Graphical abstract
Keywords: Transcatheter aortic valve replacement, Paravalvular leak, Intracardiac echocardiography
Highlights
-
•
TAVR has become a prevalent procedure.
-
•
PVL is a complication of TAVR with increased risk for mortality.
-
•
TEE, TTE, and angiography may misrepresent TAVR eccentric regurgitation for PVL.
-
•
ICE may be used to distinguish TAVR regurgitation.
Introduction
Transcatheter aortic valve replacement (TAVR) has rapidly become a compelling structural interventional management of severe aortic stenosis in adults. Since the initial use in 2002 by Cribier and colleagues, TAVR procedures are being performed at more institutions, beginning to exceed even surgical aortic valve replacement.1,2 As the use of TAVR has become more widespread, the complications associated with this intervention must be well understood to better address prevention and management. Paravalvular leak (PVL) is among the more common TAVR complications, with moderate to severe leaks seen in approximately 2% to 12% of patients.3 We describe a patient with the appearance of post-TAVR PVL on subsequent echocardiography and angiographic imaging in whom definitive diagnosis of eccentric aortic regurgitation due to frozen intra-TAVR cusp could be established only by intracardiac echocardiography (ICE).
Case Presentation
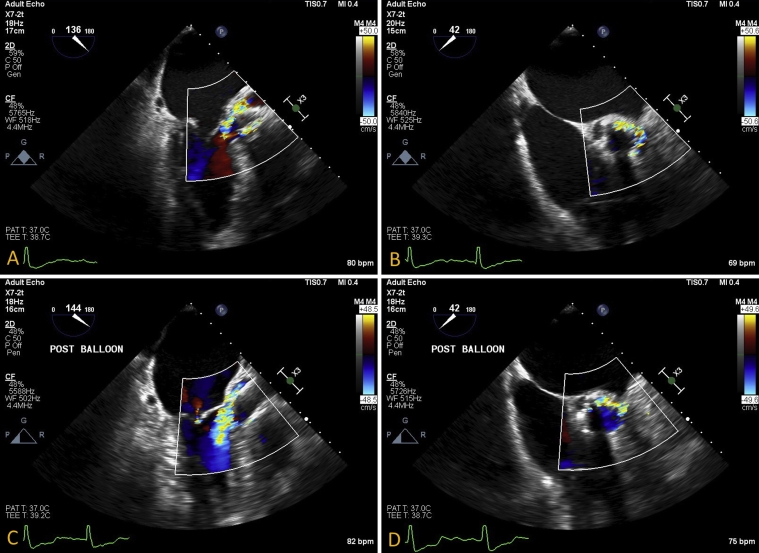
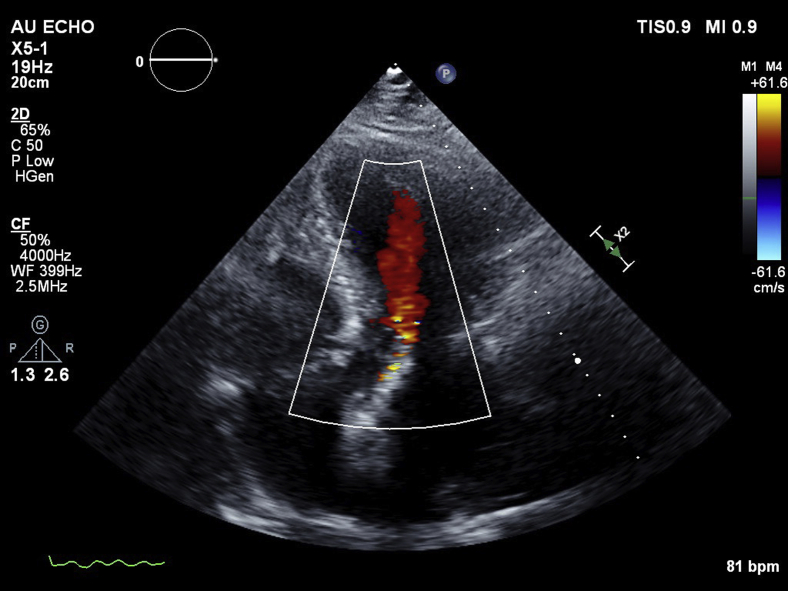
A 71-year-old man who had undergone transfemoral TAVR with a 34-mm CoreValve Evolut R bioprosthetic aortic valve (Medtronic, Minneapolis, MN) for severe symptomatic aortic stenosis with severe valvular calcification was referred for PVL device closure. His medical history included multivessel coronary artery disease with coronary artery bypass and percutaneous coronary intervention with rotational atherectomy, followed by drug-eluting stent placement to the nonrevascularized right coronary artery, paroxysmal atrial fibrillation with cryoablation, moderate to severe mitral regurgitation, and hypertension. Following initial TAVR deployment, immediate transesophageal echocardiography (TEE) was performed, demonstrating appropriate valve depth with appearance of a moderate anterior PVL, determined by the circumferential extent of regurgitation (Figures 1A and 1B, Video 1). Post-TAVR balloon dilation was performed, with notable improvement in diastolic blood pressure from the 50s to the 70s but only minimal change in the PVL appearance (Figures 1C and 1D, Video 1). Subsequent aortography showed satisfactory coronary blood flow and light opacification of the left ventricle, consistent with moderate PVL. On TEE, the aortic stenosis was resolved, with improvement in maximal velocity to 1.2 m/sec and an estimated mean gradient of 4 mm Hg. As the patient remained hemodynamically stable, the TAVR procedure was concluded, with the decision made to allow the patient to recover with close monitoring. Postprocedural transthoracic echocardiography (TTE) continued to exhibit similar moderate PVL adjacent to the mitral valve, with otherwise normal functioning bioprosthetic aortic valve (Figure 2, Video 2). There was transient diastolic flow reversal seen within the descending aorta. Quantification of regurgitation could not be determined accurately, because of significant mitral regurgitation. Left ventricular chamber size and systolic function were normal, with a left ventricular ejection fraction of 55% to 60%.
Figure 1.
Immediate TEE following TAVR. Midesophageal (A) long-axis and (B) short-axis view demonstrated appearance of moderate posterior PVL. Post–balloon dilation for PVL in (C) long-axis and (D) short-axis views with improvement in PVL appearance by the right coronary sinus of Valsalva with residual moderate leak along the left and noncoronary sinus of Valsalva.
Figure 2.
Post-TAVR follow-up TTE in apical three-chamber long-axis view demonstrated an appearance of moderate PVL with anterior directed jet.
At the patient's initial follow-up clinic visit, he reported progressive dyspnea on exertion despite optimal diuretic use, and repeat TTE showed unchanged presumed PVL. To address his symptomatic regurgitation, the patient was scheduled for percutaneous device closure of the PVL. He was brought back to the cardiac catheterization laboratory and placed under general anesthesia. During the procedure, intraoperative TEE again demonstrated the appearance of moderate anterior PVL by the location of the native left and noncoronary sinus of Valsalva. Biplane aortography in straight posteroanterior, lateral, and near en face view at 114° left anterior oblique with 28° caudal angulation demonstrated a heavily calcified aortic root with the appearance of moderate aortic regurgitation and residual contrast opacification within the sinuses of Valsalva, but insufficient for PVL diagnosis (Video 3). Then, a 260-cm, 0.035”-inch Glidewire (Terumo, Tokyo, Japan) was used to probe around the TAVR via both retrograde and antegrade approaches but was unsuccessful in demonstrating any paravalvular communication between the aortic root and left ventricle. Further interrogation was deemed necessary, for which ICE was elected to provide additional near-field views of the TAVR, as TEE, angiography, and mechanical probing did not sufficiently demonstrate PVL location.
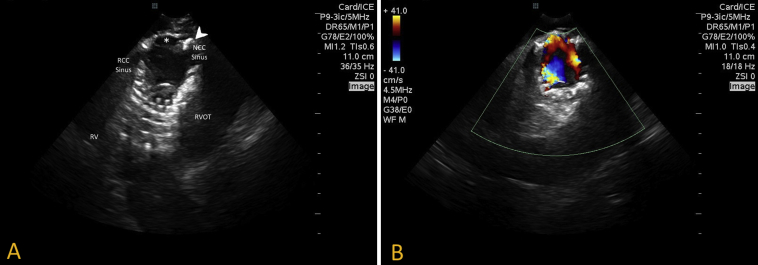
A 9-Fr ViewFlex Xtra ICE Catheter (St. Jude Medical, St. Paul, MN) was then inserted and advanced to the right atrium to profile the TAVR device. From an aortic short-axis view, ICE demonstrated limited mobility of the posterior TAVR cusp with poor coaptation and an eccentric anterior intra-TAVR regurgitant jet stream by the native left sinus of Valsalva on color Doppler, with no significant PVL visualized (Figure 3, Video 4). There was significant annular calcification, with calcium protrusion within the valve tines appearing to affect TAVR cusp mobility. No further interventions were performed at the time, and a team decision was made to bring the patient back for TAVR valve-in-valve replacement at a later date, as he was not consented for the procedure.
Figure 3.
ICE. (A) Two-dimensional short-axis view of the aortic valve demonstrated poor mobility of the TAVR leaflet by the noncoronary sinus of Valsalva (asterisk), with noted annular calcification and calcium protrusion within the tines of the TAVR scaffold (arrowhead). (B) Color Doppler interrogation demonstrated intra-TAVR anterior eccentric regurgitation propagated from the frozen TAVR cusp.
Discussion
Severe symptomatic aortic stenosis is a fatal disease with a mortality rate of up to 50% by 2 years.1 More recently, TAVR has been shown to be an effective interventional approach to treatment and has become increasingly popular in the United States.2,4 The latest generation of transcatheter aortic valves, the balloon-expandable valve (SAPIEN; Edwards Lifesciences, Irvine, CA) and the self-expanding valve (Medtronic), have been designed to improve delivery profiles and diminish potential complications, such as the addition of a bioprosthetic skirt around the transcatheter aortic valve base to enhance perivalvular seal following deployment.5
The increasing performance of such procedures warrant a solid understanding of potential TAVR-related complications and an approach to management. Immediate complications include but are not limited to bleeding, low cardiac output, coronary obstruction, stroke, heart block, PVL, and intravalvular aortic regurgitation. Despite advancements in device architecture, aortic regurgitation remains relatively common, particularly because of PVL. Moderate to severe PVL has been reported to occur in approximately 2% to 5% of cases from the newest generation of TAVR devices.3,6 Even in the recent low-risk self-expanding valve trial, moderate to severe aortic regurgitation was present in 3.5% of patients at 30 days.7
PVL may result from adverse TAVR placement technique, poor recipient substrate due to heavy valve calcification or inflammatory friability, or patient-prosthesis mismatch. The presence of PVL can lead to excessive left ventricular volume burden, hemolytic anemia, increased risk for endocarditis, and potential thrombus formation. PVL portends a poor prognosis, with multiple studies concluding a progressive evolution of regurgitation with short- and long-term risk for mortality.8 Therefore, the presentation of moderate to severe PVL signifies a serious complication requiring further investigation.
Multimodality imaging has been recommended for the intraoperative and periprocedural period of PVL diagnosis and severity on the basis of the generally accepted Valve Academic Research Consortium-2 guidelines.9 Readily available intraoperative approaches include TTE, TEE, fluoroscopy, rotational angiography, and ICE. Furthermore, advancements in fusion imaging are progressing rapidly.10 Supplemental hemodynamic assessments at time of TAVR placement may be used to signify the severity of PVL by mortality prediction.11 Per our institution's TAVR protocol, immediate post-TAVR TEE following device placement is used for evaluation before clearance from the hybrid operating room as well as subsequent TTE before hospital discharge.
After deployment, TEE can verify stent positioning, shape, and cusp motion, allowing adequate hemodynamic evaluation. On both TTE and TEE, short-axis images are essential for assessment of PVL, typically providing adequate annular and subannular views. Additionally, left ventricular outflow tract views on TEE may identify PVL and/or intravalvular regurgitant jet location and severity. Quantification may be attempted through calculation of regurgitant volumes and percentage fraction in measurements through stroke volume investigation, although accuracy may be complicated by eccentric regurgitant jet characteristics, suboptimal left ventricular function, and mitral regurgitation.11 It should also be noted that compared with cardiac magnetic resonance imaging, echocardiography has been found to underestimate the severity of PVL, but it does remain an excellent screening imaging modality.12
Aortic root angiography is at hand during the TAVR procedure, serving as a supplemental imaging modality for regurgitation assessment. Although reasonable in grading severity, if not positioned in a true en face view, which is often difficult to obtain, aortic root angiography may be restricted in establishing PVL location and is bound to regurgitant flow mechanics.8 Furthermore, additional angiographic assessment after TAVR deployment may be limited in those with renal disease because of increased risk for contrast-induced nephropathy. In our patient, limited TAVR cusp mobility and calcium intrusion produced an eccentric jet stream with Coandă effect, obscuring both transthoracic and transesophageal color Doppler imaging as well as contrast flow on aortic root angiography.
The complementary use of ICE led to our patient's definitive diagnosis of eccentric intravalvular aortic regurgitation and not PVL. Because TEE may be subject to acoustic shadowing of the anterior ring with mid- to far-field interrogation, ICE appears to provide a suitable imaging modality substitute of comparable results.5 ICE can provide immediate annular measurements, imaging of the coronary ostia and assessment of regurgitation through the aortic short-axis and long-axis views. In reviewing the utility of ICE during TAVR implantation, Bartel et al.13 described a reasonable and effective approach to TAVR device placement with the advantage of minimizing overall sedation and unnecessary dye contrast administration, while obtaining excellent near-field resolution, performed at the control of the structuralist. It is our belief that use of ICE may be generalized to the TAVR procedure as an accessible and high-fidelity means of PVL imaging in the intraoperative and postprocedural period.
Conclusion
In cases of immediate post-TAVR aortic regurgitation, especially in patients with more than mild insufficiency, it is imperative to thoroughly evaluate the etiology and severity of insufficiency using complementary imaging modalities. Imaging interrogation limitations of eccentric regurgitant jets after TAVR can result in the misdiagnosis of intravalvular regurgitation for PVLs. Therefore, we show that ICE provides a reasonable substitution to standard imaging modalities or, at the very least, a complementary imaging modality for post-TAVR implantation evaluation.
Footnotes
Conflicts of interest: The authors report no conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.11.004.
Supplementary Data
TAVR intraprocedural TEE. Initial short- and long-axis images demonstrated a severely calcific, stenotic aortic valve with central regurgitation. Immediate post-TAVR TEE demonstrated appearance of prominent moderate anterior PVL as well as by the right coronary sinus of Valsalva. Long-axis view post-TAVR balloon dilation demonstrated improvement in the appearance of PVL along the right coronary sinus with persistent anterior PVL. Midesophageal short-axis view after TAVR balloon dilation demonstrated appearance of moderate residual PVL by the left and noncoronary sinus of Valsalva.
Follow-up TAVR TTE. Parasternal long-axis view demonstrated persistent moderate anterior PVL.
Aortography demonstrated a heavily calcified aortic root with moderate contrast regurgitation along the left and noncoronary sinus of Valsalva. Angiography performed at 114° left anterior oblique with 28° caudal angulation producing a relative en face view. Light regurgitation of contrast filled the left ventricle. There was appearance of dense contrast retention within the aortic sinus of Valsalva.
Two-dimensional short-axis view of the aortic valve by ICE demonstrated a poorly mobile TAVR leaflet by the noncoronary sinus of Valsalva with significant calcium burden and impingement through the TAVR scaffold tines. Color Doppler interrogation in short-axis view demonstrated anterior eccentric regurgitation with frozen cusp of the TAVR by the noncoronary sinus. Color Doppler short-axis sweep of the TAVR apparatus exhibited flow through the tines within the sinus of Valsalva with significant calcium burden compromise of the TAVR scaffold. No PVL was visualized.
References
- 1.Arora S., Misenheimer J.A., Ramaraj R. Transcatheter aortic valve replacement: comprehensive review and present status. Texas Hear Inst J. 2017;44:29–38. doi: 10.14503/THIJ-16-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messenger J.M. Trends in United States TAVR practice. Card Interv Today. 2018;12:46–50. [Google Scholar]
- 3.Goel K., Eleid M.F. Paravalvular leak in structural heart disease. Curr Cardiol Rep. 2018;20:18. doi: 10.1007/s11886-018-0959-x. [DOI] [PubMed] [Google Scholar]
- 4.Mack M.J., Brennan J.M., Brindis R., Carroll J., Edwards F., Grover F. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 5.Hahn R.T., Little S.H., Monaghan M.J., Kodali S.K., Williams M., Leon M.B. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. JACC Cardiovasc Imaging. 2015;8:261–287. doi: 10.1016/j.jcmg.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz C.E., Hahn R.T., Berrebi A., Borer J.S., Cutlip D.E., Fontana G. Clinical trial principles and endpoint definitions for paravalvular leaks in surgical prosthesis: an expert statement. J Am Coll Cardiol. 2017;69:2067–2087. doi: 10.1016/j.jacc.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Popma J.J., Deeb G.M., Yakubov S.J., Mumtaz M., Gada H., O'Hair D. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 8.Généreux P., Head S.J., Hahn R., Daneault B., Kodali S., Williams M.R. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles' heel? A comprehensive review of the literature. J Am Coll Cardiol. 2013;61:1125–1136. doi: 10.1016/j.jacc.2012.08.1039. [DOI] [PubMed] [Google Scholar]
- 9.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Anwaruddin S. The role of preoperative and intraoperative imaging in guiding transcatheter aortic valve replacement. Interv Cardiol Clin. 2015;4:39–51. doi: 10.1016/j.iccl.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Zoghbi W.A., Asch F.M., Bruce C., Gillam L.D., Grayburn P.A., Hahn R.T. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2019;32:431–475. doi: 10.1016/j.echo.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Gopalakrishnan D., Gopal A., Grayburn P.A. Evaluating paravalvular leak after TAVR. Heart. 2014;100:1903–1904. doi: 10.1136/heartjnl-2014-306390. [DOI] [PubMed] [Google Scholar]
- 13.Bartel T., Edris A., Velik-Salchner C., Muller S. Intracardiac echocardiography for guidance of transcatheter aortic valve implantation under monitored sedation: a solution to a dilemma? Eur Heart J Cardiovasc Imaging. 2016;17:1–8. doi: 10.1093/ehjci/jev280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TAVR intraprocedural TEE. Initial short- and long-axis images demonstrated a severely calcific, stenotic aortic valve with central regurgitation. Immediate post-TAVR TEE demonstrated appearance of prominent moderate anterior PVL as well as by the right coronary sinus of Valsalva. Long-axis view post-TAVR balloon dilation demonstrated improvement in the appearance of PVL along the right coronary sinus with persistent anterior PVL. Midesophageal short-axis view after TAVR balloon dilation demonstrated appearance of moderate residual PVL by the left and noncoronary sinus of Valsalva.
Follow-up TAVR TTE. Parasternal long-axis view demonstrated persistent moderate anterior PVL.
Aortography demonstrated a heavily calcified aortic root with moderate contrast regurgitation along the left and noncoronary sinus of Valsalva. Angiography performed at 114° left anterior oblique with 28° caudal angulation producing a relative en face view. Light regurgitation of contrast filled the left ventricle. There was appearance of dense contrast retention within the aortic sinus of Valsalva.
Two-dimensional short-axis view of the aortic valve by ICE demonstrated a poorly mobile TAVR leaflet by the noncoronary sinus of Valsalva with significant calcium burden and impingement through the TAVR scaffold tines. Color Doppler interrogation in short-axis view demonstrated anterior eccentric regurgitation with frozen cusp of the TAVR by the noncoronary sinus. Color Doppler short-axis sweep of the TAVR apparatus exhibited flow through the tines within the sinus of Valsalva with significant calcium burden compromise of the TAVR scaffold. No PVL was visualized.