GABA levels detected in our study were associated with lower spatial working memory function in the short sleep duration group, suggesting a potential brain mechanism underlying impaired working memory and daily functioning following shorter sleep duration. These findings replicate and extend previous results, corroborating the critical role of GABA in sleep.
Despite its recognition as a key inhibitory neurotransmitter for sleep initiation and maintenance, the underlying mechanism of lower GABA in shorter sleep duration was not clearly defined. Since increased activity in the ACC/mPFC has been associated with insomnia severity and hyperarousal [12, 13], reduction in GABA levels may have induced an over-activation of the ACC, resulting in hyperarousal in insomnia. The roles of GABA in the ACC/mPFC have also been focused on as the ACC/mPFC is the main component of the default mode network (DMN) [32]. Among the resting-state functional networks, DMN has been frequently associated with insomnia in that over-activation of the DMN could be related to hyperarousal in insomnia. Therefore, lower inhibitory GABA levels in the ACC/mPFC detected in the current study may provide supporting evidence on the mechanism of over-activation in the DMN. In addition, lower GABA levels in the ACC/mPFC may have induced impairment in the functional decoupling between the DMN and attentional networks, thus impeding necessary deactivation of regions within DMN, considering the previous findings which reported disengagement of the DMN was required for successful performance in goal-directed cognitive tasks [33].
The study findings are in line with previous reports of lower GABA levels associated with sleep deprivation, which is an extreme form of short sleep duration [14, 17, 34]. Participants of the current study had subjective sleep complaints but did not meet the diagnostic criteria of primary insomnia. As such, our findings may imply that the GABA levels in the ACC/mPFC could be altered in the subclinical stage of insomnia. Lower GABA levels in the ACC/mPFC were associated with lower working memory function in individuals with shorter sleep duration in the current study. Considering that prefrontal GABAergic interneurons are strongly involved in working memory function [35, 36], as well as in hemodynamic brain activity [35], findings of the current study may provide additional evidence on the roles of GABAergic activities in working memory function even in the lesser severe form of insomnia.
The shorter and longer sleep duration groups in the current study may correspond to the milder forms of insomnia known as “insomnia with objective short sleep duration” and “insomnia with normal sleep duration,” respectively. In addition, the longer sleep duration group may also be classified as objective-subjective sleep discrepancy [37–39]. As such, the findings of the present study may provide additional evidence that insomnia with normal sleep duration could have distinct features in both neurotransmitters and working memory functions.
The study results should be interpreted carefully in consideration of several limitations. Since the GABA-edited MRS using MEGA-PRESS sequence requires a relatively large voxel size for a high signal-to-noise ratio signal, a more precise region-specific interpretation of the results could be limited. Furthermore, the MRS provides a static measurement of GABA and Glx levels averaged for several minutes, limiting the reflection of dynamic changes in synthesis or uptake. Regarding the cross-sectional study design, the causal relationship between GABA levels and sleep duration could not be assessed. As the time of MRS acquisitions was variable among the participants, the intrinsic circadian fluctuation of GABA may have influenced the results, although diurnal stability of using MEGA-PRESS for GABA quantification has been demonstrated [40, 41]. Finally, despite the similar proportion of sex during the recruitment of participants, there was a higher percentage of women in the longer sleep duration group. The observed discrepancy in sex proportion is in line with previous studies that reported longer sleep durations for women [42, 43]. However, other studies report contrasting results that women are more susceptible to insomnia and typically report poorer sleep quality [44, 45]. Although we included sex as a covariate in all analyses in an effort to statistically control for any potential effects, further studies are required for a better understanding of sex differences in sleep duration.
5. CONCLUSION
The present study demonstrated significantly lower GABA levels in the ACC/mPFC in association with shorter sleep duration in individuals with subjective sleep complaints. Lower GABA levels were also correlated with worse working memory function among those with shorter sleep duration. The current study findings may provide further evidence on the involvement of GABA in subclinical insomnia, specifically with sleep duration. In addition, these results may provide insight into the underlying mechanism of working memory impairment associated with shorter sleep duration in insomnia.
Supplementary Material
Highlights.
Subclinical insomnia with shorter sleep duration showed reduced brain GABA levels.
Reduced prefrontal GABA levels well correlated with lower working memory function.
Glx levels did not differ between those with shorter and longer sleep duration.
Disclosures and acknowledgments
This study was supported by the National Research Foundation of Korea grants funded by the Ministry of Science and ICT (2015M3C7A1028373 and 2015M3C7A1028376) and the Ministry of Education (2017R1D1A1B03034453) of South Korea. This study was also supported by the Field-oriented Support of Fire Fighting Technology Research and Development Program funded by the National Fire Agency (MPSS-Fire Fighting Safety-2016-86). This study applies tools developed under NIH grants R01 EB016089, R01 EB023963 and P41 EB015909; RAEE also receives salary support from these grants.
Footnotes
Authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Sheehan CM, Frochen SE, Walsemann KM, Ailshire JA. Are U.S. adults reporting less sleep?: Findings from sleep duration trends in the National Health Interview Survey, 2004–2017. Sleep. 2018;42:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–43. [DOI] [PubMed] [Google Scholar]
- [3].Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khalsa S, Hale JR, Goldstone A, Wilson RS, Mayhew SD, Bagary M, et al. Habitual sleep durations and subjective sleep quality predict white matter differences in the human brain. Neurobiol Sleep Circadian Rhythms. 2017;3:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage. 2012;60:471–5. [DOI] [PubMed] [Google Scholar]
- [7].Killgore WDS, Schwab ZJ, Weiner MR. Self-reported nocturnal sleep duration is associated with next-day resting state functional connectivity. Neuroreport. 2012;23:741–5. [DOI] [PubMed] [Google Scholar]
- [8].Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. [DOI] [PubMed] [Google Scholar]
- [9].Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6(11):475–81. [DOI] [PubMed] [Google Scholar]
- [10].Demos KE, Sweet LH, Hart CN, McCaffery JM, Williams SE, Mailloux KA, et al. The effects of experimental manipulation of sleep duration on neural response to food cues. Sleep. 2017;40:zsx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Killgore WD, Schwab ZJ, Weiner MR. Self-reported nocturnal sleep duration is associated with next-day resting state functional connectivity. Neuroreport. 2012;23:741–5. [DOI] [PubMed] [Google Scholar]
- [12].Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. [DOI] [PubMed] [Google Scholar]
- [13].Nofzinger EA, Nissen C, Germain A, Moul D, Hall M, Price JC, et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med. 2006;2:316–22. [PubMed] [Google Scholar]
- [14].Plante DT, Jensen JE, Schoerning L, Winkelman JW. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: a link to major depressive disorder? Neuropsychopharmacology. 2012;37:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gottesmann C GABA mechanisms and sleep. Neuroscience. 2002;111:231–9. [DOI] [PubMed] [Google Scholar]
- [16].Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry. 2004;65:4–7. [PMC free article] [PubMed] [Google Scholar]
- [17].Winkelman JW, Buxton OM, Jensen JE, Benson KL, O’Connor SP, Wang W, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31:1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. [DOI] [PubMed] [Google Scholar]
- [19].Alves FR, de Souza EA, de França Ferreira LG, de Oliveira Vilar Neto J, de Bruin VMS, de Bruin PFC. Sleep duration and daytime sleepiness in a large sample of Brazilian high school adolescents. Sleep Med. 2019;66:207–15. [DOI] [PubMed] [Google Scholar]
- [20].Kim J-Y, Yadav D, Ahn SV, Koh S-B, Park JT, Yoon J, et al. A prospective study of total sleep duration and incident metabolic syndrome: the ARIRANG study. Sleep Med. 2015;16:1511–5. [DOI] [PubMed] [Google Scholar]
- [21].Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. [DOI] [PubMed] [Google Scholar]
- [22].Wild CJ, Nichols ES, Battista ME, Stojanoski B, Owen AM. Dissociable effects of self-reported daily sleep duration on high-level cognitive abilities. Sleep. 2018;41:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [24].Smith ME, McEvoy LK, Gevins A. The impact of moderate sleep loss on neurophysiologic signals during working-memory task performance. Sleep. 2002;25:56–66. [PMC free article] [PubMed] [Google Scholar]
- [25].Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–34. [DOI] [PubMed] [Google Scholar]
- [27].Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Verweij IM, Romeijn N, Smit DJA, Piantoni G, Van Someren EJW, van der Werf YD. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Meyerhoff DJ, Mon A, Metzler T, Neylan TC. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep. 2014;37:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, et al. Frontal GABA levels change during working memory. PLoS One. 2012;7:e31933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yoon JH, Grandelis A, Maddock RJ. Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J Neurosci. 2016;36:11788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J Sleep Res. 2015;24:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kay DB, Karim HT, Soehner AM, Hasler BP, James JA, Germain A, et al. Subjective-objective sleep discrepancy is associated with alterations in regional glucose metabolism in patients with insomnia and good sleeper controls. Sleep. 2017;40:zsx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rezaie L, Fobian AD, McCall WV, Khazaie H. Paradoxical insomnia and subjective-objective sleep discrepancy: A review. Sleep Med Rev. 2018;40:196–202. [DOI] [PubMed] [Google Scholar]
- [40].Spiegelhalder K, Regen W, Nissen C, Feige B, Baglioni C, Riemann D, et al. Magnetic resonance spectroscopy in patients with insomnia: a repeated measurement study. PLoS One. 2016;11:e0156771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Evans CJ, McGonigle DJ, Edden RAE. Diurnal stability of γ-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–9. [DOI] [PubMed] [Google Scholar]
- [42].Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA Study. Am J Epidemiol. 2006;164:5–16. [DOI] [PubMed] [Google Scholar]
- [44].Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans Royal Soc B. 2016;371:20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang B, Wing Y-K. Sex differences in insomnia: A meta-analysis. Sleep. 2006;29:85–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.