Abstract
After the phase-out of polybrominated diphenyl ethers, their replacement compounds, organophosphate flame retardants (OPFRs) became ubiquitous in home and work environments. OPFRs, which may act as endocrine disruptors, are detectable in human urine, breast milk, and blood samples collected from pregnant women. However, the effects of perinatal OPFR exposure on offspring homeostasis and gene expression remain largely underexplored. To address this knowledge gap, virgin female mice were mated and dosed with either a sesame oil vehicle or an OPFR mixture (triphenyl phosphate, tricresyl phosphate, and tris(1,3-dichloro-2-propyl)phosphate, 1 mg/kg each) from gestational day (GD) 7 to postnatal day (PND) 14. Hypothalamic and hepatic tissues were collected from one female and one male pup per litter on PND 0 and PND 14. Expression of genes involved in energy homeostasis, reproduction, glucose metabolism, and xenobiotic metabolism were analyzed using quantitative real-time PCR. In the mediobasal hypothalamus, OPFR increased Pdyn, Tac2, Esr1, and Pparg in PND 14 females. In the liver, OPFR increased Pparg and suppressed Insr, G6pc, and Fasn in PND 14 males and increased Esr1, Foxo1, Dgat2, Fasn, and Cyb2b10 in PND 14 females. We also observed striking sex differences in gene expression that was dependent on the age of the pup. Collectively, these data suggest that maternal OPFR exposure alters hypothalamic and hepatic development by influencing neonatal gene expression in a sex-dependent manner. The long-lasting consequences of these changes in expression may disrupt puberty, hormone sensitivity, and metabolism of glucose, fatty acids, and triglycerides in the maturing juveniles.
Keywords: Endocrine disruptors, organophosphate flame retardants, maternal exposure, sex differences, gene expression, hypothalamus, liver
1. Introduction
Endocrine disrupting chemicals (EDCs) are a class of chemicals that interfere with typical endocrine system function [1,2]. Dysregulation of this system produces grave adverse developmental, reproductive, neurological, and immune effects in both human and animal populations. EDCs are ubiquitous in our environment due to their usage in household products, workplace settings, and in industrial and manufacturing plants [3]. Human daily exposures are continuous as EDCs can be found in plastic bottles, pesticides, soy products, and household goods that contain flame retardants (FRs) [4]. FRs are one of the most common EDCs as they are chemical additives applied to combustible materials to thwart the spread of fire. As such, FRs are found throughout the home and workplace, in furniture, clothing, drapery, and electronics. The constituents of FRs are not bound to these products; thus, they leach off to accumulate in household dust that is subsequently ingested or inhaled by humans, especially children [5]. The typical route of exposure in children occurs through frequent hand-to-mouth or object-to-mouth contact with contaminated dust [6]. Indeed, high levels of the widely used FR, tris(1,3-dichloro-2-propyl)phosphate (TDCPP) are observed in the urine of children [7].
For decades, polybrominated diethyl ethers (PBDEs) were used as FRs. However, their persistent presence in our environment and the associated adverse endocrine and neurological effects led to a phase-out of production in the United States since the mid to late 2000s [8]. One chemical replacement for PBDEs were compounds called organophosphate flame retardants (OPFRs). OPFRs are used as a mixture of several compounds that do not persist and bioaccumulate to the same degree as PBDEs. However, the ability of several OPFRs to interact with nuclear receptors has been recently characterized. These receptors include estrogen receptor (ER) α and peroxisome proliferator-activated receptor (PPAR) γ [9–11], both of which are involved in energy homeostasis and neuroendocrine functions. These OPFRs include, TDCPP, tricresyl phosphate (TCP), and triphenyl phosphate (TPP), which is a constituent of the common FR mixture Firemaster® 550 (FM 550). All three of these OPFRs are detectable in urine, breast milk, and blood samples from humans in a range of concentrations (1–100 ng/mL or 1–100 ng/g lipids) [5,12–16].
Dysregulation of nuclear receptor expression and downstream signaling during development (in utero and neonatal) by FRs may disrupt essential homeostatic function later in adulthood. We have previously demonstrated in mice that adult exposure to a mixture of TPP, TCP, and TDCPP (1 and 10 mg each/kg/day) for 4 weeks disrupts energy homeostasis by influencing hypothalamic and hepatic gene expression, including Esr1 (ERα) and Pparg (PPARγ) expression [17]. Both ERα and PPARγ play a critical role in the development and regulation of the hypothalamic-pituitary-gonadal (HPG) axis as well as the regulation of neuroendocrine functions related to energy homeostasis [18,19]. ERα and PPARγ are highly expressed in the arcuate nucleus, particularly within the neurons controlling energy homeostasis and reproduction: proopiomelanocortin (POMC) neurons; neurons that co-express neuropeptide Y (NPY) and agouti-related peptide (AgRP); and kisspeptin-neurokinin B (Tac2)-dynorphin (KNDy) neurons, respectively [20–23].
Numerous studies in rodents have demonstrated that mammals are particularly sensitive to the effects of EDCs in utero and neonatal development [24]. Gestational exposure to FRs may influence developmental reprogramming, as these substances pass through the placenta and are detectable in amniotic fluid in rats [16,25–27]. Studies examining human exposures indicates that OPFR metabolites are detectable in humans during the perinatal period [7] and are associated with increased risk for high body mass index (BMI) in children [28] and adverse neurological outcomes [29]. OPFRs in household dust, which are inadvertently ingested by a pregnant mother or a growing infant, are found at median concentrations of ~1–6 μg/g dust for TPP, TCP, and TDCPP [30–32]. Exposure to these compounds in two non-mammalian models, zebrafish and chicks, via water immersion (0.2–1.0 mg/L) and egg injection (9–51,600 ng/g), respectively, demonstrate OPFRs’ ability to disrupt energy homeostasis, steroidogenesis, liver functions, lipogenesis, glucose homeostasis, and activity levels and also influence hypothalamic gene expression [9,33]. Recently, maternal exposures to TPP (1 mg/kg/day via oral gavage from gestational day (GD) 6 to postnatal day (PND) 21 in CD-1 dams led to disruption of energy homeostasis (increased body weight and adiposity on a high-fat diet), increased liver weight and lipogenesis gene expression, and decreased glucose clearance in adult offspring using a dose similar to our study [34,35].
Therefore, understanding the influence of maternal OPFR exposure during prenatal and postnatal development on offspring gene expression in a mammalian model is needed. The objective of the present study is to evaluate the effects of OPFRs known to interact with ERα and PPARγ [9–11] on male and female neonatal gene expression in the hypothalamus and liver, focusing on genes involved in reproduction and energy homeostasis. We studied pups at PND 0 and PND 14 to assess differences between in utero (PND 0) and lactational exposures (PND 14) within the context of identified age and sex differences in gene expression. Both the hypothalamus and the liver are especially sensitive to developmental EDC exposures. These developmental periods are important windows for hypothalamic development, when the expression of reproductive and metabolic neuropeptides and hormone and nuclear receptors increases in the arcuate nucleus [24,36,37]. Furthermore, numerous studies using other EDCs have indicated that gene expression in the liver is susceptible during a period when the expression of metabolic and detoxification enzymes increases in this organ [38–40]. Our findings indicate that maternal OPFR exposure influences hypothalamic and hepatic gene expression in the neonate and juvenile in a sex-dependent manner in a mammalian model.
2. Material and Methods
2.1. Animal Care
Adult (>60 days old) wild-type (WT) C57BL/6J (Taconic) male and female virgin mice were bred in-house with food (breeder diet; 25% fat kCal, 3.83 kcal/g, Lab Diet 5015; Lab Diet, St. Louis, MO) and water available ad libitum. Mice were maintained under a 12-hour light/dark cycle and kept under controlled temperature regulation (25°C). Breeding pairs were established through pair housing or trio housing. The pre-pregnancy weights of females were recorded, and weights were taken throughout the duration of pregnancy. GD 0 was determined by confirmation of a vaginal plug. On GD 7, males were removed, and dams were individually housed for the duration of their gestation.
2.2. Chemicals
The OPFR mixture consisted of tris(1,3-dichloro-2-propyl)phosphate (TDCPP, Sigma Aldrich, St. Louis, MO, CAS#:13674-87-8, 95.6%), triphenyl phosphate (TPP, Sigma Aldrich, St. Louis, MO; CAS#:115-86-6, 99%), and tricresyl phosphate (TCP, AccuStandard, New Haven, CT, CAS#: 1330-78-5, 99%), each at 1 mg/kg) dissolved in an acetone:sesame oil solution (1:100). We and others have previously used similar doses of OPFRs in perinatal studies in rodents [17,34,35,41,42]. To prepare the OPFR stock, each compound was dissolved into 1 ml of acetone. One hundred (100) μl of stock acetone solution was dissolved into 10 ml of non-estrogenic sesame oil and allowed to vent for 24–48 h [17].
2.3. Maternal Exposures
Upon confirmation of a vaginal plug and sufficient weight gain of expected pregnancy on GD 3, all mice were acclimated to the vehicle (30 μl acetone:sesame oil (1:100) in powdered peanut butter (PB2®, Amazon)). To ensure that the entire OPFR dose was consumed, powdered peanut butter was used as it is highly palatable to mice and does not cause stress due to handling, gavaging, or injecting. Dams were randomly assigned to one of two exposure groups: vehicle mixed with powdered peanut butter or the OPFR mixture combined with powdered peanut butter and dosed from GD 7 to PND 14. Dams were weighed every three days to reduce the influence of handling stress. The volume of sesame oil, for both the vehicle and the OPFR mixture, added to the powdered peanut butter was determined by the most recent body weight of the dam. Powdered peanut butter (~40–50 mg) was placed on 1 inch2 weigh paper and mixed with the volume of sesame oil pipetted directly onto the powdered peanut butter to a similar consistency of regular peanut butter. Dams were monitored for consumption of peanut butter, which typically occurred within 20 min. Daily ingestion of the OPFR mixture or vehicle occurred at 1000 h.
2.4. Tissue collection and tissue processing
A total of 34 dams were dosed - 19 dams were dosed with vehicle and 15 dams were dosed with OPFR. Three dams per group did not get pregnant and 3 dams (2 vehicle and 1 OPFR) gave birth to less than 6 pups and were therefore excluded. Included in these 34 dams, were three to four litters in each exposure group that were primarily male or female. Thus, more dams were required to collect a sample size of 6 male and female PND 0 and 6 male and female PND 14 pups. On PND 7, anogenital distance (AGD), the distance from the anus to the genital tubercle, was assessed with calipers in all mice remaining in each litter and average AGD per sex per litter (7–9 litters per group). The AGD was normalized to the cube root of the body weight [43]. On PND 0 and PND 14, 1 male and 1 female pup from each litter were randomly selected, weighed, and euthanized by decapitation (n = 6/sex/exposure/age). Each brain was immediately placed in ice-cold Sorenson’s buffer. After ~30 seconds, the mediobasal hypothalamic (MBH) block, containing the arcuate nucleus and the ventromedial hypothalamus, was cut using a brain matrix (Ted Pella, Redding, CA), followed by microdissection corresponding to stereotaxic coordinates illustrated by Plates 42 (Bregma −1.34 mm) to 53 (Bregma −2.70 mm) from The Mouse Brain in Stereotaxic Coordinates [44], as we have previously described [23]. MBH was submerged in RNAlater (Life Technologies, Grand Island, NY) and stored at −80 °C. MBH RNA was extracted using RNAqueous-Micro Kits (Life Technologies) according to manufacturer’s protocol. Due to the small size of the liver in PND 0 and PND 14 pups, the whole liver was collected from the abdominal cavity from all mice, cut into pieces ~5 mm2, submerged in RNAlater, and stored at −80 °C. Liver RNA was extracted using a Trizol extraction coupled with a Macherey-Nagel NucleoSpin kit (Bethlehem, PA). To extract the hepatic RNA, ~20 mg of each liver was used. MBH and liver RNA quantity and quality were determined using a NanoDrop-2000 spectrophotometer (ThermoFisher, Waltham, MA).
2.5. Reverse Transcription and quantitative real-time PCR
As previously described [17], cDNA was synthesized from 0.5 μl of Superscript III transcriptase, 4 μl of 5x SS buffer, 1.25 μl of dNTP, 1 μl of 100 ng random hexamers, and 0.38 μl of RNasin in DEPC water to have a total volume of 20 μl. Reverse transcription was conducted using the following protocol: Incubation at 25 °C for 5 min, transcription at 50 °C for 60 min, denature at 70 °C for 15 min, and cooling for 4 min at 4 °C. Each sample was diluted using nuclease-free water at a 1:20 dilution (final concentration: MBH - 0.5 ng cDNA/μl; liver - 1.5 ng cNDA/μl). Quantitative real-time PCR (qPCR) was conducted using the primers found in Table 1. Four μl of cDNA was amplified by either Power SYBR Green (ThermoFisher) or SSO Advanced (BioRad, Hercules, CA) Master Mix using standard protocols. Relative gene expression was determined using the δδCT method calculated by the geomean of reference genes Actb and Gapdh [45,46].
Table 1.
List of primers for qPCR
| Gene | Accession # | Forward Primer | Reverse Primer |
|---|---|---|---|
| Agrp | NM_007427.2 | CTCCACTGAAGGGCATCAGAA | ATCTAGCACCTCCGCCAAA |
| Bdnf | NM_001316310.1 | TCATACTTCGGTTGCATGAAGG | AGACCTCTCGAACCTGCCC |
| Bsep | NM_021022.3 | CTGCCAAGGATGCTAATGCA | CGATGGCTACCCTTTGCTTCT |
| Cart | NM_013732 | GCTCAAGAGTAAACGCATTCC | GTCCCTTCACAAGCACTTCAA |
| Cd36 | NM_001159558.1 | GATGACGTGGCAAAGAACAG | TCCTCGGGGTCCTGAGTTAT |
| Cyp2b10 | NM_009999.4 | GACTTTGGGATGGGAAAGAG | CCAAACACAATGGAGCAGAT |
| Cyp3a11 | NM_007818.3 | ACAAACAAGCAGGGATGGAC | GGTAGAGGAGCACCAAGCTG |
| Cyp7a1 | NM_007824.2 | AACAACCTGCCAGTACTAGATAGC | GTGTAGAGTGAAGTCCTCCTTAGC |
| Cyp4a10 | NM_010011.3 | CACACCCTGATCACCAACAG | TCCTTGATGCACATTGTGGT |
| Dgat2 | NM_026384.3 | ACTCTGGAGGTTGGCACCAT | GGGTGTGGCTCAGGAGGAT |
| Esr1 | NM_007956 | GCGCAAGTGTTACGAAGTG | TTCGGCCTTCCAAGTCATC |
| Esr2 | NM_010157 | AATGTCCACCCGCTAGGCATTC | CTCCATGTCTTGCGTAGGTCTC |
| Fasn | NM_007988.3 | GGGTTCTAGCCAGCAGAGTC | TCAGCCACTTGAGTGTCCTC |
| Foxo1 | NM_019739 | CAATGGCTATGGTAGGATGG | TTTAAATGTAGCCTGCTCAC |
| G6pc | NM_013732 | TGCACCGCAAGAGCATT | GCCTCCTGTCGGATACAGAA |
| Ghsr | NM_177330 | CAGGGACCAGAACCACAAAC | AGCCAGGCTCGAAAGACT |
| Insr | NM_010568 | GTGTTCGGAACCTGATGAC | GTGATACCAGAGCATAGGAG |
| Kiss1 | NM_178260 | TGATCTCAATGGCTTCTTGGCAGC | CTCTCTGCATACCGCGATTCCTTT |
| Lepr | NM_146146.2 | AGAATGACGCAGGGCTGTAT | TCCTTGTGCCCAGGAACAAT |
| Npy | NM_023456 | ACTGACCCTCGCTCTATCTC | TCTCAGGGCTGGATCTCTTG |
| Ostb | NM_178933 | GCAGCTGTGGTGGTCATTAT | TAGGCTGTTGTGATCCTTGG |
| Pdyn | NM_018863 | AGCTTGCCTCCTCGTGATG | GGCACTCCAGGGAGCAAAT |
| Pepck | NM_011044.2 | AGCGGATATGGTGGGAAC | GGTCTCCACTCCTTGTTC |
| Pomc | NM_008895 | GGAAGATGCCGAGATTCTGC | TCCGTTGCCAGGAAACAC |
| Ppara | NM_011144.6 | CTAACCTTGGGCCACACCT | CGGGTAACCTCGAAGTCTGA |
| Pparg | NM_011146.3 | CTGCTCAAGTATGGTGTCCATGAG | GAGGAACTCCCTGGTCATGAATC |
| Shp | NM_011850.3 | TCTGCAGGTCGTCCGACTATTC | AGGCAGTGGCTGTGAGATGC |
| Tac2 | NM_001199971 | CGTGACATGCACGACTTC | CCAACAGGAGGACCTTAC |
Agrp: agouti related peptide; Bdnf: brain-derived neurotrophic factor; Bsep: bile salt export pump; Cart: cocaine and amphetamine regulatory transcript; Cd36, cluster of differentiation 36; cytochrome P450 family: cyp210, cyp3a11, cyp7a1, and cyp4a10; Dgat2: diglyceride acyltransferase; Esr1: estrogen receptor alpha; Esr2: estrogen receptor beta; Fasn: fatty acid synthase; Foxo1: forkhead box protein O1; g6pc: glucose-6-phosphatase, catalytic subunit; Ghsr: growth hormone secretagogue receptor; Insr: insulin receptor; Kiss1: kisspeptin; Lepr: leptin receptor; Npy: neuropeptide Y; Ostb: organic solute transporter beta; Pdyn: prodynorphin; Pepck: phosphoenolpyruvate carboxykinase; Pomc: proopiomelanocortin; Ppara: peroxisome proliferator-activated receptor alpha; Pparg: peroxisome proliferator-activated receptor gamma; Shp: small heterodimer partner; Tac2: neurokinin B.
2.6. Statistical Analysis
We have followed and met all the ARRIVE Guidelines for animal research. All data were analyzed within each age group by two-way ANOVA (sex and maternal exposure) followed by a post-hoc Holm-Sidak test using GraphPad Prism 8 (San Diego, CA), with tests for normality prior to ANOVA. ANOVA statistics are reported in Supplemental Table 1. All gene expression data were normalized to vehicle-treated female pups within PND 0 or PND 14 for comparison of sexes and maternal exposure. Outliers were determined using the Grubbs test, and values that exceeded 3 SDs (α<0.01) above or below the group mean. One PND 14 vehicle female hypothalamic sample and one PND 14 OPFR male hypothalamic sample were removed after being determined to be an outliner in 3 or more genes. Results were considered statistically significant at α<0.05. For all figures except Figure 1, lowercase letters denote sex differences and lowercase letters with a capped bar underneath denote OPFR effects. Data are represented as mean ± SEM.
Figure 1. Body weight and anogenital distance.

A) Body weights (BW) in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture starting on GD7. Sample size of 6 pups per group, one from each litter at each age. B) Anogenital distance (AGD) normalized to cubed root of body weight at PND 7 in female and male pups. Lowercase letters denote age differences, lowercase letters with capped bar underneath denote effects of OPFR, and uppercase letters denote sex differences (a = p < 0.05; b = p < 0.01; D/d = p < 0.0001). Data are represented as mean ± SEM, A: n = 6; B: n = 7–9 litters.
3. Results
3.1. Neonatal body weights and anogenital distance
All pups in each litter were weighed at PND 0 and again at PND 14. The average litter size included in the study was 7.2 ± 0.4 pups/litter for vehicle-treated dams and 7.2 ± 0.3 pups/litter for OPFR-treated dams. Both male and female pups gained weight from PND 0 to PND 14 (p < 0.0001, for both), regardless of maternal exposure (Figure 1A). There were no sex differences in weight; however, OPFR-exposed pups weighed more than their vehicle-treated counterparts (females: p < 0.01; males: p < 0.05). AGD, which was higher in males than females, regardless of exposure, was decreased by perinatal OPFR exposure in males (p < 0.05; Figure 1B).
3.2. Hypothalamic gene expression in WT PND 0 and PND 14 neonates
We selected estrogen-responsive hypothalamic genes that are involved in energy homeostasis and reproduction to analyze in the exposure groups. Based on their function, genes are organized as neuropeptides, genes associated with KNDy neurons, or hormone or nuclear receptors. In Figure 2A and 2B, expression of the neuropeptides Pomc and Cart were not affected by sex or OPFR. There were no effects or patterns observed on Npy expression in PND 0 pups, but Npy expression in the PND 14 pups was affected by an interaction of sex and OPFR with higher expression of Npy in vehicle-treated male pups than female pups (p < 0.05; Figure 2C). In PND 0 pups, Agrp expression was affected by OPFR with higher expression in OPFR-exposed male pups than vehicle-exposed male pups (p < 0.05; Figure 1D).
Figure 2. Hypothalamic gene expression in PND 0 and PND 14 neonates - Neuropeptides.
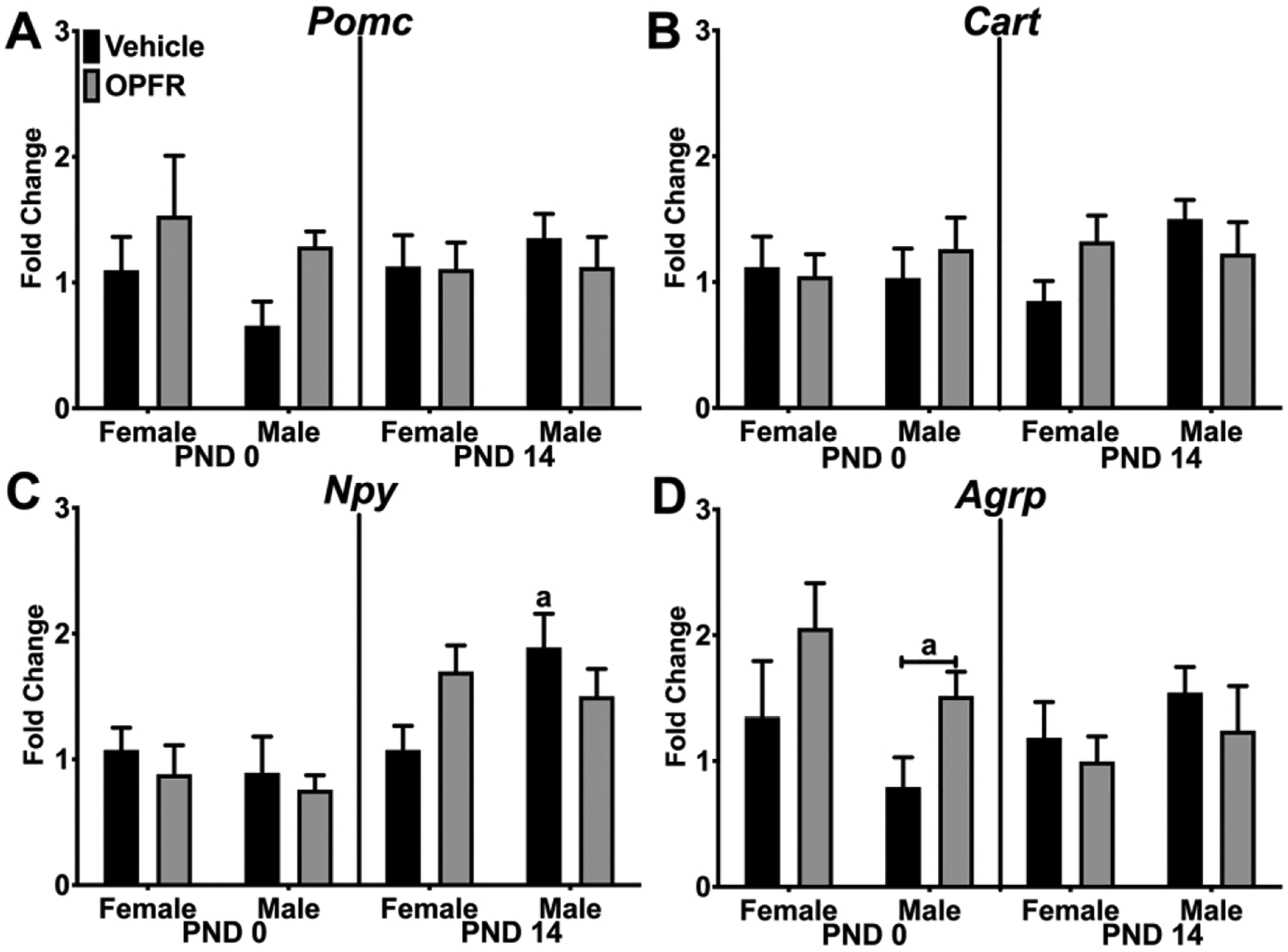
Relative gene expression in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture. A) Pomc; B) Cart; C) Npy; and D) Agrp. Lowercase letters denote sex differences and lowercase letters with capped bar underneath denote OPFR effects (a = p < 0.05). Data are represented as mean ± SEM, n = 5–6.
In Kiss1 expression, we observed an effect of sex in PND 0 pups with a 2-fold higher expression in females than males, regardless of maternal exposure (p < 0.05; Figure 3A). In PND 14 pups, Pdyn expression was affected by an interaction of OPFRs and sex (Figure 3B). Expression of Pdyn was increased ~50% in PND 14 female pups by OPFR (p < 0.05). In PND 0 pups, Tac2 expression was affected by an interaction of OPFR and sex, with OPFR-exposed males expressing more than OPFR-exposed females (p < 0.05; Figure 3C). In PND 14 pups, overall Tac2 expression was increased by OPFR exposure with a doubling in Tac2 expression in females (p < 0.01). Expression of the neurotrophin Bdnf was affected by sex in PND 14 pups with ~3-fold higher expression in vehicle-exposed males than in vehicle-exposed females (p < 0.05; Figure 3D). No effects of sex or OPFRs were observed on Foxo1 expression in either age group (Figure 3E).
Figure 3. Hypothalamic gene expression in PND 0 and PND 14 neonates - KNDy neurons.

Relative gene expression in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture. A) Kiss1; B) Pdyn; C) Tac2; D) Bdnf; and E) Foxo1. Lowercase letters denote sex differences and lowercase letters with capped bar underneath denote OPFR effects (a = p < 0.05; b = p < 0.01). Data are represented as mean ± SEM, n = 5–6.
We also examined hormone and nuclear receptors involved in energy homeostasis including ERα (Esr1), PPARγ (Pparg), insulin receptor (Insr), ghrelin receptor (Ghsr), and leptin receptor (Lepr). In PND 14 pups, expression of Esr1 was affected by OPFRs (Figure 4A). OPFR exposure increased Esr1 expression by ~3-fold in PND 14 females (p < 0.05). Expression of Esr2, Insr, and Ghsr were not affected by sex or OPFRs (Figure 4B, 4E, and 4F). In PND 14 pups, OPFR increased overall Pparg expression, and by ~3-fold in PND 14 female pups (p < 0.01; Figure 4C). In PND 0 pups, Lepr expression was affected by OPFR exposure with a decreasing (~50%) trend in expression in female pups (Figure 4D).
Figure 4. Hypothalamic gene expression in PND 0 and PND 14 neonates - Receptors.

Relative gene expression in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture. A) Esr1; B) Esr2; C) Pparg; D) Lepr; E) Insr; and F) Ghsr. Lowercase letters with capped bar underneath denote OPFR effects (a = p < 0.05; b = p < 0.01). Data are represented as mean ± SEM, n = 5–6.
3.3. Hepatic gene expression in WT PND 0 and PND 14 neonates
Because the liver regulates glucose, fatty acid, and triglyceride metabolism, we examined expression of hormone and nuclear receptors, metabolic enzymes, and transcription factors in the liver. No effects of sex or OPFR exposure were observed in Ppara expression (Figure 5A). In PND 14 pups, Pparg expression was affected by OPFRs (Figure 5B). Pparg expression was higher in OPFR-exposed males compared to vehicle-exposed males by ~50% (p < 0.05). Esr1 expression was affected by OPFRs (Figure 5C). OPFR exposure doubled Esr1 expression in PND 14 females compared to vehicle-exposed females (p < 0.01), which produced a sex difference between PND 14 OPFR-exposed female and male pups (p < 0.05). In PND 14 pups, Insr expression was affected by OPFRs, sex, and an interaction of OPFRs and sex (Figure 5D). Insr expression was suppressed by more than 50% in OPFR-exposed, PND 14 males (p < 0.0001), leading to a sex difference in Insr expression between OPFR-exposed female and male pups on PND 14 (p < 0.0001). Likewise, Lepr expression was affected by OPFRs in PND 14 pups, (Figure 5E), with ~25% lower expression in OPFR-exposed males (p < 0.05). Similarly, liver Kiss1 expression in PND 14 pups was ~50% lower in OPFR-exposed males compared to vehicle-exposed male pups (Figure 5F).
Figure 5. Hepatic gene expression in PND 0 and PND 14 neonates - Receptors.
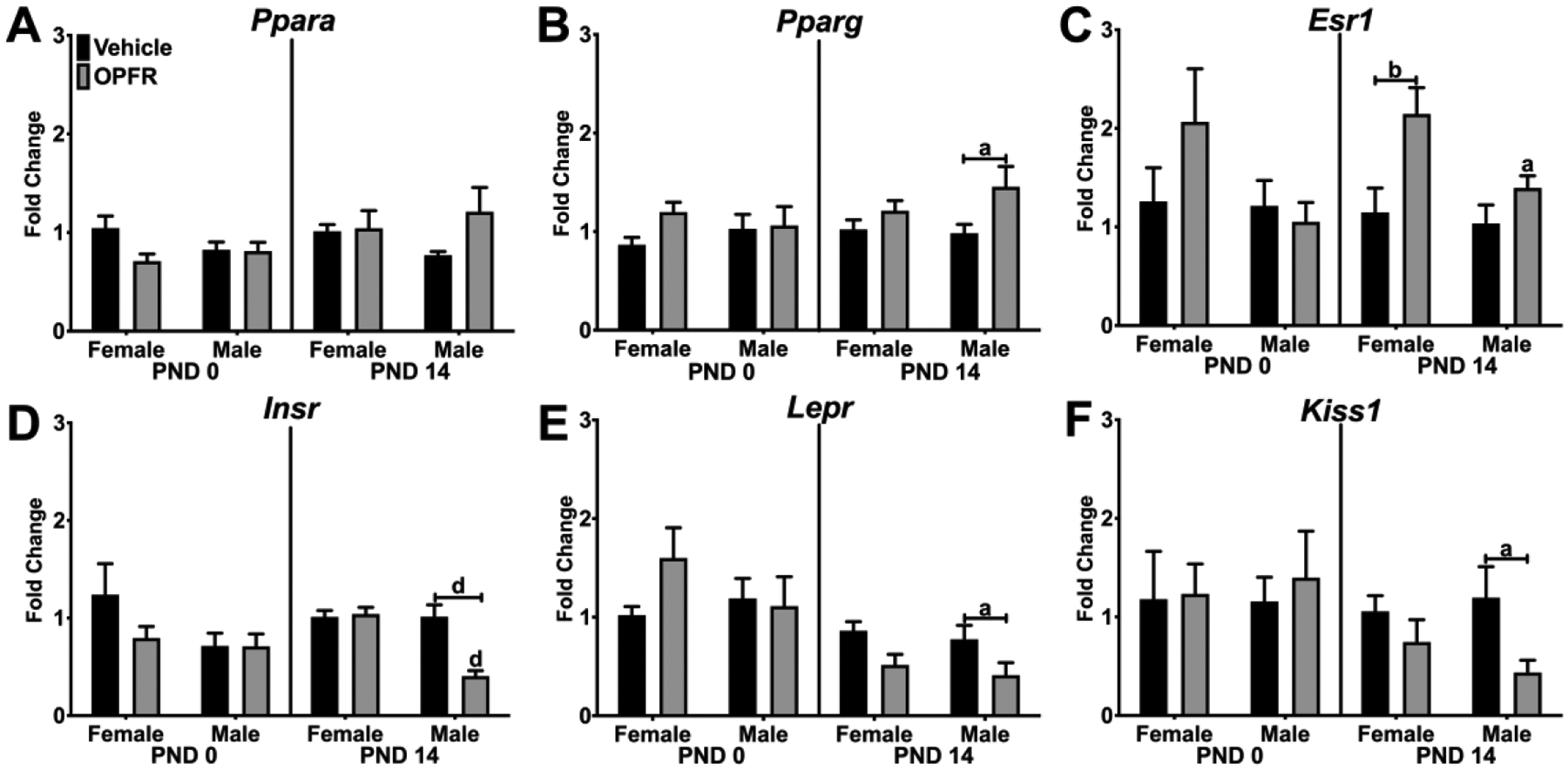
Relative gene expression in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture. A) Ppara; B) Pparg; C) Esr1; D) Insr; E) Lepr; and F) Kiss1. Lowercase letters denote sex differences and lowercase letters with capped bar underneath denote OPFR effects (a = p < 0.05; b = p < 0.01; d = p <. 0001). Data are represented as mean ± SEM, n = 5–6.
For metabolic enzymes involved in glucose metabolism, G6pc expression in PND 0 pups was not altered by OPFR exposure but there was a trend toward a sex difference (p = 0.0578; Figure 6A). In PND 14, G6pc expression was altered by OPFR exposure, sex, and an interaction of OPFRs and sex. OPFR exposure reduced G6pc expression in male pups by ~50% (p < 0.01) producing a sex difference between OPFR-exposed male and female pups (p < 0.01). Pepck expression in PND 0 pups was affected by sex with lower expression in OPFR-exposed male pups compared to OPFR-exposed female pups (p < 0.05; Figure 6B). Foxo1 expression in PND 14 pups was affected by an interaction of OPFRs and sex because OPFR exposure doubled Foxo1 expression in female pups (p < 0.05), producing a sex difference with OPFR-exposed males (p < 0.01; Figure 6C). Dgat2 expression was responsive to sex and OPFR exposure in both age groups (Figure 6D). In PND 0 pups, OPFR exposure induced a ~3-fold increase in Dgat2 expression in female pups (p < 0.001) yet suppressed expression by ~90% in male pups (p < 0.001). Thus, significant sex differences were observed in both maternal exposure groups (vehicle: p < 0.01; OPFR: p <0.0001). In PND14 pups, Dgat2 expression was ~50% lower in males compared to their PND 14 female counterparts, regardless of maternal exposure (vehicle: p < 0.01; OPFR: p < 0.01). Hepatic Fasn expression was affected by sex and an interaction of OPFR and sex in PND 14 pups (Figure 6E). OPFR exposure reduced Fasn expression in male pups by ~50% (p < 0.05), leading to lower expression than in female pups (p < 0.001).
Figure 6. Hepatic gene expression in PND 0 and PND 14 neonates - Enzymes.

Relative gene expression in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture. A) G6pc; B) Pepck; C) Foxo1; D) Dgat2; and E) Fasn. Lowercase letters denote sex differences and lowercase letters with capped bar underneath denote OPFR effects (a = p < 0.05; b = p < 0.01; c = p < 0.001; d = p <. 0001). Data are represented as mean ± SEM, n = 5–6.
Finally, we examined expression of target genes for xenobiotic receptors in the liver. Bsep, a pump for bile salts, was affected by OPFR only in PND 14 pups (Figure 7A). Bsep expression was ~25% higher in OPFR-exposed males compared to vehicle-exposed males (p < 0.05). In PND 14 pups, expression of Cd36 was affected by sex as Cd36 expression was ~50% lower in males than females, regardless of maternal exposure (vehicle: p < 0.01; OPFR: p < 0.01; Figure 7B). In PND 0 pups, Cyp2b10 expression was not affected by OPFR but there was a trend towards a sex difference (p = 0.0683), with vehicle-treated males expressing 6-fold more than females (Figure 7C). The sex difference was eliminated by OPFR exposure. In PND 14 pups, OPFR induced Cyp2b10 expression in females by 2-fold (p < 0.05), with an overall effect of OPFR exposure on Cyp2b10 expression. Cyp3a11 expression was not affected by sex or OPFR exposure (Figure 7D). Cyp4a10 expression was affected by sex with a higher expression in males than females in both age groups (Figure 7E). For example, Cyp4a10 expression in PND 14 pups was ~3-fold higher in OPFR-exposed males compared to OPFR-exposed females (p < 0.01, Figure 7E). Cyp7a1 expression was affected only by sex (Figure 7F), with males exhibiting ~3-fold higher Cyp7a1 levels than females in both age groups. No effects of OPFR or sex were observed in Ostb or Shp expression (data not shown).
Figure 7. Hepatic gene expression in PND 0 and PND 14 neonates - Xenobiotic Targets.
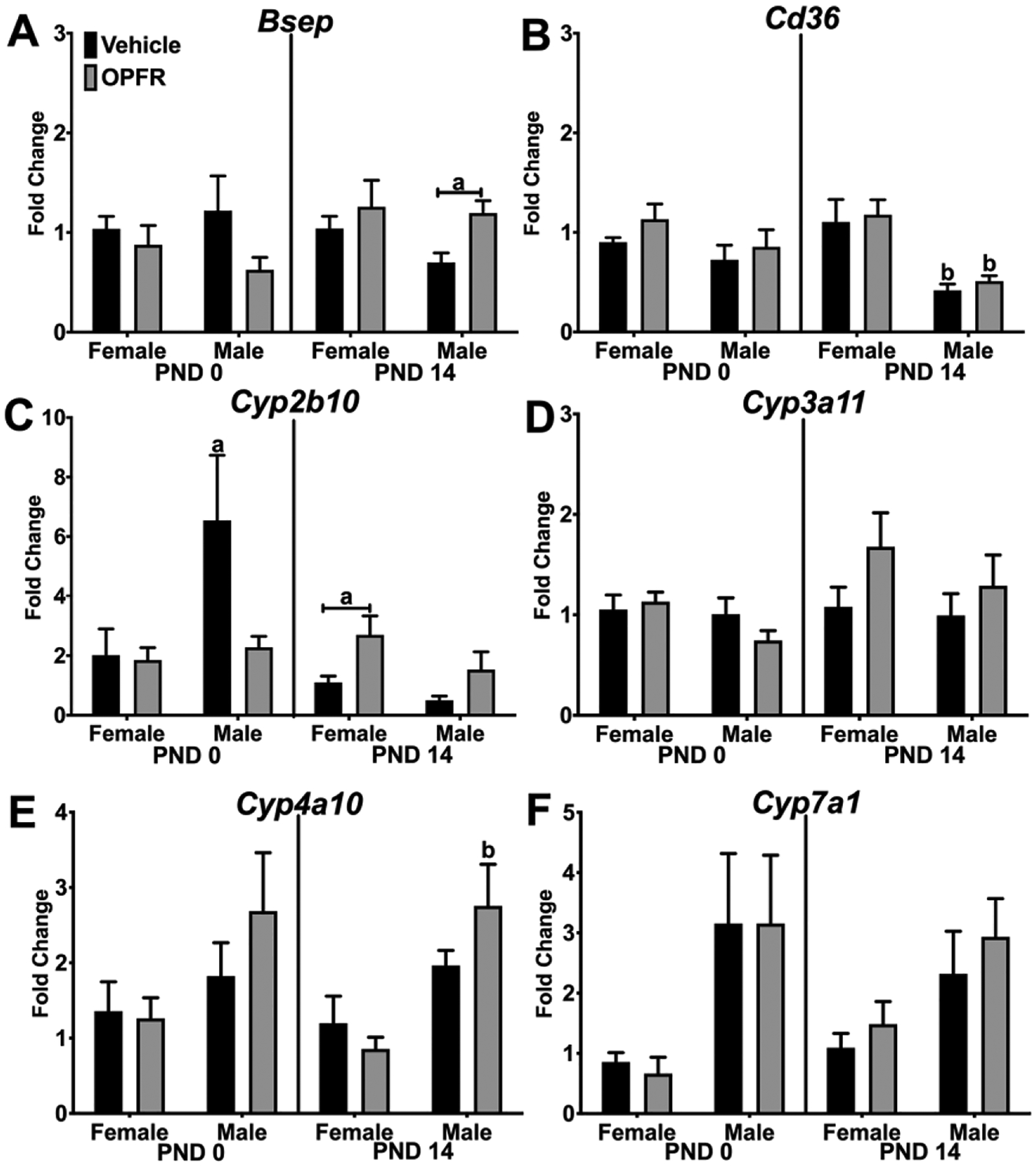
Relative gene expression in PND 0 and PND 14 female and male pups from dams orally dosed with vehicle or OPFR mixture. A) Bsep; B) Cd36; C) Cyp2b10; D) Cyp3a11; E) Cyp4a10; and F) Cyp7a1. Lowercase letters denote sex differences and lowercase letters with capped bar underneath denote OPFR effects (a = p < 0.05; b = p < 0.01).Data are represented as mean ± SEM, n = 5–6.
4. Discussion
As OPFRs are increasingly detectable in home and work environments and, thus, in human samples [5,7], it is imperative to understand their impact on mammalian physiology and gene expression, especially during sensitive developmental periods. Few studies have examined OPFR exposure on neonatal gene expression in a mammalian model. Several studies in fish and chicks have demonstrated that developmental exposures to OPFRs alter both hypothalamic and hepatic gene expression [47–49]. Furthermore, few studies have compared the different sensitivities of female and male neonates to these compounds during the early neonatal period. Therefore, in the present study, we exposed pregnant dams to an OPFR mixture (TPP, TCP, TDCPP) from GD7 to PND14 and measured the expression of a range of genes involved in energy balance, reproduction, nutrient metabolism, and xenobiotic metabolism in the hypothalamus and the liver. Rather than discussing individual genes and their specific roles, we will focus on the interactions of age, sex, and OPFR exposure in our discussion. As such, we observed both age- and sex-dependent effects on gene expression in both the hypothalamus and liver, as well as the sensitivity of these tissues to OPFR, illustrating the need for further targeted investigations into the effects of maternal OPFR exposure on offspring physiology.
The mechanisms underlying OPFR endocrine disruption are not fully characterized in mammalian systems. Our data indicates that these mechanisms include estrogenic or anti-androgenic endocrine disruption because AGD, a hormone- and EDC-sensitive anatomical measurement [50], was reduced in male pups by maternal OPFR exposure. Furthermore, these mechanisms may also alter body weight and metabolism, at least in the neonate. Both 14-day-old male and female pups from OPFR-treated dams weighed more than pups from vehicle-treated dams. Indeed, as both ERα and PPARγ are critical for the control of energy homeostasis, modulation of receptor expression by OPFRs during development, which is a mechanism of toxicity different than receptor activation [51], may play a part in OPFR-induced endocrine disruption.
While our primary focus were the effects of OPFR on gene expression, we also observed numerous sex differences in a number of genes in the hypothalamus. Sex was a factor in the expression of Npy, Kiss1, Pdyn, Tac2, and Bdnf, depending upon pup age. By analyzing expression within each age group, we found that Npy expression was higher in vehicle-treated males at PND14 which was eliminated by OPFR exposure. The transition to consuming chow around PND 14 is associated with expression of arcuate neuropeptides that control feeding; thus, the sex difference in expression may underlie the sex difference in feeding during this transition [52,53]. Our data, reveals higher Kiss1 expression in neonatal females than males, which supports earlier findings that juvenile female mice (PND16–18) express more Kiss1 in the mediobasal hypothalamus than male mice [54]. We also observed OPFR-induced sex differences in Tac2 expression in neonatal mice and sex differences in Pdyn expression in juvenile mice. Due to their roles in sexual maturation, the sex differences in these genes underlie differences in the onset of puberty and the beginnings of luteinizing hormone pulsatility in juvenile mice [55,56]. We also found sex differences in Bdnf expression in PND 14 pups, again with vehicle-treated males expressing more than females. These differences may be due to the effects of estrogens and ERα (Esr1) in controlling Bdnf expression in the brain [57] and the apparent sex differences in the regulation of BDNF-TrkB signaling during development [58]. Since BDNF is necessary for neural plasticity and neurogenesis in the developing brain [59], the differences may influence the sensitivities of both sexes to exogenous compounds that alter neuronal development.
Another relevant sex difference was the response to OPFR exposure. In the hypothalamus, female pups were more sensitive to maternal OPFR exposure than male pups. At PND 14, two neuropeptides (Pdyn and Tac2) and two nuclear receptors (Esr1 and Pparg) were expressed at higher levels in OPFR-exposed females than vehicle-exposed females, while there were no differences between male groups at that age. Only Agrp expression was sensitive to OPFR in neonatal male pups. Furthermore, OPFR exposure eliminated the apparent sex difference in Esr1 expression (males > females) in the MBH. Interestingly, in rats, Esr1 expression via in situ hybridization is equal between the sexes on PND 0, greater in females than males during the early postnatal period (PND 2–7) in the arcuate and ventromedial hypothalamus (VMH). However, by PND 19, Esr1 expression equalizes between the sexes in the arcuate but not in the VMH, where males continue to express less than females [60]. We found no significant sex differences in Esr1 expression at PND 0 and PND 14 in the vehicle-exposed groups. Because expression of these genes in OPFR-exposed female pups was similar to the expression in vehicle-exposed males, we interpret this as a masculinization of hypothalamic expression patterns of hormone-sensitive genes by maternal OPFR exposure. This interpretation is further supported by the well-known effects of the postnatal testosterone surge in male pups, which is converted into 17β-estradiol in the brain by aromatase [61]. The neural production of 17β-estradiol activates ERα and other estrogen receptors to modulate gene expression as well as neurogenesis and brain development [62–64]. We hypothesize that the increase in Esr1 expression in PND 14 female pups and the potential interactions of OPFR with ERα influences subsequent hypothalamic gene expression, at least transiently, and may have lasting consequences for reproduction, energy balance, and a range of hypothalamic physiological and neurological functions.
Another organ that is especially sensitive to exogenous compounds and endocrine disruption is the developing liver [65]. For a number of hepatic genes, sex is also an important factor in expression patterns. In our study of neonates and juveniles, Cyp7a1 expression was higher in male pups than female pups, while in studies using adults, the female mice have higher Cyp7a1 expression and higher bile acid levels than males [66–68]. We also observed sex differences in three other xenobiotic receptor targets: Cd36, Cyp2b10, and Cyp4a10. In the case of Cd36, males had lower expression than females at PND 14, but in adults, the reverse is true especially when fed a high-fat diet [68,69]. In pups from our study, males expressed more Cyp4a10 than females; however, in adult mice, Cyp4a10 is expressed more in female livers than in male livers [67,70]. Many of the sex differences in our study were due to induction of expression in females by maternal OPFR exposure including Esr1, Foxo1, Dgat2, Fasn, and Cyp2b10. This interaction is no more apparent than in the case of Dgat2 and Fasn. Diglyceride acyltransferase (DGAT) activity is the terminal reaction in the production of triglycerides [71], while fatty acid synthase (FASN) is necessary for the production of palmitate, a long-chain fatty acid [72]. While sex differences in hepatic Dgat2 and Fasn expression have been described in adult rodent models driven in part by steroids [71–73], little is known about its expression in neonates. In our study, OPFR induced a modest increase in Dgat2 expression in PND 14 female pups but not in males at that age. However, in PND 0 pups, OPFR exposure induced Dgat2 expression in females and suppressed it in males. For Fasn, females at PND 14 also expressed more than males, which was exaggerated by OPFR exposure. Such sensitivity to OPFR indicates that triglyceride and fatty acid metabolism may be a primary target for OPFR exposure in mammals, at least in neonates and juveniles. One final point to make about sex differences is the direction of the gene response to OPFR exposure. In females, OPFR exposure tended to induce expression, while in males, OPFR exposure tended to reduce expression. Thus, the pattern of induction between the sexes suggests that there are sex differences in the mechanisms of OPFR toxicity in neonatal mice.
In terms of OPFR effects, receptors, enzymes, and xenobiotic receptor targets were modulated, both positively and negatively, by maternal OPFR exposure. As in the hypothalamus, Esr1 was upregulated in OPFR-exposed female pups at PND 14, again indicating that ERα and its regulatory gene network are important targets for OPFR exposure. This is relevant as ERα activity, in cooperation with androgen receptors, drives the sexually dimorphic expression of hepatic genes in utero and through to early adulthood [74]. We also observed an increase in Pparg due to OPFR exposure, but this time in juvenile male pups. Therefore, OPFRs interact with PPARγ in both sexes but in a tissue-dependent manner, which differentially impact the offspring by altering the central and peripheral control of physiology. For example, the observed decrease in Insr and G6pc by OPFR in PND 14 males, but not females, may lead to disruption of glucose homeostasis in juvenile males and perhaps later in life. OPFR also induced Cyp2b10 expression in females, indicating that constitutive androstane receptor (CAR) is a primary xenobiotic receptor activated by OPFR in females pups [75]. However, OPFR induced Bsep expression in livers from male pups suggesting that farnesoid X receptor (FXR) may also be a target [76]. In our previous publication on adult OPFR exposure in wild-type mice, we also found CAR and pregnane X receptor (PXR) activation by OPFR in the adult liver [17]. Collectively, these data suggest that multiple xenobiotic receptors are a target for OPFRs which is dependent on developmental stage and sex of the mouse.
5. Conclusions
In summary, our study demonstrates that maternal OPFR exposure alters gene expression in the brain and the liver, two organs sensitive to endocrine disruption. These effects of OPFR exposure are also dependent on the sex and age of the pups as OPFR-induced changes in gene expression in PND 0 pups were not observed, but were in 14-day-old pups, the last day of maternal dosing. Many of the genes altered are involved in the hypothalamic control of energy balance and reproduction and the metabolism of glucose, triglycerides, and fatty acids in the liver. Our data also support a recent study demonstrating that direct OPFR exposure from PND 1 to 10 in mice alters endocrine and lipid profiles [34]. However, our study suggest that female pups are more susceptible than males, at least in terms of gene expression. Furthermore, our data suggest that these effects occur via direct modulation of metabolic enzymes or via modulation of nuclear and hormone receptors that control these metabolic pathways. Future maternal exposure studies will follow the exposed pups into adulthood and measure metabolic, reproductive, and behavioral outcomes that are regulated by these receptors, neuropeptides, and metabolic enzymes.
Supplementary Material
Highlights.
OPFR exposures increase pup body weight and reduce male anogenital distance.
Hypothalamic expression of hormone receptors and neuropeptides was altered by OPFR.
Hepatic expression of hormone receptors and metabolic enzymes was altered by OPFR.
Effects of OPFR was dependent on the developmental age of the pups.
Striking sex differences in the response to OPFR in the hypothalamus and liver.
Acknowledgements
The authors would like to thank Katherine Manger for careful editing of the manuscript. This work was supported by the US Department of Agriculture-National Institute of Food and Agriculture (NJ06195) and the National Institutes of Health (R21ES027119 to TAR; R01GM104037 to GLG; P30ES005022 to TAR, GLG). SA was funded by R25ES020721 and KW was funded by T32ES007148.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Thomas Zoeller R, Brown TR, Doan LL, Gore a. C., Skakkebaek NE, Soto a. M., Woodruff TJ, Vom Saal FS, Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society, Endocrinology. 153 (2012) 4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bergman Å, Becher G, Blumberg B, Bjerregaard P, Bornman R, Brandt I, Casey SC, Frouin H, Giudice LC, Heindel JJ, Iguchi T, Jobling S, Kidd KA, Kortenkamp A, Lind PM, Muir D, Ochieng R, Ropstad E, Ross PS, Skakkebaek NE, Toppari J, Vandenberg LN, Woodruff TJ, Zoeller RT, Manufacturing doubt about endocrine disrupter science - A rebuttal of industry-sponsored critical comments on the UNEP/WHO report “State of the Science of Endocrine Disrupting Chemicals 2012,” Regul Toxicol Pharmacol. 73 (2015) 1007–1017. doi: 10.1016/j.yrtph.2015.07.026. [DOI] [PubMed] [Google Scholar]
- [3].Giesy J, Hilscherová K, Jones PD, Kannan K, Machala M, Cell bioassay for detection of aryl hydrocarbon (AhR) and estrogen receptor (ER) madiated activity in enviromental samples, Mar Pollut Bull. 45 (2002) 3–16. file:///C:/Users/Kdd/AppData/Local/Temp/1-s2.0-S0025326X02000978-main.pdf. [DOI] [PubMed] [Google Scholar]
- [4].Wong KH, Durrani TS, Exposures to Endocrine Disrupting Chemicals in Consumer Products—A Guide for Pediatricians, Curr Probl Pediatr Adolesc Health Care. 47 (2017) 107–118. doi: 10.1016/j.cppeds.2017.04.002. [DOI] [PubMed] [Google Scholar]
- [5].Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, Stapleton HM, Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States, Environ Sci Technol Lett. 4 (2017) 112–118. doi: 10.1021/acs.estlett.6b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xue J, Zartarian V, Moya J, Freeman N, Beamer P, Black K, Tulve N, Shalat S, A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure, Risk Anal. 27 (2007) 411–420. doi: 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- [7].Hoffman K, Butt CM, Chen A, Limkakeng AT, Stapleton HM, High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products, Environ Sci Technol. 49 (2015) 14554–14559. doi: 10.1021/acs.est.5b03577. [DOI] [PubMed] [Google Scholar]
- [8].Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ, Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California, Environ Sci Technol. 47 (2013) 11776–11784. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu X, Cai Y, Wang Y, Xu S, Ji K, Choi K, Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish, Ecotoxicol Environ Saf. 170 (2019) 25–32. doi: 10.1016/j.ecoenv.2018.11.058. [DOI] [PubMed] [Google Scholar]
- [10].Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM, In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components, Toxicol Lett. 228 (2014) 93–102. doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu X, Ji K, Choi K, Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish, Aquat Toxicol. 114–115 (2012) 173–181. doi: 10.1016/j.aquatox.2012.02.019. [DOI] [PubMed] [Google Scholar]
- [12].Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM, Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers., Environ Sci Technol. 48 (2014) 10432–8. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- [13].V Dishaw L, J Macaulay L, Roberts SC, Stapleton HM, Exposures, mechanisms, and impacts of endocrine-active flame retardants, Curr Opin Pharmacol. 19 (2014) 125–133. doi: 10.1016/j.coph.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sundkvist AM, Olofsson U, Haglund P, Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk., J Environ Monit. 12 (2010) 943–951. doi: 10.1039/b921910b. [DOI] [PubMed] [Google Scholar]
- [15].Kim J-WW, Isobe T, Muto M, Tue NM, Katsura K, Malarvannan G, Sudaryanto A, Chang K-HH, Prudente M, Viet PH, Takahashi S, Tanabe S, Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries., Chemosphere. 116 (2014) 91–97. doi: 10.1016/j.chemosphere.2014.02.033. [DOI] [PubMed] [Google Scholar]
- [16].Hoffman K, Daniels JL, Stapleton HM, Urinary metabolites of organophosphate flame retardants and their variability in pregnant women, Environ Int. 63 (2014) 169–172. doi: 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krumm EA, Patel VJ, Tillery TS, Yasrebi A, Shen J, Guo GL, Marco SM, Buckley BT, Roepke TA, Organophosphate Flame-Retardants Alter Adult Mouse Homeostasis and Gene Expression in a Sex-Dependent Manner Potentially Through Interactions With ERα, Toxicol Sci. 162 (2018) 212–224. doi: 10.1093/toxsci/kfx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fernandez MO, Sharma S, Kim S, Rickert E, Hsueh K, Hwang V, Olefsky JM, Webster NJG, Obese neuronal PPARγ knock-out mice are leptin sensitive but show impaired glucose tolerance and fertility, Endocrinology. 158 (2017) en.2016–1818. doi: 10.1210/en.2016-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Couse JF, Korach KS, Estrogen receptor null mice: What have we learned and where will they lead us?, Endocr Rev. 20 (1999) 358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- [20].Bosch MA, Xue C, Rønnekleiv OK, Kisspeptin expression in guinea pig hypothalamus: Effects of 17β-estradiol, J Comp Neurol. 520 (2012) 2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner R. a., Molecular properties of kiss1 neurons in the arcuate nucleus of the mouse, Endocrinology. 152 (2011) 4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Proudan N, Peroski M, Grignol G, Merchenthaler I, Dudas B, Juxtapositions between the somatostatinergic and growth hormone-releasing hormone (GHRH) neurons in the human hypothalamus, Neuroscience. 297 (2015) 205–210. doi: 10.1016/j.neuroscience.2015.03.054. [DOI] [PubMed] [Google Scholar]
- [23].Yang J, Yasrebi A, Snyder M, Roepke T, The interaction of fasting, caloric restriction, and diet-induced obesity with 17β-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse, Mol Cell Endocrinol. 437 (2016) 35–50. doi: 10.1016/j.mce.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schneider JE, Brozek JM, Keen-Rhinehart E, Our stolen figures: The interface of sexual differentiation, endocrine disruptors, maternal programming, and energy balance, Horm Behav. 66 (2014) 104–119. doi: 10.1016/j.yhbeh.2014.03.011. [DOI] [PubMed] [Google Scholar]
- [25].Yang F, Ding J, Huang W, Xie W, Liu W, Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter, Environ Sci Technol. 48 (2014) 63–70. doi: 10.1021/es403186z. [DOI] [PubMed] [Google Scholar]
- [26].Baldwin KR, Horman B, Phillips AL, McRitchie SL, Watson S, Deese-Spruill J, Jima, Sumner S, Stapleton H, Patisaul H, EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain, Endocr Connect. (2018) EC-17–0373. doi: 10.1530/EC-17-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM, Transplacental and Lactational Transfer of Firemaster® 550 Components in Dosed Wistar Rats., Toxicol Sci. (2016) 1–12. doi: 10.1093/toxsci/kfw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boyle M, Buckley JP, Quirós-Alcalá L, Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013–2014, Environ Int. 127 (2019) 754–763. doi: 10.1016/j.envint.2019.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, Olshan AF, Daniels JL, Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study, Neurotoxicology. 73 (2019) 150–160. doi: 10.1016/j.neuro.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fan X, Kubwabo C, Rasmussen PE, Wu F, Simultaneous determination of thirteen organophosphate esters in settled indoor house dust and a comparison between two sampling techniques, Sci Total Environ. 491–492 (2014) 80–86. doi: 10.1016/j.scitotenv.2013.12.127. [DOI] [PubMed] [Google Scholar]
- [31].Meeker JD, Cooper EM, Stapleton HM, Hauser R, Urinary Metabolites of Organophosphate Flame Retardants: Temporal Variability and Correlations with House Dust Concentrations, Environ Health Perspect. 121 (2013) 580–585. doi: 10.1289/ehp.1205907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF, Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers, Environ Int. 55 (2013) 56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Farhat A, Buick JK, Williams A, Yauk CL, O’Brien JM, Crump D, Williams KL, Chiu S, Kennedy SW, Tris(1,3-dichloro-2-propyl) phosphate perturbs the expression of genes involved in immune response and lipid and steroid metabolism in chicken embryos, Toxicol Appl Pharmacol. 275 (2014) 104–112. doi: 10.1016/j.taap.2013.12.020. [DOI] [PubMed] [Google Scholar]
- [34].Wang D, Zhu W, Chen L, Yan J, Teng M, Zhou Z, Neonatal triphenyl phosphate and its metabolite diphenyl phosphate exposure induce sex- and dose-dependent metabolic disruptions in adult mice, Environ Pollut. 237 (2018) 10–17. doi: 10.1016/j.envpol.2018.01.047. [DOI] [PubMed] [Google Scholar]
- [35].Wang D, Yan S, Yan J, Teng M, Meng Z, Li R, Zhou Z, Zhu W, Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis, Environ Pollut. 246 (2019) 630–638. doi: 10.1016/j.envpol.2018.12.053. [DOI] [PubMed] [Google Scholar]
- [36].Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson S. a., Patisaul HB, Prenatal bisphenol a exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala, Toxicol Sci. 133 (2013) 157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Adewale HB, Todd KL, J. a. Mickens, H.B. Patisaul, The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat, Neurotoxicology. 32 (2011) 38–49. doi: 10.1016/j.neuro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gordillo M, Evans T, Gouon-Evans V, Orchestrating liver development, Dev. 142 (2015) 2094–2108. doi: 10.1242/dev.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Heindel JJ, Blumberg B, Environmental Obesogens: Mechanisms and Controversies, Annu Rev Pharmacol Toxicol. 59 (2019) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Muscogiuri G, Barrea L, Laudisio D, Savastano S, Colao A, Obesogenic endocrine disruptors and obesity: myths and truths, Arch Toxicol. 91 (2017) 3469–3475. doi: 10.1007/s00204-017-2071-1. [DOI] [PubMed] [Google Scholar]
- [41].Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB, Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats, Sci Rep. 7 (2017) 7118. doi: 10.1038/s41598-017-07216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patisaul H, Roberts S, Mabrey N, McCaffrey K, Gear R, Braun J, Belcher S, Stapleton H, Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment, J Biochem Mol Toxicol. 27 (2013) 124–136. doi: 10.1002/jbt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wise LD, Vetter CM, Anderson CA, Antonello JM, Clark RL, Reversible effects of triamcinolone and lack of effects with aspirin or L-656,224 on external genitalia of male Sprague-Dawley rats exposed in utero, Teratology. 44 (1991) 507–520. doi: 10.1002/tera.1420440505. [DOI] [PubMed] [Google Scholar]
- [44].Paxinos G, Franklin KBJ, The Mouse Brain in Stereotaxic Coordinates, Compact, Third Edition: The coronal plates and diagrams, 3rd ed., Academic Press, London, UK, 2008. [Google Scholar]
- [45].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method., Methods. 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [46].Schmittgen TD, Livak KJ, Analyzing real-time PCR data by the comparative C(T) method., Nat Protoc. 3 (2008) 1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- [47].Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM, Developmental Exposure to Organophosphate Flame Retardants Elicits Overt Toxicity and Alters Behavior in Early Life Stage Zebrafish (Danio rerio), Toxicol Sci. 142 (2014) 445–454. doi: 10.1093/toxsci/kfu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bradley M, Rutkiewicz J, Mittal K, Fernie K, Basu N, In ovo exposure to organophosphorous flame retardants: Survival, development, neurochemical, and behavioral changes in white leghorn chickens, Neurotoxicol Teratol. 52 (2015) 228–235. doi: 10.1016/j.ntt.2015.08.003. [DOI] [PubMed] [Google Scholar]
- [49].Zheng XB, Luo XJ, Zeng YH, Wu JP, Chen SJ, Mai BX, Halogenated flame retardants during egg formation and chicken embryo development: Maternal transfer, possible biotransformation, and tissue distribution, Environ Toxicol Chem. 33 (2014) 1712–1719. doi: 10.1002/etc.2588. [DOI] [PubMed] [Google Scholar]
- [50].Liu C, Xu X, Huo X, Anogenital distance and its application in environmental health research, Environ Sci Pollut Res. 21 (2014) 5457–5464. doi: 10.1007/s11356-014-2570-z. [DOI] [PubMed] [Google Scholar]
- [51].La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, Guyton KZ, Kortenkamp A, Cogliano VJ, Woodruff TJ, Rieswijk L, Sone H, Korach KS, Gore AC, Zeise L, Zoeller RT, Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification, Nat Rev Endocrinol. 16 (2019). doi: 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zimmer MR, Fonseca AHO, Iyilikci O, Pra RD, Dietrich MO, Functional Ontogeny of Hypothalamic Agrp Neurons in Neonatal Mouse Behaviors, Cell. 178 (2019) 1–16. doi: 10.1016/j.cell.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Coupe B, Bouret SG, Development of the hypothalamic melanocortin system, Front Endocrinol (Lausanne). 4 (2013) 1–7. doi: 10.3389/fendo.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA, Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: Implications for the timing of puberty, Am J Physiol - Endocrinol Metab. 297 (2009) 1212–1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Plant TM, The role of KiSS-1 in the regulation of puberty in higher primates., Eur J Endocrinol. 155 Suppl (2006) S11–S16. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- [56].Kumar D, Periasamy V, Freese M, Voigt A, Boehm U, In utero development of kisspeptin/GnRH neural circuitry in male mice, Endocrinology. 156 (2015) 3084–3090. doi: 10.1210/EN.2015-1412. [DOI] [PubMed] [Google Scholar]
- [57].Furuta M, Numakawa T, Chiba S, Ninomiya M, Kajiyama Y, Adachi N, Akema T, Kunugi H, Estrogen, predominantly via estrogen receptor α, attenuates postpartum-induced anxiety and depression-like behaviors in female rats, Endocrinology. 154 (2013) 3807–3816. doi: 10.1210/en.2012-2136. [DOI] [PubMed] [Google Scholar]
- [58].Byerly MS, Swanson RD, Wong GW, Blackshaw S, Stage-specific inhibition of TrkB activity leads to long-lasting and sexually dimorphic effects on body weight and hypothalamic gene expression, PLoS One. 8 (2013) 1–11. doi: 10.1371/journal.pone.0080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fargali S, Sadahiro M, Jiang C, Frick AL, Indall T, Cogliani V, Welagen J, Lin WJ, Salton SR, Role of neurotrophins in the development and function of neural circuits that regulate energy homeostasis, J Mol Neurosci. 48 (2012) 654–659. doi: 10.1007/s12031-012-9790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cao J, Patisaul HB, Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats, J Comp Neurol. 519 (2011) 2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Clarkson J, Herbison AE, Hypothalamic control of the male neonatal testosterone surge, Philos Trans R Soc B Biol Sci. 371 (2016). doi: 10.1098/rstb.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Küppers E, Krust A, Chambon P, Beyer C, Functional alterations of the nigrostriatal dopamine system in estrogen receptor-α knockout (ERKO) mice, Psychoneuroendocrinology. 33 (2008) 832–838. doi: 10.1016/j.psyneuen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [63].Semaan SJ, Kauffman AS, Sexual differentiation and development of forebrain reproductive circuits, Curr Opin Neurobiol. 20 (2010) 424–431. doi: 10.1016/j.conb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Brannvall K, Estrogen-Receptor-Dependent Regulation of Neural Stem Cell Proliferation and Differentiation, Mol Cell Neurosci. 21 (2002) 512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- [65].Treviño LS, Katz TA, Endocrine Disruptors and Developmental Origins of Nonalcoholic Fatty Liver Disease, Endocrinology. 159 (2018) 20–31. doi: 10.1210/en.2017-00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen JM, Zhang QS, Li XY, Gong X, Ruan YJ, Zeng SJ, Lu LL, Qi XX, Wang Y, Hu M, Zhu LJ, Liu ZQ, Tissue Distribution and Gender-Specific Protein Expression of Cytochrome P450 in five Mouse Genotypes with a Background of FVB, Pharm Res. 35 (2018). doi: 10.1007/s11095-018-2389-2. [DOI] [PubMed] [Google Scholar]
- [67].Lu Y-F, Jin T, Xu Y, Zhang D, Wu Q, Zhang Y-KJ, Liu J, Sex differences in the circadian variation of cytochrome p450 genes and corresponding nuclear receptors in mouse liver., Chronobiol Int. 30 (2013) 1135–43. doi: 10.3109/07420528.2013.805762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tanaka Y, Ikeda T, Yamamoto K, Masuda S, Ogawa H, Kamisako T, Gender-divergent expression of lipid and bile acid metabolism-related genes in adult mice offspring of dams fed a high-fat diet, J Biosci. 43 (2018) 329–337. doi: 10.1007/s12038-018-9750-9. [DOI] [PubMed] [Google Scholar]
- [69].Lorbek G, Perše M, Horvat S, Björkhem I, Rozman D, Sex differences in the hepatic cholesterol sensing mechanisms in mice, Molecules. 18 (2013) 11067–11085. doi: 10.3390/molecules180911067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Y, Klaassen CD, Hormonal regulation of Cyp4a isoforms in mouse liver and kidney, Xenobiotica. 43 (2013) 1055–1063. doi: 10.3109/00498254.2013.797622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sanguino E, Bejarano R, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC, Sexual dimorphism in lipid metabolic phenotype associated with old age in Sprague-Dawley rats, Exp Gerontol. 39 (2004) 1295–1306. doi: 10.1016/j.exger.2004.06.007. [DOI] [PubMed] [Google Scholar]
- [72].González-Granillo M, Helguero LA, Alves E, Archer A, Savva C, Pedrelli M, Ahmed O, Li X, Domingues MR, Parini P, Gustafsson JÅ, Korach-André M, Sex-specific lipid molecular signatures in obesity-associated metabolic dysfunctions revealed by lipidomic characterization in ob/ob mouse 11 Medical and Health Sciences 1103 Clinical Sciences, Biol Sex Differ. 10 (2019) 1–17. doi: 10.1186/s13293-019-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Besse-Patin A, Léveillé M, Oropeza D, Nguyen BN, Prat A, Estrogen Signals Through Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α to Reduce Oxidative Damage Associated With Diet-Induced Fatty Liver Disease, Gastroenterology. 152 (2017) 243–256. doi: 10.1053/j.gastro.2016.09.017. [DOI] [PubMed] [Google Scholar]
- [74].Zheng D, Wang X, Antonson P, Gustafsson JÅ, Li Z, Genomics of sex hormone receptor signaling in hepatic sexual dimorphism, Mol Cell Endocrinol. 471 (2018) 33–41. doi: 10.1016/j.mce.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Honkakoski P, Palvimo JJ, Penttilä L, Vepsäläinen J, Auriola S, Effects of triaryl phosphates on mouse and human nuclear receptors, Biochem Pharmacol. 67 (2004) 97–106. doi: 10.1016/j.bcp.2003.08.037. [DOI] [PubMed] [Google Scholar]
- [76].Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ, Human Bile Salt Export Pump Promoter Is Transactivated by the Farnesoid X Receptor/Bile Acid Receptor, J Biol Chem. 276 (2001) 28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


