Graphical abstract

Keywords: Transesophageal echocardiography, Watchman device, Infectious endocarditis
Highlights
-
•
The authors report a rare case of infected Watchman device 4 months after placement.
-
•
A high index of suspicion allowed timely diagnosis of Watchman device infection.
-
•
Transesophageal echocardiography led to diagnosis of an infected Watchman device.
-
•
High clinical morbidity is associated with Watchman device endocarditis.
Introduction
The Watchman left atrial appendage (LAA) occlusion device is approved for patients with atrial fibrillation requiring anticoagulation and unacceptable bleeding risk, and since its approval in 2015, >100,000 devices have been implanted.
We present a case report of a patient in whom transesophageal echocardiography (TEE) was instrumental in diagnosing an infected Watchman (Boston Scientific, Marlborough, Massachusetts) device. At this time, there is no formal recommendation regarding bacterial endocarditis prophylaxis with Watchman placement, and there are only limited case studies citing infection in the literature.
Case Presentation
A 74-year-old man had a Watchman device implanted to limit anticoagulation use. He had a history of persistent atrial fibrillation and prior percutaneous intervention with drug-eluting stents followed by massive gastrointestinal bleeding on triple-antiplatelet therapy and cardioembolic strokes off warfarin. Following Watchman placement, he was successfully treated with 45 days of warfarin plus aspirin. Forty-five-day postprocedural TEE showed correct device position and complete occlusion of the LAA. At that time, warfarin was discontinued, and 81 mg aspirin was continued with prasugrel.
Thirteen weeks following Watchman placement, the patient was admitted to the hospital with fever, chills, and back pain secondary to sigmoid diverticulitis. He was treated with intravenous antibiotics and discharged home on amoxicillin/clavulanic acid. Fifteen weeks following Watchman placement, he presented to the hospital again with repeat fever. Abdominal computed tomography showed uncomplicated diverticulitis. The infectious disease team was consulted and discharged the patient on oral amoxicillin/clavulanic acid given clinical improvement. He continued to have intermittent fevers and persistent weakness while on antibiotics.
Twenty weeks after implantation, the patient was again admitted to the hospital with fevers and chills. Chest radiography showed left lower lobe infiltrate. Blood cultures returned positive for Gram-positive and Gram-negative pathogens speciated to Enterococcus and Enterobacter. Computed tomography of the chest, abdomen, and pelvis showed splenic necrosis. Magnetic resonance imaging of the brain showed multiple acute and subacute embolic infarcts. The patient was transferred to a tertiary care facility where TEE was completed given the high index of suspicion of endocarditis in the setting of an indwelling device and metastatic infection.
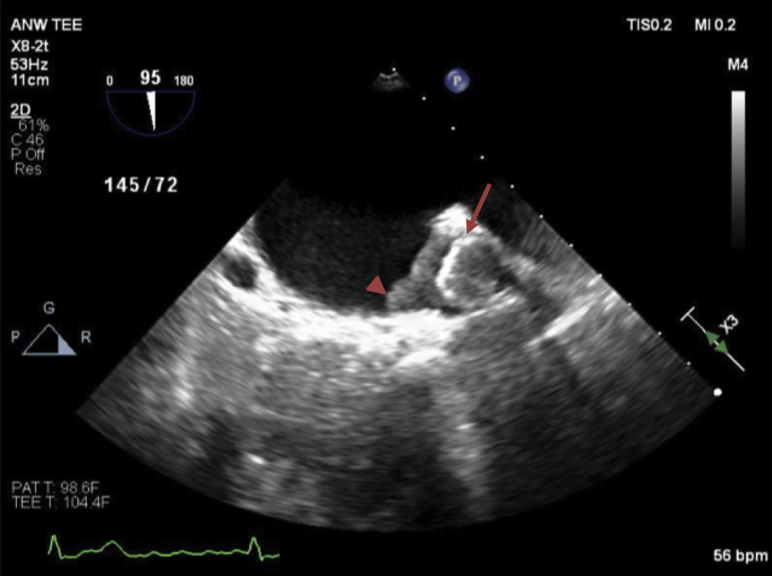
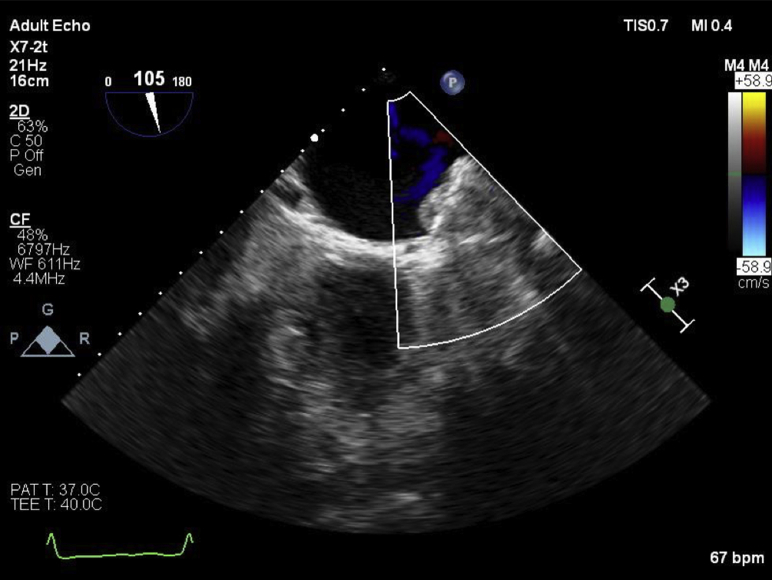
TEE revealed severe biatrial enlargement with a well-visualized Watchman device properly positioned in the LAA and a large, dense, multilobulated irregular mass adherent to the device with some mobile components (Figures 1 and 2, Videos 1 and 2) without evidence of any valvular involvement. The echogenicity was similar to the myocardium. There was no evidence of flow into the LAA by color or pulsed-wave Doppler (Figure 3, Video 3). Following multidisciplinary case discussion involving cardiology, infectious diseases, and cardiac surgery, the decision was made to recommend removal of the occluder device after trial of therapeutic heparin and antibiotics. However, cardiac intervention was delayed for 1 month because of patient instability for septic shock and acute renal failure, secondary to complete spleen necrosis complicated by abscess formation. Pathology examination after splenectomy was positive for infection by Enterococcus and Enterobacter. Transthoracic echocardiography was unable to visualize device or mass, and TEE repeated after a 21-day trial of intravenous therapeutic heparin therapy showed no change and increased prominence of the mass attached to the Watchman device (Figures 4 and 5).
Figure 1.
Midesophageal transesophageal echocardiographic image of Watchman device (arrow) with echogenic mass (arrowhead) at initial diagnosis (95°).
Figure 2.
Midesophageal full-volume three-dimensional transesophageal echocardiographic image of Watchman device with multilobulated mass (arrow) adhered to device with anterior leaflet of the mitral valve (arrowhead) for reference (95°).
Figure 3.
Preoperative midesophageal transesophageal echocardiographic image showing no flow as assessed by color flow Doppler in the LAA (105°).
Figure 4.
Preoperative midesophageal transesophageal echocardiographic image with echogenic mass measuring 1.38 × 1.58 cm (134°).
Figure 5.
Preoperative midesophageal transesophageal echocardiographic biplane image demonstrating the echogenic mass (75° and −32°).
Surgical removal of the infected Watchman device and closure of the LAA was completed without complication using a left atrial approach through the Waterston groove (Figures 6 and 7). The pathology report was notable for fibrinopurulent adhesions and polymorphonuclear neutrophils within the mass attached to the occluder device. Intraoperative cultures were negative.
Figure 6.
Postbypass midesophageal transesophageal echocardiographic image demonstrating surgical ligation (arrow) of Watchman device and LAA (71°).
Figure 7.

Explanted Watchman device with adherent thrombus.
The patient had a prolonged postprocedural course with multiple complications, including acute hemothorax requiring evacuation via tube thoracostomy and anticoagulation reversal, encephalopathy, occlusive right axillary vein thrombosis, nonocclusive subclavian vein thrombosis, respiratory failure requiring reintubation, and ultimately tracheostomy placement. He was discharged to a long-term acute care hospital, and he is alive at 10 months after discharge.
Discussion
We present a unique case report of a patient with an infected Watchman device 4 months following placement despite appropriate postprocedural antiplatelet therapy.
As noted with other indwelling hardware, including prosthetic valves, TEE is recommended with class I evidence for patients with a moderate to high index of suspicion for subacute bacterial endocarditis.1,2 In this case, early transfer of care to a tertiary center was appropriate given strong suspicion for infection. At the tertiary center, early-stage evaluation was indicated with an “endocarditis team” (including cardiology, cardiac surgery, and infectious diseases) as well as imaging with TEE.1 In this case the decision for surgery was supported by the finding of a large irregular multilobulated mass with evidence of ongoing bacteremia and systemic septic embolization. The mass was believed to be a vegetation or a combination of infection and thrombus on the basis of its shape, echogenicity, mobility, and probable growth in size despite anticoagulation.3 The distinction between thrombus and vegetation may not be possible by echocardiography alone.3 The pathologic specimen was consistent with subacute bacterial endocarditis with negative intraoperative cultures reflecting the prolonged antibiotics course before device removal. The septic splenic emboli provide supportive evidence for subacute bacterial endocarditis. Although there are no data specifically looking at Watchman device infections, complete removal is recommended for all other cardiac device–related infective endocarditis, as medical therapy alone has been associated with high mortality and risk for recurrence.4
As the number of LAA devices increases, more infections will be seen in clinical practice. Although no data currently support early diagnosis leading to superior outcomes, it can be hypothesized that morbidity can be reduced with prompt treatment. This is especially important because cardiac device– and prosthetic valve–related infections are associated with high morbidity and mortality.
There are rare case reports in the literature regarding LAA device infection given low rates of reported infectious complications thus far. The EWOLUTION study of 1,021 subjects reported only one infection as a significant adverse event related to the procedure, with no further information provided.5 Other examples have reported infection either very early, within the first week of device implantation,6 or very late, 30 months after device implantation, thought to be associated with a catheter-related infection.7 This case falls between the prior reports but happened outside the window of endothelialization, when infection risk would presumably be highest. At this time there is no guideline regarding endocarditis prophylaxis after placement of LAA occluder devices given the lack of high-quality evidence, though expert opinion hypothesizes that 6 months of endocarditis prophylaxis may be warranted.8
The currently under way PRAGUE-17 study is evaluating 400 patients with a primary composite end point that includes procedure or device-related complications and is set to complete in May 2020.9 This may shed more light on true rates of infection, which could help shape future guidelines.
Conclusion
Infectious complications of LAA closure devices are rare. Clinicians should maintain a high degree of suspicion when a patient with a device presents with infectious symptoms. A multidisciplinary team consisting of cardiology, cardiac surgery, and infectious diseases should be formed, as well as early imaging with TEE followed by device removal if indicated.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.01.008.
Supplementary Data
Midesophageal transesophageal echocardiographic image (116°) demonstrating echogenic mass (arrowhead) attached to Watchman device (arrow).
Midesophageal full-volume three-dimensional transesophageal echocardiographic image (95°) of Watchman device in appropriate position with multilobulated mass (arrow) adhered to device at initial diagnosis with anterior leaflet of the mitral valve (arrowhead) for reference.
Transesophageal echocardiographic image with color Doppler (63°) showing no flow in echogenic mass.
References
- 1.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.P., Del Zotti F. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 2.Harris K.M., Li D.Y., L'Ecuyer P., Moon K.E., German M., Fraser V. The prospective role of transesophageal echocardiography in the diagnosis and management of patients with suspected infective endocarditis. Echocardiography. 2003;20:57–62. doi: 10.1046/j.1540-8175.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 3.Feigenbaum H., Armstrong W.F., Ryan T. Feigenbaum's echocardiography. Lippincott Williams & Wilkins; Philadelphia: 2005. Infective endocarditis; pp. 375–398. [Google Scholar]
- 4.Baddour L.M., Bettmann M.A., Bolger A.F., Epstein A.E., Ferrieri P., Gerber M.A. Nonvalvular cardio-vascular device-related infections. Circulation. 2003;108:2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 5.Boersma L.V.A., Schmidt B., Betts T.R., Sievert H., Tamburino C., Teiger E. Implant success and safety of left atrial appendage closure with the Watchman device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khumri T.M., Thibodeau J.B., Main M.L. Transesophageal echocardiographic diagnosis of left atrial appendage occluder device infection. Eur J Echocardiogr. 2007;9:565–566. doi: 10.1016/j.euje.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Boukobza M., Smaali I., Duval X., Laissy J.-P. Convexity subarachnoid hemorrhage, pseudomonas aeruginosa (PA) infective endocarditis and left atrial appendage occluder (LAAO) device infection. A case report. Open Neuroimaging J. 2017;11:26. doi: 10.2174/1874440001711010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hijazi Z.M., Saw J. Nonpharmacologic therapy to prevent embolization in patients with atrial fibrillation. UpToDate. https://www.uptodate.com/contents/nonpharmacologic-therapy-to-prevent-embolization-in-patients-with-atrial-fibrillation Available at:
- 9.Osmancik P., Tousek P., Herman D., Neuzil P., Hala P., Stasek J. Interventional left atrial appendage closure vs novel anticoagulation agents in patients with atrial fibrillation indicated for long-term anticoagulation (PRAGUE-17 study) Am Heart J. 2017;183:108–114. doi: 10.1016/j.ahj.2016.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Midesophageal transesophageal echocardiographic image (116°) demonstrating echogenic mass (arrowhead) attached to Watchman device (arrow).
Midesophageal full-volume three-dimensional transesophageal echocardiographic image (95°) of Watchman device in appropriate position with multilobulated mass (arrow) adhered to device at initial diagnosis with anterior leaflet of the mitral valve (arrowhead) for reference.
Transesophageal echocardiographic image with color Doppler (63°) showing no flow in echogenic mass.