Brain iron deficiency (BID) particularly in the substantia nigra (SN) is a well-documented biological abnormality of the restless legs syndrome (RLS). It has been documented in 24 of 26 reports. These include: 7 Magnetic resonance imaging (MRI) [1–7], 7 midbrain ultrasound [4, 8–13], 4 cerebral spinal fluid (CSF) [14–17] and 5 autopsy studies [18–22]. Only 2 of all 26 studies of brain iron in RLS failed to show BID [23, 24]. These were both MRI studies using the less accurate R2 rather than R2* or R2’ measure [6] with small RLS sample sizes (n=11, 12).
Two major features of BID in RLS patients define the pattern of the pathophysiology. First, The BID occurs despite normal peripheral iron stores [5, 14–16]. The critical pathophysiology is the brain and not peripheral iron status. Second, the BID of RLS involves regional more than total brain iron. It occurs mostly for some brain regions, particularly the substantia nigra (SN) in 5 of the 7 MRI studies [1–3, 5, 6], all 7 of the ultrasound studies [4, 8–13] and all 4 of the autopsy studies that evaluated SN iron [18–21]}.
The ubiquity in RLS of BID, particularly of the SN, raises the putative hypothesis that the biology of the RLS BID produces the RLS symptoms. Association, however, does not prove such a direct relation. One approach to study the nature of the BID biology relation to RLS would be an experimental study of an animal model biologically developed to reproduce the RLS BID pattern noted above. This animal model could be experimentally evaluated for effects of factors known to alter clinical RLS, e.g. dopaminergic treatments, increasing iron deficiency. These are both evaluated in the study reported in this paper. The study uses 1) an animal known to have an RLS-like iron biological pattern and 2) an established RLS-like behavioral phenotype appropriate for that animal.
First, the study uses an animal model based on the RLS iron biology pattern noted above. Forward genetic studies of the BXD inbred strains has identified one strain that has the experimentally induced RLS BID pattern of ventral midbrain (VMB) (containing the substantia nigra) Iron deficiency (ID) without peripheral ID. Twenty-two recombinant BXD strains and their parental strains (C57BL/6J and DBA/2J) upon weaning at post-natal day 21 were placed for the next 100 days on the same pellet diets except these were either iron sufficient (240 ppm) or iron deficient (3–5 ppm) pellets (Teklad, Inc). The VMB iron between mice on sufficient vs. deficient diets varied by 35% between the strains [25]. Moreover, across the 22 strains there were no correlation between the VMB and peripheral iron loss with the iron deficiency diet [26] indicating different genetic regulation of peripheral and brain iron. Strain 40 females were identified as having the RLS iron biological pattern of significant VMB iron loss with little change in Hgb [25, 27, 28]. (Note that in the following the reference to BXD40f is used to indicate BXD40 females. BXD40 males did not show the same iron management profile as the females and thus were not evaluated in the study.)
Second, the study evaluates experimental effects on a previously established RLS-like behavioral phenotype, i.e. increased motor activity in the last part of the active cycle [29]. This RLS-like behavior is based on the RLS clinical diagnostic crtierion of a circadian pattern (worse at the end of the day) of an urge or awareness of a drive to move [30].
The study evaluates the RLS-like behaviors in the BXD40f animal model of the RLS iron biology. It tests two important experimental hypotheses linking BXD40f BID to RLS-like behavioral changes, i.e. 1) increasing BXD40f BID will increase the RLS-like behaviors following the clinical pattern seen for increased RLS severity, and 2) Dopamine agonist treatment of the BXD40f will reduce the RLS-like behaviors produced with increased BID. These hypotheses if confirmed would add to the validation of the BXD40f as a useful animal model to study RLS and also support the putative link of the BID biology to RLS.
Methods
Animals
The study follows the animal care guidelines of the National Institutes of Health with Penn State Institutional Animal Care and Use committee approval. The BXD40f were maintained under constant light-dark cycle (05:00– 17:00 hours, lights-off) and housed with 40% relative humidity and 23 ± 2 degrees C ambient temperature. Mice were group housed 3–4 per cage from weaning until the G2 E-Mitter telemetry devices were implanted 10 days prior to the beginning of data collection. After surgery, the mice were housed one per cage.
Dietary treatment
13 BxD40 females upon weaning at post-natal day 21 were randomly assigned 6 to iron-sufficient and 7 to iron-deficient pellet diet (120 ppm or 3–5 ppm) (Teklad, Inc.) given ad libitum. Deionized distilled water was also provided ad libitum. This followed the procedures previously demonstrated to produce iron deficiency (ID) in the ventral midbrain without significant peripheral iron loss for these BXD40f on the iron-deficient diet [25, 27, 28] . The sample sizes were similar to the 5 −9 mice per condition that showed statistically significant RLS-like behaviors in a prior study [29]. The effect size from this prior study was a mean difference of approximately 1.5 standard deviations giving 80% power for samples sizes of 6 comparing means from 2 samples with a one-sided test [31, 32].
RLS-like behavioral measures
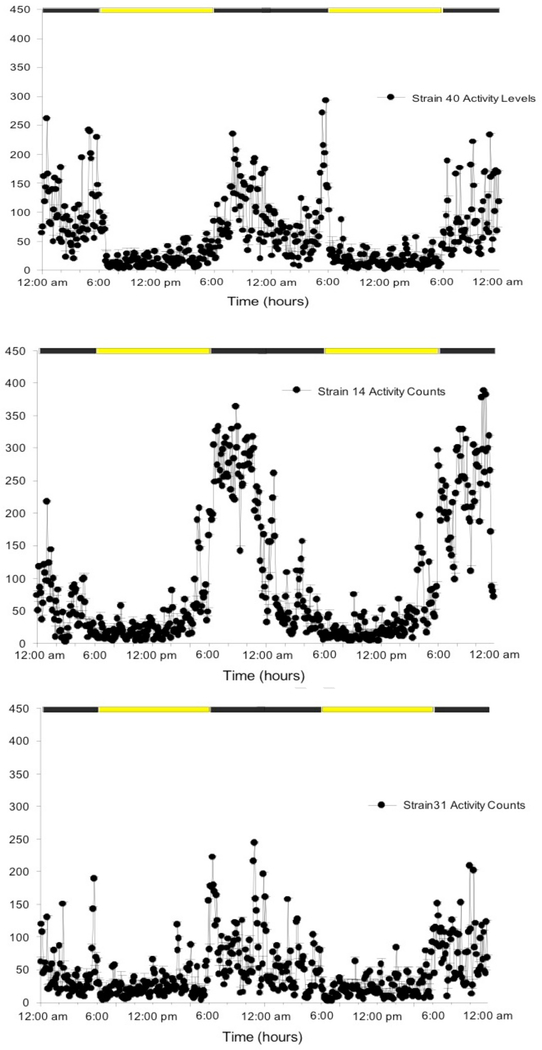
A prior study [29] comparing BXD40f to other BXD strains maintained on iron-sufficient diets identified a significant RLS-like behavior. This was based on essential diagnostic features defining RLS, i.e. a strong circadian increase in the urge or awareness of the drive to move when inactive at the end of the normal waking or active period of the day [30]. This diagnostic feature of RLS leads to a clinical pattern of increased restlessness and motor activity at the end of the waking period [33, 34]. Not only is the RLS restlessness and urge or drive to move expressed more in the evening but also as the condition becomes more severe its expression occurs earlier in the day as most clearly shown by RLS augmentation [35]. This strong circadian feature of RLS was used by one of the first RLS scales which defined severity by how early in the day symptoms usually start [36]. Thus, for animals one major RLS-like behavioral phenotype involves increased motor activity in the end of the active period. A corollary RLS-like behavioral phenotype would be decreased rest periods in the end of active period consistent with the essential RLS diagnostic criteria of rest inducing the RLS drive to move [30]. The RLS-like behavior of increased activity in the last 2 hours of the active cycle was shown in a prior study to be a unique feature of the BXD40f and not other BXD strains examined when fed an iron-sufficient diet [29]. (See figure 1) These BXD40f also had the largest circadian change in VMB iron producing the lowest VMB iron content in the active but not inactive period. Thus, the BXD40f showed both the lowest active-period VMB iron levels and the greatest end-of-active-period activity when on an iron sufficient diet.
Figure 1:
Average total activity counts for every 6-minute period over 2 days for 3 female BXD strains n=8 for strains 40 and 31, n=7 for strain 14).
The lights off active period is from 6 PM to 6 AM. Note for BXD40f only (top graph) the repeated peak increases in activity during the last 2 hours of the active cycle from 4 – 6 AM on each day. This one period of time, 2 hours before the end of the active period, has consistently greater activity for the BXD40f compared to all seven other strains evaluated as reported by Earley et. al, Sleep Med, 2019. (Graphs provided by Erica Unger.) The two other strains shown here for comparison are chosen for overall high (strain 14) or low (strain 31) activity, neither shows increased activity at 4– 6 AM.
Hypothesis testing
It was hypothesized that the BXD40f on an ID diet known to reduce VMB iron but not Hgb would show an RLS-like behavioral pattern similar to the clinical pattern of worsening RLS [36], i.e. onset of the increased activity at the end of the active period occurring earlier than that observed for the iron-sufficient diet condition. Similar changes were hypothesized to occur for decreased resting time. It was also hypothesized that dopamine agonist treatment would reduce ID diet effects on both of these RLS-like behaviors. This study was thus planned assuming the RLS-like behaviors for the BXD40f would occur one hour earlier on the ID diet than the 2-hours before end of the active period previously observed on the iron-sufficient diet shown in figure 1 [29].
Activity and rest measurements
Activity levels were measured using implanted G2 E-Mitter telemetry devices (Respironics/Mini Mitter Company, Bend OR). Mice were anesthetized on postnatal day 92–93 using 3% isoflurane, and telemetry devices were implanted into the peritoneal cavity following the manufacturer’s protocol. The mice were then housed one per cage and had a 7-day recovery period. Activity recordings were then obtained by placing home cages on ER4000 energizers/receivers. Gross body movement events were determined by changes in signal strength as the mouse moved on the receiver board. This counts gross body movements but does not indicate nature or velocity of the movements. The total activity count for body movements was recorded for each 6-minute period for 24 hours of each day in the study using the VitalView program.
The primary outcome measures were the total activity counts for each hour and rest time occurring during each hour. Rest time was defined as any 6-minute period with no activity. A rest epoch was defined as any consecutive sequence of 6- minute rest periods that could be as short as one 6- minute period. The total minutes included in rest periods and the number of rest epochs were evaluated for each hour.
Medication Treatment
It was hypothesized, as noted above, that an ID diet would worsen the RLS-like state with an onset of the RLS-like circadian behavioral phenotype earlier than displayed by BXD40f fed an iron-sufficient diet [29]. The medications were accordingly given IP at 3 rather than 2 hours before the end of the dark (active) period starting at 100 days post-weaning. Five treatments were used: saline (vehicle only), 12.5 mg levodopa, 25 mg levodopa, 0.5 mg quinpirole, and 1 .0 mg quinpirole. Each mouse received all 5 treatments in the order given above, but each was started at a different point in this order. Two animals were started at each of 0.5 mg quinpirole and at 25 mg levodopa doses and one animal was started at each of 1.0 mg quinpirole, saline, and 12.5 mg levodopa doses. There were two days between each treatment administration.
Data analyses
The analyses focused on the a priori hypothesized times of expected maximal effect sizes. Further analyses explored possible differences during the remaining times studied.
The hypothesized earlier onset of increased activity for the iron-deficient compared to iron-sufficient diet BXD40f mice was tested by a between subjects one-tailed unequal variance t-test of activity levels for the 3rd hour before lights on. It was hypothesized that this would be greater for the iron-deficient (ID) than the iron-sufficient diet animals after saline injection. The differences in the other 2 hours before lights and the differences for each 6- minute period across the 24-hour day were evaluated as exploratory analyses of ID compared to iron-sufficient diet animals. The rest measures were also examined, but floor effects prohibited statistical analyses.
The hypothesized benefits for each of the dopaminergic treatments were similarly evaluated for the first hour after the treatment for total activity, minutes of rest time and number of rest periods using within-subjects unequal variance t-tests. It was hypothesized that the treatment would decrease overall activity and increase resting time and number of rest periods. Exploratory analyses examined dose effects and the persistence of effect over the remaining 2 hours before lights on.
Results
The analyses confirmed the hypothesis of significantly increased activity during the 3rd hour before the end of the active period for animals on the ID compared to control diet (see table 1). There were, however, no significant differences between animals on the ID vs. control diets for the remaining 2 hours of the active period. Both ID and control animals show the same increased activity for these last 2 hours of the active period that is unique for the BXD40f (see figure 1). There were no minutes of resting for either ID or iron-sufficient diet animals in the 3rd hour before end of the active period and only 3 of the 13 animals had any rest activity for the 2nd hour. All but 3 of the animals (2 with ID and 1 with iron-sufficient diet) had some rest time in the last hour before lights-on but there were no significant differences in rest measures between ID and iron-sufficient diet animals (average ± sd: minutes resting 11.1 ±9.4 vs. 11.0 ±12.8, p>0.1, number rest epochs 1.4 ±1.1 vs. 1.2 ±1.0, p> 0.1, respectively).
Table 1:
Activity counts/1000 average ± standard deviation during each of the last 3 hours of the active period for mice on iron-deficient or iron-sufficient diets treated with saline.
| Hours before end of active period | 2– 3 hours | 1 – 2 hours | 0 – 1 hour |
|---|---|---|---|
| Iron-sufficient diet | 2.0 ± 0.7 | 2.1 ± 0.4 | 0.6 ± 0.4 |
| Iron-deficient (ID) diet | 3.8 ± 1.1* | 2.5 ± 2.2 | 1.4 ± 0.2 |
Difference between iron-deficient and iron-sufficient p=0.002, t test, unequal variance
There were no other significant differences between diet groups(p<0.05).
Almost all of the animals on both iron-deficient and iron-sufficient diets had no rest times for the 2–3 and 1–2 hours before end of the active period these and showed no difference in rest minutes or epochs for the last hour before end of the active period.
Activity counts: Sum for each animal of number of gross body movements in each 6-minute period detected by the G2 E-Mitter telemetry. Average and SD total activity determined for 6 animals on iron-sufficient diet and 7 on iron-deficient diet.
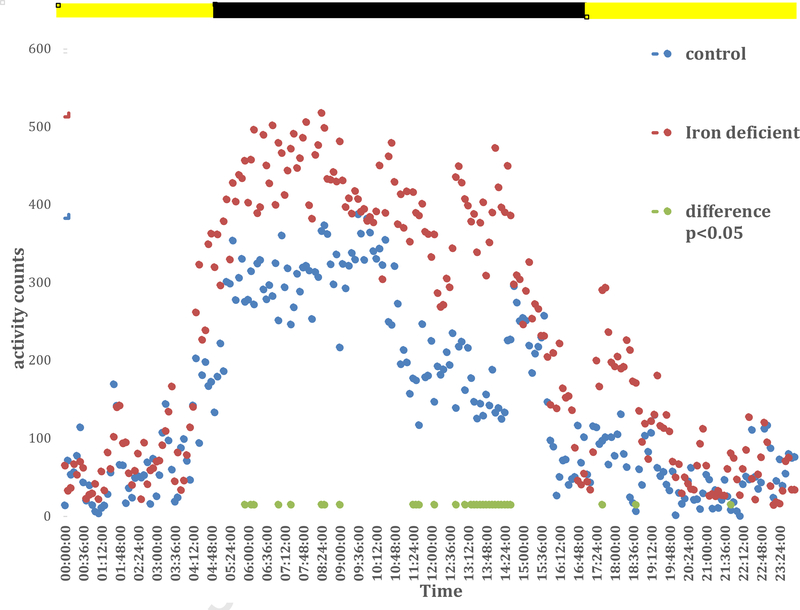
Exploratory analyses compared the 24-hour pattern of activity levels of animals on ID vs. iron-sufficient (control) diets. The ID compared to iron-sufficient diet animals had generally more activity throughout the active but not the inactive period (see figure 2), but the differences were not persistently statistically significant except for a 90-minute period from 13:06 to 14:36. Figure 2 shows all the times with statistically significant differences (p<0.05, separate unequal variance t-tests for each time period.)
Figure 2:
Average total activity for every 6 minutes for the day with saline injection for BXD40f on Iron-sufficient (control, n=6) and iron-deficient (ID, n=7) diets.
Lights off active period is from 5 AM to 5 PM shown by the dark bar at the top of the figure. Note the control replicates the increased activity to the levels seen earlier in the day for the 2-hour period before lights on (17:00 – 05:00) reported in a prior study (Earley et al, 2019) and shown in figure 1. The ID show the same pattern except it starts about 2 hours earlier and persists even into the very first part of the inactive period. Overall activity during the active period is increased for the ID compared to the control but this is a consistent statistically significant difference only during 13:06 – 14:36, the 4th and 3rd hours before end of the active cycle.
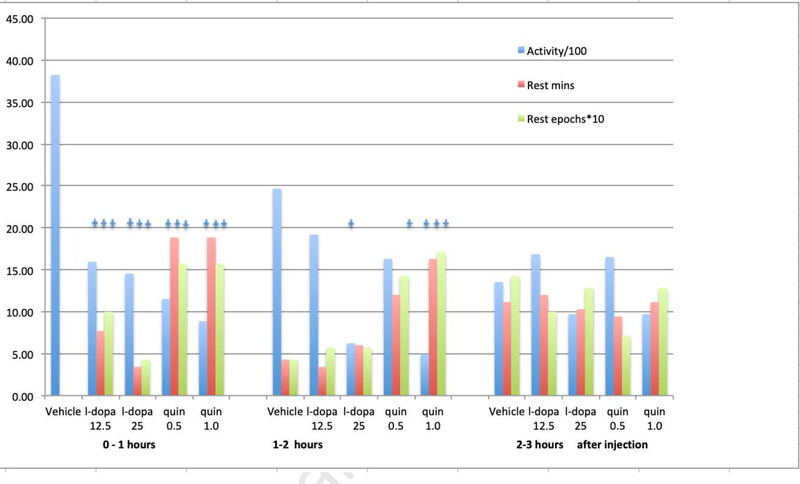
The analyses also confirmed the hypothesized dopaminergic treatment effects for the 1st hour after treatment. Average activity significantly decreased and the rest minutes and number of rest periods significantly increased for all treatments. The animals on the 25 mg but not the 12.5 mg dose of levodopa showed significant persistence of effect for reducing activity in the 2nd hour after treatment but neither dose produced significant differences from saline at 3 hours after treatment. The 1.0 mg quinpirole dose showed persistent significant effect for reducing activity and increasing both rest time and number of rest periods for the 2nd hour after treatment. The 0.5 mg quinpirole dose compared to placebo showed significant increase in number of rest periods but not in the other measures for the 2nd hour after treatment (See table 2 and figure 3).
Table 2.
Average ± SD of total activity counts/1000, minutes resting and number of rest periods for the each of the 3 hours after each treatment – last 3 hours of the active period for the BXD40f on an ID diet.
| 1st hour | 2nd hour | 3rd hour | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Activity/1000 | Mins rest | # rest periods | Activity/1000 | Mins rest | # rest periods | Activity/1000 | Mins rest | # rest periods |
| Saline | 3.8 ±1.1 | 0.0 ± 0 | 0.0 ± 0 | 2.5 ± 2.2 | 4.3 ±5.7 | 0.4 ± 0.5 | 1.4 ± 1.6 | 11.1 ± 9.4 | 1.4 ± 1.1 |
| 12.5 mg L-dopa | 1.6 ±1.1**** | 7.7 ± 8.3 * | 1.0 ± 1.0 * | 1.9 ± 1.2 | 3.4 ± 4.7 | 0.6 ± 0.8 | 1.7 ±1.5 | 12.0 ± 9.8 | 1.0 ± 0.8 |
| 25.0 mg L-dopa | 1.5 ± 1.0*** | 3.4 ±4.7* | 0.4 ± 0.5* | 0.6 ± 0.2* | 6.0 ± 10.0 | 0.6 ± 1.0 | 0.9 ± 0.7 | 10.3 ± 8.3 | 1.3 ± 0.8 |
| 0.5 mg Quin | 1.2 ± 0.8**** | 18.9 ± 16.0** | 1.6 ± 1.1*** | 1.6 ±1.2 | 12.0 ± 9.8 | 1.4 ± 1.0* | 1.7 ± 1.2 | 9.4 ± 12.0 | 0.7 ± 1.0 |
| 1.0 mg Quin | 0.9 ± 0.7**** | 18.9 ± 15.3** | 1.6 ± 1.1** | 0.5 ± 0.2* | 16.3 ± 9.6* | 1.7 ± 1.0* | 1.0 ± 0.5 | 11.1 ± 4.1 | 1.3 ± 0.5 |
Significance for within subject t test compared to saline
p<0.001
p< 0.005
p< 0.01
p< 0.05
Activity counts: Sum for each animal of number of gross body movements in each 6-minute period detected by the G2 E-Mitter telemetry. Average and SD total activity determined for 6 animals on iron-sufficient diet and 7 on iron-deficient diet.
Figure 3:
RLS circadian phenotype behavior for each treatment condition and each of the 3 hours after treatment – ending at onset of the inactive (light) period. (n=7 Iron deprived BxD40f)
The stars over the bars indicate statistically significant differences (p<0.05) compared to vehicle (saline). The same 7 mice (BXD40f) after post-weaning dietary iron deprivation received each of the five treatments in the same order but with rotating starting points in the order. There was one day between each treatment.
Discussion
These data provide strong support for the hypothesis that the biology of RLS BID produces RLS symptoms. The BXD40f were selected because they uniquely show the two basic BID features of RLS: i.e. regional BID involving VMB and BID without peripheral ID [25, 27]. A prior study showed that the BXD40f also uniquely showed the circadian RLS-like behaviors that were not shown by other strains without the BID features of RLS[29]. The RLS-like behaviors were present only when the BID features of RLS were present. This current study further demonstrated that dietary ID known to make BID worse in the BXD40f strains [25, 27] also made the RLS-like behaviors occur earlier following the same circadian pattern for effects of increasing RLS severity, i.e. starting earlier in the evening more than persisting longer after starting [36]. Finally, levodopamine and dopamine agonist treatments known to be dramatically effective for reducing RLS symptoms [37–39] similarly reduced the RLS-like behaviors in these BXD40f. Thus, the BXD40f provide a useful animal model for RLS serving to support the hypothesized relation between RLS BID biology and RLS symptoms. How the BID biology produces these symptoms remains to be further explored, but the BXD40f provide a useful animal model for exploring these relations.
Four features of this BXD40f model deserve special note. First, the model was based on considerations of RLS iron biology attempting to produce the brain iron deficiency without peripheral iron deficiency. The observed behavioral circadian changes cannot be explained by anemia or peripheral iron changes. They reflect an effect of brain iron deficiency on central nervous system (CNS) functioning. It should be noted, however, that concurrent anemia maybe less of a problem for studies in other rodents of BID effects not involving behaviors, e.g. such studies have established BID effects on brain dopamine biology similar to that seen in RLS patients [40–43]. The peripheral anemia seems unlikely to alter these relations within the brain. The genetic differences, however, may have some effect on these relations, but this remains to be evaluated.
Second, the model does not involve any direct manipulation of dopamine or dopaminergic systems. Nonetheless, the dopaminergic treatments reducing RLS circadian urge to move also reduce the RLS-like circadian pattern of excessive activity and lack of resting time in these mice. This may be a non-specific effect of dopaminergic treatment but it is also consistent with the large body of literature relating brain iron deficiency to the dopamine status in RLS [41, 44–47]. Particularly striking are the findings that in rats VMB BID produces RLS-like abnormalities in striatal dopamine [46, 48, 49] that can be reversed by directly infusing iron into the VMB [50]. The RLS-like behavioral response to dopaminergic treatment thus supports viewing the BXD40f as an animal model producing the putative iron-dopamine relations to RLS symptoms.
Third, the behavioral measurements used in this study involve not just changes in activity but also an attempt to measure the duration of the animals’ resting periods. This is unfortunately limited by the technology used that cumulated activity over consecutive 6-minute epochs. Mice often have much shorter rest periods, even one-minute periods with sleep. Finer grain analyses of inactive resting periods might, therefore, improve sensitivity of this measurement approach. But the data obtained here are nonetheless interesting. The BXD40f with or without ID diet had in the last part of the wake period almost no 6-minute periods without activity. Six-minute rest periods were, however, seen for all of the animals after levodopa and dopamine agonist treatment indicating somewhat dramatic treatment effects similar to that reported by RLS patients treated with levodopa and dopamine agonist drugs. It deserves note that the RLS diagnosis specifies not only the increased drive/urge to move in the last part of the day but also that it is relieved by movement and brought on by rest [30]. Thus the roles of rest and activity differ in clinical RLS, one engenders and the other decreases the RLS drive to move. While related these measure somewhat different aspects of RLS. In this study of the RLS-like rest and activity behaviors the activity measure accounted for only about 50 −55% of the variance in the rest measures. It may be useful to explore both of these RLS-like behaviors in future studies.
Fourth, the 24-hour activity patterns reveal two major effects. First, the data from the animals on iron-sufficient diets replicate the findings previously reported of this RLS-like increased activity in the last two hours of the active period [29]. Second, this RLS-like pattern of increased activity in the last part of the active period starts as expected earlier in the day. This study was designed assuming a one-hour earlier onset, but the data showed about a two-hour earlier onset of the RLS-like behavior, starting at about 13:00. The ID animals’ activity remained overall high compared to controls for the remainder of the hours before end of the active period. There was, however, also some indication of an overall increase in activity levels for the ID compared to control animals across the active but not the inactive period. These differences were generally not statistically significant except for the 4th and 3rd hour before the end of the active period and may not replicate in future studies. The ID diet may, however, produce a general increase in activity levels during the active period, but even then, the RLS-like pattern persists with the unique for BXD40f increases in activity during the last hours of the day that does not occur for the other BXD strains. The BXD40f on the ID diet have this increase occurring much earlier in the last part of the active period and persisting possibly even into the very initial part of inactive period. (see fig 2). This pattern matches well the unique clinical feature of increased RLS severity producing earlier onset of symptoms with some less prominent extension later in the day. This feature was used to develop one of the first scales for RLS severity [36].
This study has some significant Limitations. There was only one day of treatment for each condition and no repeated measure for the treatment. The animals had not been pre-conditioned to an IP injection at 14:00 each day. The initial experience and the expectation of this repeating likely altered their behavior. The order of medication dosing was systematically altered to counterbalance these effects across the animals, but this remains a potential problem for these data. These problems, overall, tend to work against finding significant treatment differences as seen in this study.
This study is also limited by the inherent differences between animals and humans, particularly for diseases involving sensory symptoms. Animal RLS-like behaviors were established based on the hypothesis that the animal’s response to an RLS-like circadian rest-induced urge/drive to move will at the end of the active period lead to increased activity and also disruption of resting reducing time in resting periods. RLS patients show these behaviors [33, 34]. The assumptions defining the RLS-like behaviors are similar to assumptions accepted for other animal models of sensory symptoms including those defining anxiety-like behaviors in animals, e.g. open field activity [51, 52].
This study was limited to only the BXD40f strain. Future studies evaluating behavioral effects of the ID diet for other strains without the low VMB iron would have been informative. The prior study [29] documented that on the iron-sufficient diet these other strains unlike the BXD40f do not show the RLS-like behavior (See figure 1). Presumably the other strains would not develop the RLS-like behavior even when on the ID diet since they do not have significant VMB iron loss with the ID diet. Similarly, the dopaminergic treatment with these strains would be expected to have little specific effect on the activity patterns late in the active phase (RLS-like behaviors). This remains to be evaluated in future studies.
Finally, the brain and peripheral iron status was not directly tested for these animals. Prior studies, however, have clearly established that the methods used in this study would produce in BXD40f the brain without peripheral iron deficiency [25, 27, 28]. The results in this study, therefore, need to be interpreted in the context of the extensive literature on the BXD40 strains and in particular the BXD40f strain.
Conclusions
Overall the major hypotheses for this study were confirmed. The BXD40f circadian RLS-like behaviors are both exacerbated by iron deficiency and reduced by levodopa and dopamine agonist treatment similar to these effects on RLS symptoms. These results provide support for viewing the BXD40f as a useful animal model for RLS and support the putative role of the RLS brain iron deficiency biology producing RLS symptoms.
Supplementary Material
Highlights.
Iron deprived BXD40f mice show RLS-like low brain with normal peripheral iron.
RLS-like behaviors occur earlier for BXD40f on iron-deficient vs. sufficient diets.
Dopaminergic treatment reduces RLS-like behaviors of iron-deprived BXD40f
Increasing brain iron deficiency in BXD40f increases RLS-like behaviors
BXD40f provide a useful animal model of RLS
Acknowledgements
This work was funded in part by National Institute on Aging grant: PO1-AG21190
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- [1].Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–5. [DOI] [PubMed] [Google Scholar]
- [2].Earley CJ, P BB, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:458–61. [DOI] [PubMed] [Google Scholar]
- [3].Astrakas LG, Konitsiotis S, Margariti P, Tsouli S, Tzarouhi L, Argyropoulou MI. T2 relaxometry and fMRI of the brain in late-onset restless legs syndrome. Neurology. 2008;71:911–6. [DOI] [PubMed] [Google Scholar]
- [4].Godau J, Klose U, Di Santo A, Schweitzer K, Berg D. Multiregional brain iron deficiency in restless legs syndrome. Mov Disord. 2008;23:1184–7. [DOI] [PubMed] [Google Scholar]
- [5].Rizzo G, Manners D, Testa C, Tonon C, Vetrugno R, Marconi S, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov Disord. 2013;28:1886–90. [DOI] [PubMed] [Google Scholar]
- [6].Moon HJ, Chang Y, Lee YS, Song H, Chang HW, Ku J, et al. A comparison of MRI tissue relaxometry and ROI methods used to determine regional brain iron concentrations in restless legs syndrome. Med Devices (Auckl). 2015;8:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li X, Allen RP, Earley CJ, Liu H, Cruz TE, Edden RA, et al. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016;22:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schmidauer C, Sojer M, Seppi K, Stockner H, Hogl B, Biedermann B, et al. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol. 2005;58:630–4. [DOI] [PubMed] [Google Scholar]
- [9].Godau J, Schweitzer KJ, Liepelt I, Gerloff C, Berg D. Substantia nigra hypoechogenicity: definition and findings in restless legs syndrome. Mov Disord. 2007;22:187–92. [DOI] [PubMed] [Google Scholar]
- [10].Godau J, Wevers AK, Gaenslen A, Di Santo A, Liepelt I, Gasser T, et al. Sonographic abnormalities of brainstem structures in restless legs syndrome. Sleep Med. 2008;9:782–9. [DOI] [PubMed] [Google Scholar]
- [11].Godau J, Manz A, Wevers AK, Gaenslen A, Berg D. Sonographic substantia nigra hypoechogenicity in polyneuropathy and restless legs syndrome. Mov Disord. 2009;24:133–7. [DOI] [PubMed] [Google Scholar]
- [12].Kwon DY, Seo WK, Yoon HK, Park MH, Koh SB, Park KW. Transcranial brain sonography in Parkinson’s disease with restless legs syndrome. Mov Disord. 2010;25:1373–8. [DOI] [PubMed] [Google Scholar]
- [13].Ryu JH, Lee MS, Baik JS. Sonographic abnormalities in idiopathic restless legs syndrome (RLS) and RLS in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:201–3. [DOI] [PubMed] [Google Scholar]
- [14].Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–700. [DOI] [PubMed] [Google Scholar]
- [15].Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–7. [DOI] [PubMed] [Google Scholar]
- [16].Earley CJ, Connor JR, Beard JL, Clardy SL, Allen RP. Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep. 2005;28:1069–75. [DOI] [PubMed] [Google Scholar]
- [17].Clardy SL, Earley CJ, Allen RP, Beard JL, Connor JR. Ferritin subunits in CSF are decreased in restless legs syndrome. J Lab Clin Med. 2006;147:67–73. [DOI] [PubMed] [Google Scholar]
- [18].Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. [DOI] [PubMed] [Google Scholar]
- [19].Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–7. [DOI] [PubMed] [Google Scholar]
- [20].Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, et al. Thy1 expressionin the brain is affected by iron and is decreased in Restless Legs Syndrome. J Neuro Sci. 2004;220:59–66. [DOI] [PubMed] [Google Scholar]
- [21].Snyder AM, Wang X, Patton SM, Arosio P, Levi S, Earley CJ, et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 2009;68:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Connor JR, Ponnuru P, Lee BY, Podskalny GD, Alam S, Allen RP, et al. Postmortem and imaging based analyses reveal CNS decreased myelination in restless legs syndrome. Sleep Med. 2011;12:614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Margariti PN, Astrakas LG, Tsouli SG, Hadjigeorgiou GM, Konitsiotis S, Argyropoulou MI. Investigation of Unmedicated Early Onset Restless Legs Syndrome by Voxel-Based Morphometry, T2 Relaxometry, and Functional MR Imaging during the Night-Time Hours. AJNR Am J Neuroradiol. 2012;33:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Knake S, Heverhagen JT, Menzler K, Keil B, Oertel WH, Stiasny-Kolster K. Normal regional brain iron concentration in restless legs syndrome measured by MRI. Nat Sci Sleep. 2010;2:19–22. [PMC free article] [PubMed] [Google Scholar]
- [25].Jellen LC, Unger EL, Lu L, Williams RW, Rousseau S, Wang X, et al. Systems genetic analysis of the effects of iron deficiency in mouse brain. Neurogenetics. 2012;13:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yin L, Unger EL, Jellen LC, Earley CJ, Allen RP, Tomaszewicz A, et al. Systems genetic analysis of multivariate response to iron deficiency in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1282–R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jones BC, Reed CL, Hitzemann R, Wiesinger JA, McCarhy KA, Buwen JP, et al. Quantitative genetic analysis of ventral midbrain and liver iron in BXD recombinant inbred mice. Nutr Neurosci. 2003;6:369–77. [DOI] [PubMed] [Google Scholar]
- [28].Allen RP, Donelson NC, Jones BC, Li Y, Manconi M, Rye DB, et al. Animal models of RLS phenotypes. Sleep Med. 2017;31:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Earley C, Allen R, Jones B, Unger E. Behavioral Model of Restless Legs Syndrome Utilizing Natural Circadian Variances in BxD Mice Strains. Sleep Med. submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15:860–73. [DOI] [PubMed] [Google Scholar]
- [31].Rosner B Fundamentals of biostatistics 7th ed: Brooks/Cole; 2010. p. 302–3. [Google Scholar]
- [32].Chow B, Shao J, Wamg H Sqamqple Size Calculations in Clinical Research. 2nd ed: Chapman & Hall/CRC; 2008. p. 58. [Google Scholar]
- [33].Trenkwalder C, Hening WA, Walters AS, Campbell SS, Rahman K, Chokroverty S. Circadian rhythm of periodic limb movements and sensory symptoms of restless legs syndrome. Mov Disord. 1999;14:102–10. [DOI] [PubMed] [Google Scholar]
- [34].Hening WA, Walters AS, Wagner M, Rosen R, Chen V, Kim S, et al. Circadian rhythm of motor restlessness and sensory symptoms in the idiopathic restless legs syndrome. Sleep. 1999;22:901–12. [DOI] [PubMed] [Google Scholar]
- [35].Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–13. [DOI] [PubMed] [Google Scholar]
- [36].Allen RP, Earley CJ. Validation of the Johns Hopkins restless legs severity scale. Sleep Med. 2001;2:239–42. [DOI] [PubMed] [Google Scholar]
- [37].Wilt TJ, MacDonald R, Ouellette J, Khawaja IS, Rutks I, Butler M, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA internal medicine. 2013;173:496–505. [DOI] [PubMed] [Google Scholar]
- [38].Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev. 2011:CD006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stiasny K, Wetter TC, Trenkwalder C, Oertel WH. Restless legs syndrome and its treatment by dopamine agonists. 2000;7:21–5. [DOI] [PubMed] [Google Scholar]
- [40].Ferre S, Garcia-Borreguero D, Allen RP, Earley CJ. New Insights into the Neurobiology of Restless Legs Syndrome. Neuroscientist. 2019;25:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Connor JR, Wang X, Allen RP, Beard J, Wiesinger JA, Felt BT, et al. Altered Dopaminergic Profile in the Putamen and Substantia Nigra in Restless Leg Syndrome. Brain. 2009;132:2403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Youdim M, Ben-Shacher D, Ashkenazi R, Yehuda S. Brain iron and dopamine receptor function In: Mandel P, De-Feudis F, editors. CNS Receptors-from Molecular Pharmacology to Behavior. New York: Raven Press; 1983. p. 309–21. [PubMed] [Google Scholar]
- [43].Youdim M Brain iron metabolism: Biochemical and behavioral aspects in relation to dopaminergic neurotransmission In: Lajtha A, editor. Handbook of neurochemistry. New York: Plenium Press; 1982. p. 731–55. [Google Scholar]
- [44].Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas R, Brasic J, et al. The Dopamine Transporter is Decreased in the Striatum of Subjects with Restless Legs Syndrome. Sleep. 2011;34:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas RE, Brasic JR, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Earley CJ, Connor J, Garcia-Borreguero D, Jenner P, Winkelman J, Zee PC, et al. Altered Brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis-Ekbom Disease). Sleep Med. 2014;15:1299–301. [DOI] [PubMed] [Google Scholar]
- [47].Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48:621–4. [DOI] [PubMed] [Google Scholar]
- [49].Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–8. [DOI] [PubMed] [Google Scholar]
- [50].Unger EL, Bianco LE, Jones BC, Allen RP, Earley CJ. Low brain iron effects and reversibility on striatal dopamine dynamics. Exp Neurol. 2014;261:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hang S, Huh JR. The Immune-Mind Connection. Cell. 2019;179:803–5. [DOI] [PubMed] [Google Scholar]
- [52].Fan KQ, Li YY, Wang HL, Mao XT, Guo JX, Wang F, et al. Stress-Induced Metabolic Disorder in Peripheral CD4(+) T Cells Leads to Anxiety-like Behavior. Cell. 2019;179:864–79 e19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.