Neutrophils have a short lifespan that is extended after exposure to granulocyte macrophage colony stimulating factor (GM-CSF) or lipopolysaccharide (LPS)1. While the survival is regulated by BCL-2 family proteins2, it is not known which pro-survival proteins are involved. GM-CSF stimulation in neutrophils upregulates A1, but A1-deficient mice showed no defects in this cell type3. MCL-1 is critical for the survival of quiescent neutrophils4,5, but it is not known whether the same holds true after activation. We hypothesized that A1 and MCL-1 have overlapping roles in the survival of activated neutrophils.
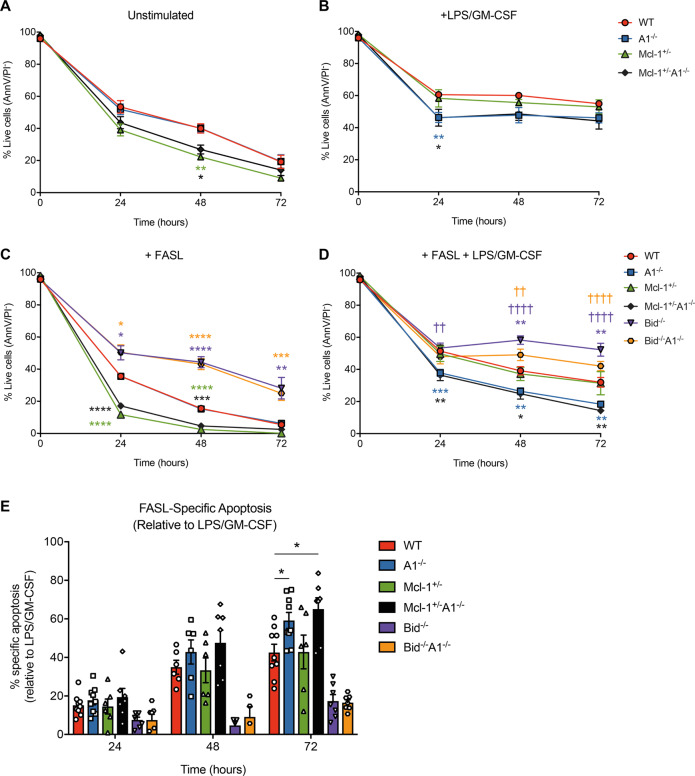
We generated mutant mice deficient for A1 and lacking one allele of Mcl-1 (Mcl-1+/–A1–/–). Mcl-1+/–A1–/– mice are grossly normal in the haematopoietic compartment, with only a small reduction in lymphocyte numbers, similar to Mcl-1+/– mice6 (Supplementary Fig. 1A). Loss of A1 did not cause a survival defect in GM-CSF-stimulated neutrophils. Here, we examined the survival of neutrophils activated with LPS plus GM-CSF from A1–/–, Mcl-1+/–, and Mcl-1+/–A1–/– mice. Without stimulation, Mcl-1+/– neutrophils had a significant survival disadvantage compared to their wild-type and A1–/– counterparts and no further decrease in cell survival was observed in Mcl-1+/–A1–/– neutrophils (Fig. 1a). Presumably, this increased apoptosis observed in Mcl-1+/– neutrophils is due to the in vitro conditions, as we saw normal neutrophil numbers in vivo in Mcl-1+/– or Mcl-1+/–A1–/– mice (Supplementary Fig. 1B). After activation with LPS plus GM-CSF, the A1−/− and Mcl-1+/–A1–/– neutrophils exhibited significantly poorer survival, whilst Mcl-1+/– neutrophils behaved similarly to wild-type cells (Fig. 1b). LPS treatment alone was ineffective at promoting a survival advantage and failed to induce neutrophil blasting or upregulate pro-survival MCL-1 expression (Supplementary Fig. 2A–C). GM-CSF treatment alone promoted survival, blasting, and MCL-1 upregulation in wild-type and A1–/– cells3. GM-CSF is known to induce expression of the TLR4 co-receptor CD147. We observed marked upregulation of CD14 on neutrophils after GM-CSF stimulation, and more so after treatment with GM-CSF plus LPS (Supplementary Fig. 2C). Hence, the survival defect of LPS plus GM-CSF-stimulated A1−/− neutrophils could be due to a lack of increased A1 expression, contributing to the survival of activated neutrophils8,9.
Fig. 1.
Survival analysis of neutrophils from mice with the indicated genotypes cultured in a simple medium (no added cytokines), b after stimulation with 10 ng/mL GM-CSF plus 10 ng/mL LPS, c after treatment with Fc-FASL (0.6 ng/mL), and d after stimulation with LPS plus GM-CSF (10 ng/mL each) and Fc-FASL (0.6 ng/mL). e FASL-specific apoptosis when compared to survival of cells stimulated with LPS plus GM-CSF. Data are from five combined experiments (WT n = 9, A1–/– n = 9, Mcl-1+/– n = 6, Mcl-1+/–A1–/– n = 7, Bid–/– n = 7, and Bid–/–A1–/– n = 7 mice). Statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) was determined using Student’s t-test at each timepoint compared to WT (*) or A1–/– (†).
Neutrophils are highly sensitive to FAS-induced apoptosis1, but this death is delayed when they are activated by LPS plus GM-CSF1. We analyzed FASL-induced apoptosis with and without LPS plus GM-CSF stimulation in neutrophils from A1 and Mcl-1 mutant mice. Additionally, FASL-induced apoptosis in neutrophils is dependent on caspase-8-mediated activation of the pro-apoptotic BCL-2 family member BID (called tBID)10, which A1 binds to with high affinity11. We therefore also included Bid–/– mice12 as a control in our experiments and, furthermore, generated Bid–/–A1–/– mice in order to examine whether any effects seen in the A1–/– cells were dependent on A1–tBID interactions.
Mcl-1+/– (and Mcl-1+/–A1–/–) neutrophils died quicker than wild-type cells after FASL treatment (Fig. 1c). FASL-induced apoptosis was greater than basal apoptosis in culture (Supplementary Fig. 3). Bid–/– neutrophils were protected from FASL-induced apoptosis10. LPS plus GM-CSF protected both wild-type and Mcl-1+/– neutrophils against FASL-induced killing (Fig. 1d). In contrast, A1–/– and Mcl-1+/–A1–/– neutrophils exhibited significantly more apoptosis across all time points after treatment with FASL in LPS plus GM-CSF-activated neutrophils. Taking into account the increase in apoptosis after LPS plus GM-CSF stimulation in A1–/– neutrophils. We observed a trend towards more FASL-specific apoptosis in the A1-deficient cells, although this only reached statistical significance at 72 h (Fig. 1e). The amount of FASL-specific apoptosis did not differ between Bid–/– and Bid–/– A1–/– cells, indicating that the increased sensitivity of activated A1–/– neutrophils to FASL killing is mediated by tBID. Bid–/–A1–/– neutrophils displayed lower viability than their Bid–/– counterparts, both after LPS plus GM-CSF stimulation (Supplementary Fig. 4) and with the combination of LPS, GM-CSF, and FASL (Fig. 1d), fitting with the role we showed for A1 in promoting cell survival after LPS plus GM-CSF stimulation alone.
Collectively, we demonstrate that upregulation of A1 after stimulation imparts a survival advantage in neutrophils, including FASL-induced apoptosis. However, A1’s role is relatively small, and other factors must also regulate the survival of activated neutrophils. These results suggest a previously unrecognized role for A1 in promoting neutrophil survival in an inflammatory context.
Supplementary information
Acknowledgements
We acknowledge the invaluable contributions of the animal caretaker staff at the Walter & Eliza Hall Institute for animal husbandry, namely Giovanni Siciliano, Krystal Hughes and Daniel Fayle. We also acknowledge the flow cytometry facilities of the Walter & Eliza Hall Institute, led by Simon Monard and his team. This work was supported by grants and fellowships from the Australian National Health and Medical Research Council (NHMRC) (Project Grants 1186575 and 1145728 to M.J.H., 1143105 to M.J.H. and A.S., 1159658 to M.J.H., Program Grant 1016701 to A.S. and Fellowships 1020363 to A.S., 1156095 to M.J.H.), the Leukemia and Lymphoma Society of America (LLS SCOR 7001-13 to A.S. and M.J.H.), the Cancer Council of Victoria (project grant 1147328 to M.J.H., 1052309 to A.S., and Venture Grant to M.J.H. and A.S.), as well as by operational infrastructure grants through the Australian Government Independent Research Institute Infrastructure Support Scheme (361646 and 9000220) and the Victorian State Government Operational Infrastructure Support Program.
Author contributions
R.L.S. performed and designed most experiments and wrote the manuscript; L.G. helped to perform experiments and write the manuscript; K.E.L. helped with discussions and advice on neutrophil experiments and write the manuscript; L.A.O. provided reagents and helped with advice on FASL experiments and write the manuscript; A.S. and M.J.H. planned the project, were involved in experimental design and helped to write the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-2676-9).
References
- 1.O’Donnell JA, et al. Fas regulates neutrophil lifespan during viral and bacterial infection. J. Leukoc. Biol. 2015;97:321–326. doi: 10.1189/jlb.3AB1113-594RR. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenk RL, et al. Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ. 2017;24:534–545. doi: 10.1038/cdd.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzhagalov I, John AS, He Y-W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csepregi JZ, et al. Myeloid-specific deletion of Mcl-1 yields severely neutropenic mice that survive and breed in homozygous form. J. Immunol. 2018;201:3793–3803. doi: 10.4049/jimmunol.1701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann K, et al. The combination of reduced MCL-1 and standard chemotherapeutics is tolerable in mice. Cell Death Differ. 2017;24:2032–2043. doi: 10.1038/cdd.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurt-Jones EA, et al. Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–1868. doi: 10.1182/blood.V100.5.1860.h81702001860_1860_1868. [DOI] [PubMed] [Google Scholar]
- 8.Vier J, Groth M, Sochalska M, Kirschnek S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016;7:e2103. doi: 10.1038/cddis.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renshaw SA, et al. Inflammatory neutrophils retain susceptibility to apoptosis mediated via the Fas death receptor. J. Leukoc. Biol. 2000;67:662–668. doi: 10.1002/jlb.67.5.662. [DOI] [PubMed] [Google Scholar]
- 10.Wicki S, et al. Loss of BID delays FASL-induced cell death of mouse neutrophils and aggravates DSS-induced weight loss. Int. J. Mol. Sci. 2018;19:684. doi: 10.3390/ijms19030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner AB, Vries E, de, Tait SWG, Bontjer I, Borst J. Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit its collaboration with pro-apoptotic Bak or Bax. J. Biol. Chem. 2002;277:22781–22788. doi: 10.1074/jbc.M201469200. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann T, et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.