Graphical abstract
Keywords: Atrial septal aneurysm, Supraventricular tachycardia, Left atrial ablation, Transthoracic echocardiography, Intracardiac echocardiography
Highlights
-
•
Atrial septal aneurysm is a largely underdiagnosed phenomenon.
-
•
Septal anatomy is best delineated using intracardiac echocardiography.
-
•
Septal anatomy is clinically relevant with procedures involving transseptal puncture.
-
•
Atrial septal aneurysms may be associated with supraventricular tachycardias.
Introduction
An atrial septal aneurysm (ASA) is defined by redundant tissue of the central interatrial septum, encompassing the fossa ovalis, with resultant hypermobility of the septum and protrusion >15 mm into the left or right atrium. It is often asymptomatic and clinically insignificant when existing as an isolated finding without other atrial septal defects. We present a case of a 62-year-old female patient with a chronic ischemic cardiomyopathy who presented with palpitations. An electrophysiology (EP) study was notable for difficult intracardiac electrogram mapping on the interatrial septum due to difficulty in obtaining stable electrograms; with placement of an intracardiac echocardiography (ICE) catheter, the patient was found to have a large ASA not appreciated on previous transthoracic echocardiography (TTE). Ultimately, the presence of the ASA dramatically increased the risk of transseptal puncture, and the case was aborted. In the ever-expanding world of catheter-based left atrial procedures, it becomes even more important to correctly recognize abnormal septal anatomy.
Case Presentation
A 62-year-old Hispanic female with coronary artery disease, remote bypass grafting, ischemic cardiomyopathy, chronic systolic heart failure, status postprimary-prevention defibrillator, with subsequent improvement of her measured left ventricular ejection fraction to 35%-40%, presented with sudden-onset palpitations, lightheadedness, and chest tightness. She reported five episodes of sudden-onset palpitations shortly after completing light housework, each lasting a few minutes, with no other associated symptoms. On physical examination, vital signs were within normal limits. Cardiac auscultation was normal, and there was no evidence of acute heart failure.
Device interrogation showed multiple events of a supraventricular tachycardia (SVT), likely either atypical atrioventricular nodal reentrant tachycardia or an atrial tachycardia. As these symptomatic events occurred in the setting of metoprolol therapy, she was referred for ablation. Prior to the ablation, a TTE was notable for left ventricular ejection fraction of 35%-40% and mild paradoxical motion of the ventricular septum consistent with a paced rhythm (Figure 1 and Videos 1 and 2). No other abnormalities were noted.
Figure 1.
TTE, four-chamber view in early diastole, showing no obvious abnormalities of the intra-atrial septum. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
During the EP study, tachycardia was easily induced, and atrial tachycardia was diagnosed as the mechanism of her arrhythmia through electrophysiologic maneuvers. The earliest activation of the tachycardia in the right atrium was mapped to the midseptum. It was noted that there was difficulty in maintaining consistent electrograms on the septum despite apparent catheter stability. Nonetheless, a cluster of radiofrequency ablation lesions was placed on the septum. This did not terminate or alter the cycle length of the tachycardia. As the earliest signal on the septum was broad, and ablation in this area did not terminate the tachycardia, the decision was made to perform a transseptal puncture to map the left atrium as this appeared to be the most likely location of the origin of the tachycardia.
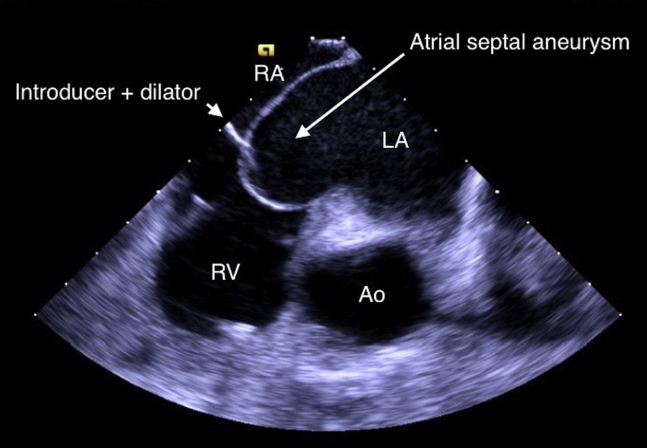
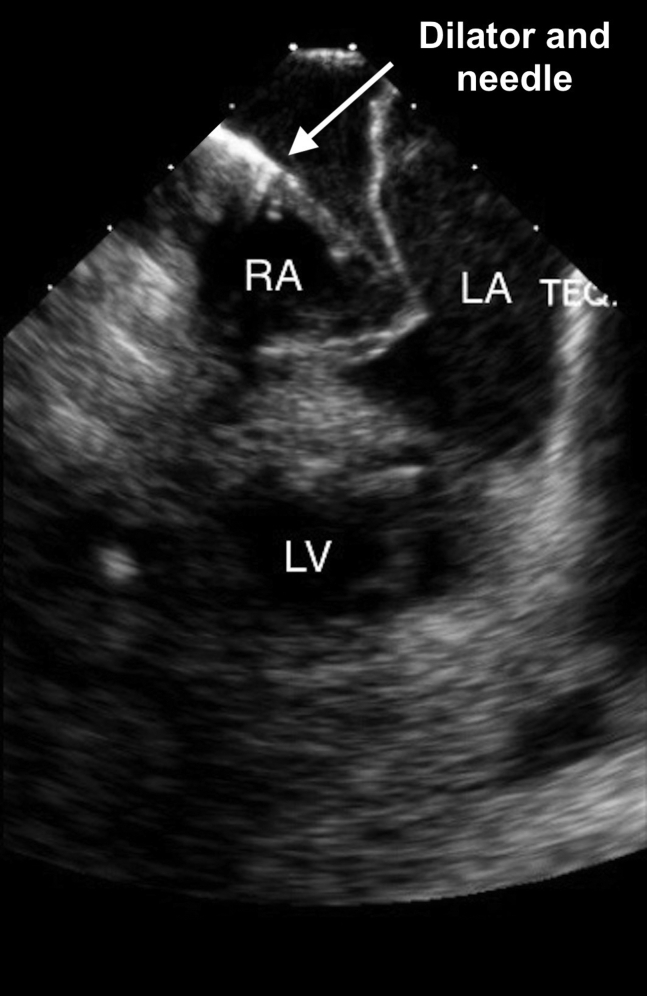
When an ICE catheter was placed in the right atrium, attention was turned to the septum for transseptal puncture. It was noted that the patient had a markedly aneurysmal septum (Figure 2, Figure 3, Figure 4 and Videos 3 and 4). In fact, when engaging the septum with the transseptal sheath and dilator, the necessary amount of force on the septum for passage of the transseptal needle placed the tip of the dilator very near the lateral left atrial wall (Figure 5 and Video 5). Passage of the needle out of the transseptal sheath would have placed the needle tip close to the lateral left atrial wall, posing a much higher risk than is typical for transseptal puncture for atrial perforation.
Figure 2.
ICE, home view, showing large intra-atrial septal aneurysm (arrow). This is a diffuse aneurysm with dimensions of 2.4 cm × 3.0 cm. There is no visible thrombus attached to the aneurysm. Ao, Aortic outflow; LA, left atrium; RA, right atrium; RV, right ventricle.
Figure 3.
ICE, home view with color Doppler, showing large intra-atrial septum aneurysm (arrow) and the absence of atrial septal defect or patent foramen ovale. Ao, Aortic outflow; LA, left atrium; RA, right atrium; RV, right ventricle.
Figure 4.
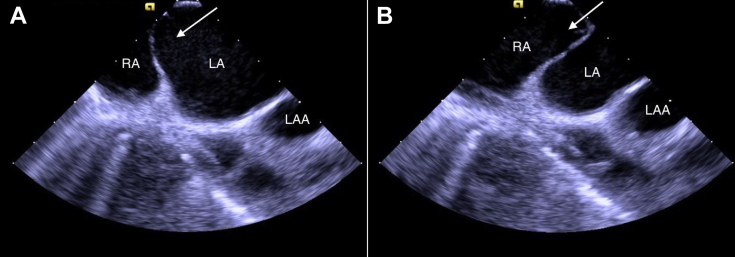
ICE, home view, clockwise rotated compared with Figures 2 and 3, showing another view of the large intra-atrial septum (arrow) in dynamic motion, bowing towards the RA (A) and then the LA (B). LA, Left atrium; LAA, left atrial appendage; RA, right atrium.
Figure 5.
ICE, home view, showing the introducer and dilator (arrow) engaging the intra-atrial septum and the dynamic movements of the septum. LA, Left atrium; LV, left ventricle; RA, right atrium.
Rather than proceed with this higher than expected risk with transseptal puncture, the procedure was aborted. A plan was made to focus on medical therapy by increasing metoprolol dosing. On follow-up office visit 2 months later, the patient denied a recurrence of palpitations.
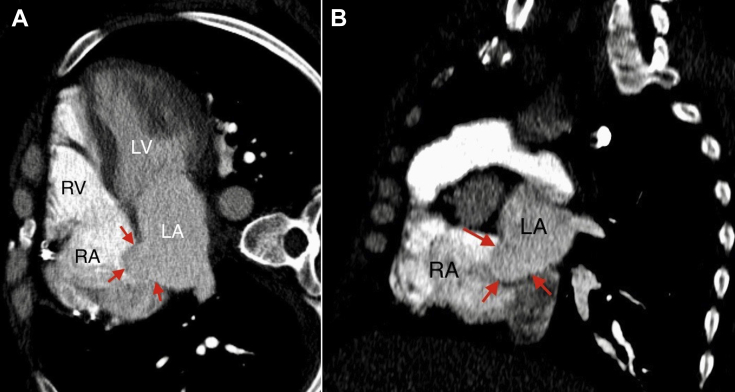
We also present images from a previous chest computed tomography angiography done 4 years ago to exclude pulmonary embolism. Retrospectively, we can identify the ASA emerging out of the fossa ovalis and protruding into the right atrium (Figure 6). It was not reported at the time, probably because it was not a dedicated cardiac study and the reader might have mistaken that for venous admixture from inferior vena cava. Because ASAs of these magnitudes are rare, these lesions are often initially misdiagnosed.1 ASA can also be evaluated using other tomographic modalities such as dedicated cardiac computed tomography and magnetic resonance imaging.
Figure 6.
Computed tomography angiography of the chest, multiplanar reconstruction—horizontal long-axis view of the heart with four chambers (A) and short-axis view of the heart at the atrial level (B)—showing intra-atrial septum bulging into the right atrium cavity (arrows). Aneurysm measures 20 mm × 17 mm. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Discussion
Atrial septal aneurysm is defined as redundant tissue and abnormal mobility of the fossa ovalis membrane protruding >15 mm toward the left or right atrium.2 The prevalence of incidentally noted ASA among patients undergoing TTE is approximately 2%.3,4 This number can be as high as 27.7% in stroke patients, mainly due to the strong association with other atrial defects such as patent foramen ovale and atrial septal defect.5
In asymptomatic patients in whom ASA is incidentally identified, the clinical significance remains unknown. However, in the ever-expanding world of catheter-based left atrial procedures, such as atrial fibrillation ablation, left atrial appendage occlusion device implants, and transcatheter mitral valve repair, septal anatomy becomes highly relevant, as the presence of ASA may pose unique challenges and an increased risk of complications of atrial septal puncture. A series of 30 patients with ASA undergoing balloon mitral valvotomy described failure of transseptal puncture in 10% of the patients due to introducer instability, left atrial perforation, or suboptimal transseptal location precluding crossing of the mitral valve.6 While that was only a small series, it shows a much higher rate of failure or complication with transseptal puncture than is observed in the general population.7 Other complications, including right hemothorax caused by septal puncture in the context of ASA, have been described.8 Given the greater procedural risk associated with transseptal puncture in the presence of an ASA, identification of patients with ASA is extremely important prior to procedures involving atrial septal punctures. As the informed consent process requires discussion of potential risks of any given procedure, identification of this patient's ASA in advance would have informed the consent process, specifically regarding the risk of transseptal puncture.
Interestingly, the role of identifying ASAs prior to atrial septal punctures becomes especially important given the emerging studies suggesting an association between SVT and ASA. The prevalence of SVTs in patients with ASA is 17%-24%,9,10 perhaps related to greater P-wave dispersion, an indicator of delayed or more variable conduction properties through the left atrial myocardium.11
Echocardiography remains the primary method of identifying ASAs. As transesophageal echocardiography is superior to TTE in elucidating cardiac anatomy, as borne out in a 5- to 13-fold increase in detection rates of atrial structural abnormalities, the true prevalence of ASA may actually be much higher than the approximately 2% prevalence currently reported in the literature.12,13 ICE, which offers advantages by providing a larger field of view and superior soft-tissue contrast, is comparable to transesophageal echocardiography in cost and effectiveness, and avoids the added complications of esophageal intubation.14 As a result, there are growing applications for ICE beyond enhancing the safety of transseptal puncture, including for improved clarity in imaging of atrial, ventricular, and valvular structures.15 In this patient, the degree of the ASA was underappreciated on TTE, resulting in unexpected anatomical findings during a cardiac procedure.
Despite the reported increased risk of septal puncture in patients with ASA,6, 7, 8 there exist multiple techniques to reduce the risks in such a setting. Specifically, resourcefulness with existing instruments and advances in transseptal puncture technology can allow for safer procedures. The Brockenbrough needle, which mechanically traverses the intra-atrial septum, has long been used for transseptal puncture, but can pierce the lateral left atrial wall if used alone in a patient with a severe ASA. However, to reduce the amount of pressure required to traverse the septum, and thus the “tenting” toward the lateral left atrial wall, Bovie can be applied to the needle to perforate the intra-atrial septum and gain access to the left atrium.16 The radiofrequency-powered transseptal needle (NRG Transseptal Needle, Baylis Medical, Montreal, ON, Canada) is another alternative that uses radiofrequency applied directly to the fossa ovalis membrane to reduce tenting of the septum. The radiofrequency-powered needle may further mitigate risk as it has a blunter needle tip.17 Use of guide wires can also reduce risk in performing transseptal puncture in patients with ASA. A Brockenbrough needle used in conjunction with a coronary guide wire or J-tipped guide wire inserted through the needle can be safely advanced into the left atrium.18 Even J-tipped wires can cause trauma in the left atrium, specifically in the left atrial appendage, but risk is significantly reduced in ASA patients.19 Similarly, the J-tipped SafeSept needle-free wire (Pressure Products, San Pedro, CA), which has a sharp tip and a radiopaque distal coil, and the TORAYGUIDE wire (Tokyo, Japan), which coils on itself after entering the left atrium, can be passed through a dilator without the need for a standard transseptal needle.20 The decision to proceed with transseptal puncture when facing a pronounced ASA should be based on an assessment of the risks and benefits, and knowledge of various transseptal techniques that can mitigate risk is critical.
Conclusion
We report a dramatic example of an ASA that was underestimated by TTE, but was well visualized on ICE and markedly increased the risk of routine transseptal puncture. This case highlights the increased clinical relevance of ASA in the world of catheter-based left atrial procedures, and the critical role of ICE in detailing important aspects of atrial anatomy and facilitating the safety of catheter-based cardiac procedures.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.11.005.
Supplementary Data
TTE, four-chamber view, showing difficulty in visualizing the large ASA in this echocardiography modality.
TTE, subcostal view, showing an absence of an obvious ASA. Note the presence of the implantable cardioverter defibrillator lead in the right atrium.
ICE, home view clockwise rotated, showing large intraseptal aneurysm dramatically waving from right atrium to left atrium. The ablation dilator is resting in the right atrium.
ICE, home view clockwise rotated with Dopplers, showing the intraseptal aneurysm and the absence of intra-atrial connections such as patent foramen ovale or atrial septal defect.
ICE, home view, showing the introducer and dilator engaging the intra-atrial septum and the dynamic movement of the septum. Note the proximity of the probe to the lateral left atrial wall. The shortest measured distance between the probe and the lateral left atrial wall was 0.7 cm.
References
- 1.Malik S.B., Kwan D., Shah A.B., Hsu J.Y. The right atrium: Gateway to the heart—anatomic and pathologic imaging findings. Radiographics. 2015;35:14–31. doi: 10.1148/rg.351130010. [DOI] [PubMed] [Google Scholar]
- 2.Olivares-Reyes A., Chan S., Lazar E.J., Bandlamudi K., Narla V., Ong K. Atrial septal aneurysm: A new classification in two hundred five adults. J Am Soc Echocardiogr. 1997;10:644–656. doi: 10.1016/s0894-7317(97)70027-0. [DOI] [PubMed] [Google Scholar]
- 3.Yetkin E., Atalay H., Ileri M. Atrial septal aneurysm: Prevalence and covariates in adults. Int J Cardiol. 2016;223:656–659. doi: 10.1016/j.ijcard.2016.08.220. [DOI] [PubMed] [Google Scholar]
- 4.Agmon Y., Khandheria B.K., Meissner I., Gentile F., Whisnant J.P., Sicks J.D. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation. 1999;99:1942. doi: 10.1161/01.cir.99.15.1942. [DOI] [PubMed] [Google Scholar]
- 5.Mattioli A.V., Aquilina M., Oldani A., Longhini C., Mattioli G. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J. 2001;22:261–268. doi: 10.1053/euhj.2001.2293. [DOI] [PubMed] [Google Scholar]
- 6.Goel P.K., Kumar A.S., Kapoor A., Umeshan C.V., Gupta D.K. Inoue balloon mitral valvotomy in patients with atrial septal aneurysm. Int J Cardiol. 2001;78:127–134. doi: 10.1016/s0167-5273(00)00478-2. [DOI] [PubMed] [Google Scholar]
- 7.Salghetti F., Sieira J., Chierchia G.B., Curnis A., de Asmundis C. Recognizing and reacting to complications of trans-septal puncture. Expert Rev Cardiovasc Ther. 2017;15:905–912. doi: 10.1080/14779072.2017.1408411. [DOI] [PubMed] [Google Scholar]
- 8.Nakano T., Hirata A., Sotomi Y., Higuchi Y. Right hemothorax caused by septal puncture during catheter ablation for atrial fibrillation: A case with atrial septal aneurysm and pulmonary emphysema. JACC Clin Electrophysiol. 2018;4:555–556. doi: 10.1016/j.jacep.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Mügge A., Daniel W.G., Angermann C., Spes C., Khandheria B.K., Kronzon I. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1999;91:2785–2792. doi: 10.1161/01.cir.91.11.2785. [DOI] [PubMed] [Google Scholar]
- 10.Janion M., Kurzawski J. Atrial fibrillation in patients with atrial septal aneurysm. Cardiol J. 2007;14:580–584. [PubMed] [Google Scholar]
- 11.Deveci O.S., Aytemir K., Okutucu S., Tulumen E., Aksoy H., Kaya E.B. Evaluation of the relationship between atrial septal aneurysm and cardiac arrhythmias via P-wave dispersion and signal-averaged P-wave duration. Ann Noninvasive Electrocardiol. 2010;15:157–164. doi: 10.1111/j.1542-474X.2010.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum A., Reisner S., Farbstein Y. Transesophageal echocardiography (TEE) vs. transthoracic echocardiography (TTE) in assessing cardio-vascular sources of emboli in patients with acute ischemic stroke. Med Sci Monit. 2004;10:CR521–CR523. [PubMed] [Google Scholar]
- 13.Chu W., Wang H. Transesophageal echocardiography in cardiogenic embolic cerebral infarction. Pak J Med Sci. 2018;34:58–61. doi: 10.12669/pjms.341.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanchetta M., Rigatelli G., Pedon L., Zennaro M., Maiolino P., Onorato E. Role of intracardiac echocardiography in atrial septal abnormalities. J Interv Cardiol. 2003;16:63–77. doi: 10.1046/j.1540-8183.2003.08004.x. [DOI] [PubMed] [Google Scholar]
- 15.Ruisi C.P., Brysiewicz N., Asnes J.D., Sugeng L., Marieb M., Clancy J. Use of intracardiac echocardiography during atrial fibrillation ablation. Pacing Clin Electrophysiol. 2013;36:781–788. doi: 10.1111/pace.12030. [DOI] [PubMed] [Google Scholar]
- 16.McWilliams M.J., Tchou P. The use of a standard radiofrequency energy delivery system to facilitate transseptal puncture. J Cardiovasc Electrophysiol. 2009;20:238–240. doi: 10.1111/j.1540-8167.2008.01323.x. [DOI] [PubMed] [Google Scholar]
- 17.Smelley M., Shah D., Weisberg I., Kim S., Lin A., Beshai J. Initial experience using a radiofrequency powered transseptal needle. J Cardiovasc Electrophysiol. 2010;21:423–427. doi: 10.1111/j.1540-8167.2009.01656.x. [DOI] [PubMed] [Google Scholar]
- 18.Khan J.M., Rogers T., Eng M.H., Lederman R.J., Greenbaum A.B. Guidewire electrosurgery-assisted trans-septal puncture. Catheter Cardiovasc Interv. 2018;91:1164–1170. doi: 10.1002/ccd.27311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki H., Yamagami F., Machino T., Kuroki K., Sekiguchi Y., Aonuma K. Perforation of the left atrial appendage caused by inadvertent deployment of a soft J-tipped guidewire during radiofrequency hot-balloon ablation. Circ J. 2018;82:1476–1477. doi: 10.1253/circj.CJ-17-0733. [DOI] [PubMed] [Google Scholar]
- 20.Wadehra V., Buxton A.E., Antoniadis A.P., McCready J.W., Redpath C.J., Segal O.R. The use of a novel nitinol guidewire to facilitate transseptal puncture and left atrial catheterization for catheter ablation procedures. Europace. 2011;13:1401–1405. doi: 10.1093/europace/eur155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE, four-chamber view, showing difficulty in visualizing the large ASA in this echocardiography modality.
TTE, subcostal view, showing an absence of an obvious ASA. Note the presence of the implantable cardioverter defibrillator lead in the right atrium.
ICE, home view clockwise rotated, showing large intraseptal aneurysm dramatically waving from right atrium to left atrium. The ablation dilator is resting in the right atrium.
ICE, home view clockwise rotated with Dopplers, showing the intraseptal aneurysm and the absence of intra-atrial connections such as patent foramen ovale or atrial septal defect.
ICE, home view, showing the introducer and dilator engaging the intra-atrial septum and the dynamic movement of the septum. Note the proximity of the probe to the lateral left atrial wall. The shortest measured distance between the probe and the lateral left atrial wall was 0.7 cm.