Graphical abstract
Keywords: Cardiology, Veterinary, Congenital heart disease
Highlights
-
•
Aberrant insertion of both vena cava is previously unreported in dogs.
-
•
The dog presented with severe polycythemia and severe exercise intolerance.
-
•
It is caused by flow abnormalities in-utero associated with a sinus venosus ASD.
-
•
A systemic to pulmonary shunt provided clinical benefit and normalisation of PCV.
Introduction
Left atrial insertion of the cranial (i.e., superior) and caudal (i.e., inferior) venae cavae is a rare disorder in humans and is unreported in the domestic dog (Canis lupus familiaris). In humans, it most often manifests as unexplained cyanosis in patients without other signs of heart disease. Herein, we report the case of a young French bulldog presented with cyanosis and marked exercise intolerance, in which a diagnosis of left atrial drainage of the cranial and caudal venae cavae was made, with an atrial septal defect (ASD) and aorticopulmonary collateral vessels.
Case Presentation
A female French bulldog aged 1 year, 7 months, weighing 12.6 kg, was presented to a veterinary teaching hospital for evaluation of unexplained severe exercise intolerance and polycythemia (packed cell volume [PCV] 72%, reference interval 38%–48%). On physical examination, no heart murmur was detectable, and rhythm was regular. The dog could walk no more than a few steps without becoming breathless and sitting down. Therapeutic phlebotomy was performed, followed by intravenous crystalloid fluid administration.
Echocardiography was performed with the patient unsedated, using a Vivid e95 r2 and 6S phased-array probe (GE Systems, Hatfield, United Kingdom). The dog was somewhat anxious during restraint, so image quality was overall suboptimal. Studies showed a subjectively large left heart with a small right side (Figure 1A). The tricuspid and pulmonic valves were difficult to image clearly but appeared reduced in size, in line with the reduced size of the right heart (Figures 1B and 1G). The right atrium was identified as a discrete chamber. No two-dimensional or color Doppler evidence of tricuspid or pulmonic valve stenosis was identified, but midventricular obstruction was present in the right ventricle, with aliasing of color flow mapping and a pressure gradient approximating 25 mm Hg (Figures 1C and 1D). Doppler interrogation of pulmonary artery flow was not possible. A vascular structure was imaged between the left and right atria, with flow apparent into the left atrium (Figures 1E and 1F).
Figure 1.
Echocardiographic images illustrating abnormalities detected. The right heart appeared hypoplastic from a right parasternal long-axis view and was difficult to image clearly (A). A hypoplastic pulmonary artery was visible in short-axis views (B). Midventricular obstruction of the right ventricle was apparent in short axis, evident on color flow mapping (C) and continuous-wave Doppler (D). A vascular structure was present at the heart base, between the left and right atrium, with an obvious connection to the left atrium (E, F). Left apical oblique views identified that the right atrium was small but contained a tricuspid valve, and a distinct partition appeared to be present between the vascular structure and the right atrium in these views (G, arrows). # denotes cranial vena cava. Ao, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium.
The left heart was dominant, and chamber dimensions were compared with 95% reference intervals derived from a wide range of dog breeds.1 Left ventricular internal diameter in diastole normalized for body weight was enlarged at 1.62 (reference interval 1.15–1.55),1 and maximum left atrial diameter normalized for body weight was within the reference interval (1.19–1.56) at 1.46.1
Contrast studies performed using agitated saline were undertaken from both the thoracic limb (injection in the right cephalic vein) and the pelvic limb (injection in the left lateral saphenous vein). In studies of both the cranial (i.e., superior) and caudal (i.e., inferior) venae cavae, saline contrast was seen to enter the left atrium densely, followed by a lower density entering the right atrium. This established an abnormal venoatrial connection between the systemic veins and the left atrium, both cranial (superior) and caudal (inferior) to the heart, excluding the possibility of the vascular structure being part of a divided right atrium (cor triatriatum dexter).
Computed tomographic (CT) imaging was performed to ascertain any additional anomalies of thoracic or abdominal anatomy, using a Siemens Somatom eMotion 16-slice scanner (Siemens Healthcare, Camberley, United Kingdom). A contrast study from a thoracic limb injection identified venous flow into the right heart and pulmonary circulation, which again appeared to be of very small volume compared with the left heart (Figure 2A). The connection between the left atrium and venae cavae was apparent as before. Several tortuous aorticopulmonary collateral vessels were identified alongside the midthoracic esophagus (Figures 2B and 2C). The azygous vein was considered to be of normal size and drained into the cranial (superior) vena cava. Abdominal organ anatomy was normal, excluding the presence of heterotaxy disorders.
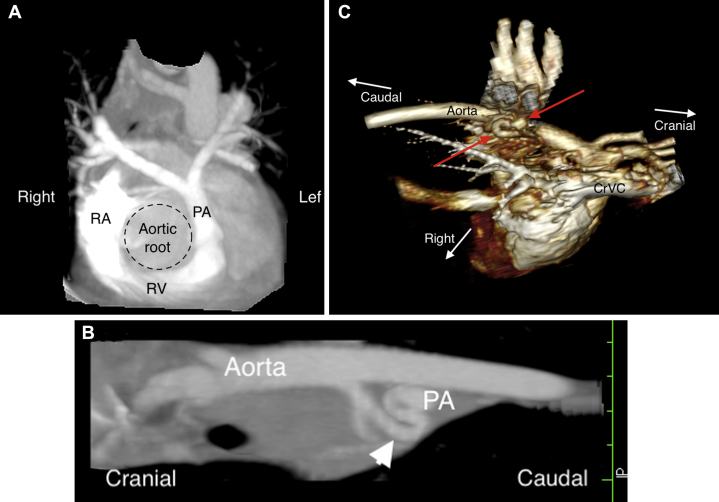
Figure 2.
CT images from the case, displayed using multiplanar reconstructions (MPR) and three-dimensional volume-rendering algorithms. The right heart was confirmed to be significantly smaller than the left heart, with a hypoplastic main pulmonary artery compared with the size of the aortic root (A). Aorticopulmonary collateral vessels were present between the descending aorta and main pulmonary artery, seen clearly in both an oblique MPR (B, arrowhead) and volume-rendered (C, arrows) images. CrVC, Cranial vena cava; PA, pulmonary artery.
Cardiac magnetic resonance (CMR) imaging, using a Philips D-sterm Ingenia 1.5-T system and pediatric receiver coil (Philips, Guilford, United Kingdom), confirmed drainage of the cranial vena cava into the left atrium and caudal vena cava into both atria via an ASD, presumed to be a sinus venosus–type ASD (Figures 3A–3C). All pulmonary veins drained into the morphologic left atrium, but the right cranial pulmonary vein shared a common orifice with the cranial vena cava (Figure 3D). The right heart and pulmonary artery appeared small. Phase-contrast two-dimensional fast-field-echo velocity-encoded flow studies were used to quantify the shunt severity. Stroke volume through the ascending aorta was 24.4 mL and through the main pulmonary artery was 4. 3 mL, a Qp/Qs ratio of 0.18 (normal, 1:1).
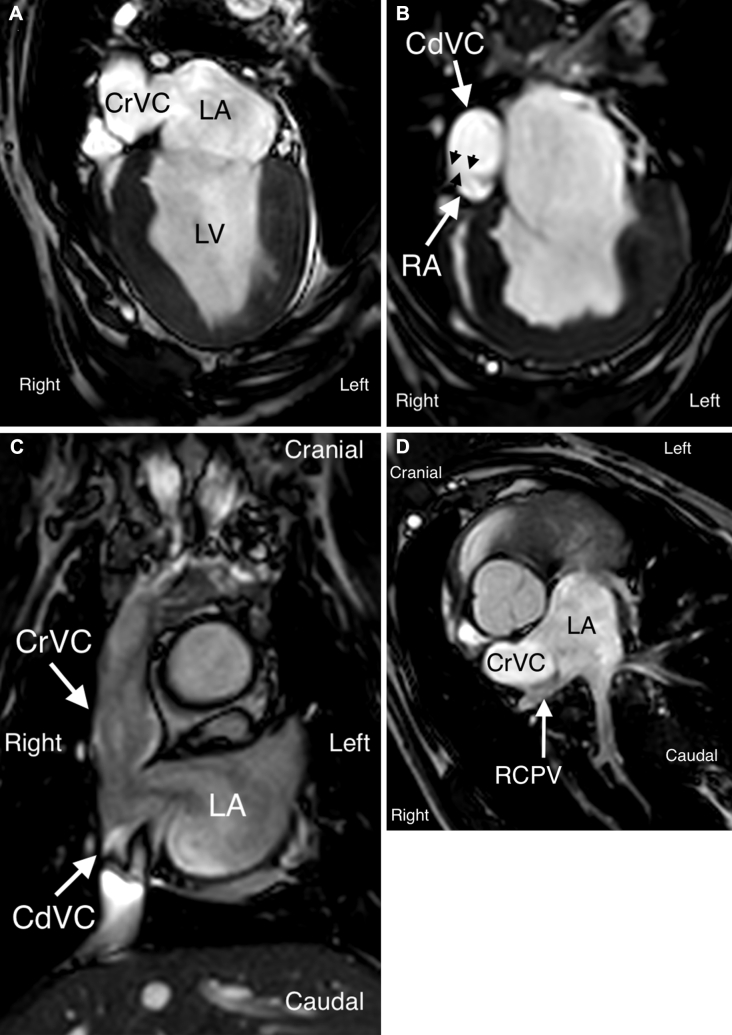
Figure 3.
CMR images confirming abnormal venoatrial connections. A four-chamber view identified communication between the cranial vena cava and left atrium through a large orifice (A). The caudal vena cava was identified to communicate with both the right atrium in a more caudal four-chamber view (B; black arrowheads) and the left atrium through a common orifice with the cranial vena cava in a coronal projection of the heart base (C). The right cranial pulmonary vein entered the left atrium through a common orifice with cranial vena cava, best seen in a tangential projection through the shunt (D). CdVC, Caudal vena cava; CrVC, cranial vena cava; LA, left atrium; LV, left ventricle; RA, right atrium; RCPV, right cranial pulmonary vein.
Repeat phlebotomy was required within 1 month to control clinical signs attributable to recurrence of polycythemia (PCV 73%). Palliative surgery was elected to reduce the severity of systemic hypoxemia. A modified Blalock-Thomas-Taussig shunt procedure was performed through a left intercostal thoracotomy: in short, a 6-mm Gore-Tex stretch vascular graft (Gore & Associates, Dundee, United Kingdom) was used to connect the left subclavian artery to the main pulmonary artery through a side-to-side anastomosis. The graft was sutured with 6-0 polypropylene (Ethicon Endo-Surgery, Somerville, NJ). The dog recovered uneventfully and was discharged 5 days postoperatively, receiving oral antiplatelet drugs to reduce the risk for conduit thrombosis (clopidogrel 37.5 mg/day, aspirin 6.3 mg/day). At reexamination 4 weeks later, exercise tolerance had vastly increased, up to 10 min uninterrupted and unrestricted exercise, and PCV had reduced to 44%. Echocardiography revealed patency of the conduit at 4 and 16 weeks after surgery (Figure 4), and 12 months later the dog remained well and free of clinical signs, with conduit patency confirmed echocardiographically and PCV measured at 48%.
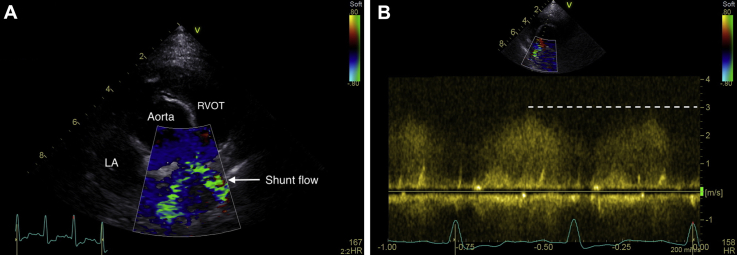
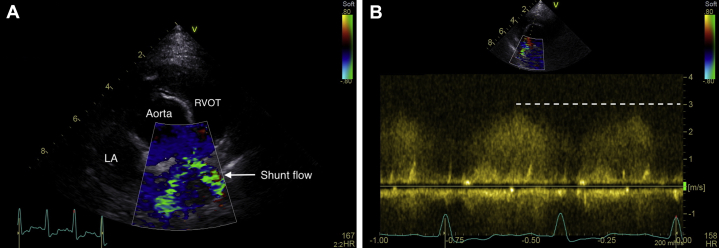
Figure 4.
Echocardiography performed at 4 weeks after Blalock-Thomas-Taussig shunt procedure. Shunt flow is visible in a right parasternal short-axis view (A), in which systolic flow in the pulmonary artery is coded as blue and aliasing flow via the shunt can be identified as a green mosaic pattern through the shunt, from the left subclavian artery to the pulmonary artery in systole; continuous-wave Doppler (B) identified continuous flow with a maximum pressure gradient of 36 mm Hg.
Discussion
To our knowledge, this is the first reported case of left atrial drainage of both venae cavae in a domestic dog. Left atrial drainage of a persistent left cranial vena cava has been previously reported, also in a French bulldog,2 but that case differed from the present case in a number of ways. In the previously reported case, blood from the caudal vena cava drained as expected into the right atrium. Second, the cranial vena cava was left sided, whereas in the present case both cranial and caudal cavae were right sided. In addition, the drainage of a persistent left cranial cava into left atrium is most likely to be caused by an unroofed coronary sinus (type I) in the previous case.3,4 In the present case, in which both cranial and caudal venae cavae were right sided and drained into the left atrium, we presume that the mechanism of embryogenesis was related to a sinus venosus defect. Similar cases are occasionally reported in humans,5 and primary surgical repair is the treatment of choice.
Complex congenital heart diseases in dogs are infrequently diagnosed, perhaps because veterinary examinations are often delayed until the age of first vaccination (around 6–12 weeks). Neonatal puppy death is often attributed by laypersons (owners or breeders) to pneumonia or a viral infection (so-called fading puppy syndrome).6 The proportion of neonatal canine fatalities that are due to complex congenital heart disease is unknown. In the case reported here, we presume that the dog survived into early adulthood before clinical signs of severe hypoxemia were detected because of sufficient pulmonary flow through the small connection between the caudal vena cava and right atrium through a presumed sinus venosus ASD, in addition to pulmonary artery flow through aorticopulmonary collateral vessels.
The embryologic origin of vena cava to left atrial connections is complex. In patients with upper sinus venosus defects, the wall between the superior (cranial in the dog) vena cava and the left upper (cranial) pulmonary vein may be lacking, as shown on CMR in this case, and the pulmonary vein orifice becomes an interatrial communication. Under particular flow circumstances in utero, depending on the difference in atrial compliance and pressure, the cranial vena caval–to–left atrial shunt may predominate, and the orifice with the right atrium may become reduced or atretic, not favouring underdevelopment of the right heart,7 as in this case. Connection of the inferior (caudal) vena cava to the left atrium is more difficult to understand. The right horn of the sinus venosus cannot be incorporated fully into the true left atrium, and it is likely that many cases of previously suspected caudal caval drainage into the left atrium in fact represent a left-sided morphologic right atrium.8 Here, the dog appeared to have a morphologic, left-sided left atrium.9 Rather than a direct connection between caudal vena cava and left atrium, the flow presumably was into both atria through a large sinus venosus defect, but left atrial flow more florid. We could not confidently identify a sinus venosus ASD on echocardiography in this case, because of a relatively poor echocardiographic window and the dominance of the left atrium and vena cava, but we presume that this was the reason for right heart inflow from the caudal vena cava at the pretricuspid level. In rare human cases, drainage of the inferior vena cava into the left atrium can occur iatrogenically, after incorrect ASD closure technique.10
Although the right heart was markedly reduced in size compared with the left heart, there was no evidence of tricuspid or pulmonary valve anomalies on echocardiographic or CMR imaging, so we cannot conclusively make a diagnosis of hypoplastic right heart syndrome in this case.11 The moderate midventricular gradient in the right heart was presumably caused by an extremely underloaded chamber, and we suspect that it was not a primary component of pathology.
Treatment for children with left atrial drainage of either (or both) cavae consists of open heart surgery to restore normal flow dynamics (i.e., deoxygenated systemic venous blood returning through the venae cavae and draining into the pulmonary vasculature). This is achieved through a combination of resection and anastomosis of vessels or the use of conduits or baffles. A partial or temporary solution may be achieved using a Glenn procedure, in which anastomosis of the superior vena cava and pulmonary artery is performed, bypassing the right heart entirely.12 Neither of these options was selected in this dog. In part this was because of owner factors (financial limitations, aversion to risk) but also because of the lack of widespread veterinary experience with these procedures. The use of a modified Blalock-Thomas-Taussig shunt was selected as a fair option to help control clinical signs by allowing a proportion of desaturated arterial blood a second pass through the pulmonary capillaries.13 This was deemed realistic in terms of cost and risk and proved to be successful, at least to date (14 months after surgery), in the management of this case to improve quality of life and abolish clinical signs.
It is worth noting several limitations of this case, based on our reflections. First, transesophageal echocardiography was not performed but may have helped in confirmation of the classification of ASD in this case (presumed to be sinus venosus on the basis of embryologic knowledge of the venous drainage anomalies detected). Second, cardiac catheterization and angiographic studies may have been helpful to evaluate hemodynamics further. If postmortem examination had been available in this patient, it would have provided confirmation of the anatomic abnormalities and further insights into embryologic origin.
In this case, we were not confident to make the diagnosis using echocardiography alone, because of technical imaging limitations due to patient intolerance of the procedure. Multimodal imaging was required because of the highly unusual nature of the malformation and the lack of published reference reports in veterinary patients. The use of CT and CMR imaging allowed us not only to confirm the diagnosis but to exclude concurrent anatomic pathology, which may have limited treatment options. We did not have access to electrocardiographic gating on CT imaging, limiting the utility of this modality alone for our clinic; it is likely that electrocardiographically gated CT imaging would have provided the diagnosis without the need for CMR. However, CMR made it possible to accurately quantify the shunt, which would not have been possible using CT imaging. With details of the imaging in this case now available, it is possible that clinicians may be able to be more confident in a diagnosis based on echocardiography alone, but CMR offers the advantage of allowing complete assessment of pulmonary venous anatomy and accurate quantification of shunt ratio to assess disease severity and act as a baseline for monitoring and surgical planning.
Conclusion
Left atrial drainage of the cranial and caudal venae cavae is a very rare congenital heart disease but may occur in dogs presenting with unexplained polycythemia. Diagnosis may be suspected on the basis of echocardiographic imaging, but CMR is useful to identify pulmonary venous anatomy and quantify shunt severity. If primary surgical repair is not considered a realistic option, the modified Blalock-Thomas-Taussig procedure offers hope for reducing clinical signs and improving quality of life in dogs.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
References
- 1.Visser L.C., Ciccozzi M.M., Sintov D.J., Sharp A.N. Echocardiographic quantification of left heart size and function in 122 dogs: a prospective study proposing reference intervals and assessing repeatability. J Vet Intern Med. 2019;33:1909–1920. doi: 10.1111/jvim.15562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zani A., Becchetti E., Leonardi P., Sinatra A. Persistent left atrial vena cava draining into the left atrium associated with pulmonary stenosis in a French Bulldog. J Vet Cardiol. 2014;16:121–125. doi: 10.1016/j.jvc.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Ootaki Y., Yamaguchi M., Yoshimura N., Oka S., Yoshida M., Hasegawa T. Unroofed coronary sinus syndrome: diagnosis, classification and surgical treatment. J Thorac Cardiovasc Surg. 2003;126:1655–1656. doi: 10.1016/s0022-5223(03)01019-5. [DOI] [PubMed] [Google Scholar]
- 4.Kong P.K., Ahmad F. Unroofed coronary sinus and persistent superior vena cava. Eur Heart J Cardiovasc Imaging. 2007;8:398–401. doi: 10.1016/j.euje.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Hulten E.A., Pinto G., Weissman G., Fuisz A. Anomalous vena caval return to the left atrium. Circulation. 2012;125:e525–e528. doi: 10.1161/CIRCULATIONAHA.111.019521. [DOI] [PubMed] [Google Scholar]
- 6.Casal M. Pediatric care during the postpartum period. In: Ettinger S.J., Feldman E.C., Cote E., editors. Textbook of veterinary internal medicine. 8th ed. Elsevier Saunders; Philadelphia: 2016. pp. 4597–4601. [Google Scholar]
- 7.Shapiro E.P., Al-Sadir J., Campbell N.P., Thilenius O.G., Anagnostopoulos C.E., Hays P. Drainage of the right superior vena cava into both atria. Circulation. 1981;63:712–717. doi: 10.1161/01.cir.63.3.712. [DOI] [PubMed] [Google Scholar]
- 8.Geva T. Abnormal systemic venous connections. In: Allan H.D., Shaddy R.E., Penny D.J., Feltes T.F., Cetta F., editors. Moss and Adams' heart disease in infants, children and adolescents. 9th ed. Wolters Kluwer; London: 2016. pp. 911–933. [Google Scholar]
- 9.Anderson R.H., Becker A.E., Freedom R.M., Macartney F.J., Quero-Jiminez M., Shinebourne E.A. Sequential segmental analysis of congenital heart disease. Pediatr Cardiol. 1984;5:281–288. doi: 10.1007/BF02424973. [DOI] [PubMed] [Google Scholar]
- 10.Beitzke D., Koestenberger M., Knez I., Beitzke A. Anomalous connection of the inferior vena cava to the left atrium: a surgical error in closing an atrial septal defect. Clin Res Cardiol. 2008;97:191–193. doi: 10.1007/s00392-007-0628-3. [DOI] [PubMed] [Google Scholar]
- 11.Prasad K., Singh M., Radhakrishnan S. Hypoplastic right ventricle with mild pulmonary stenosis in an adult. Int J Cardiol. 1992;37:260–262. doi: 10.1016/0167-5273(92)90219-s. [DOI] [PubMed] [Google Scholar]
- 12.Pridjian A.K., Mendelsohn A.M., Lupinetti F.M., Beekman R.H., Dick M., Serwer G. Usefulness of the bidirectional Glenn procedure as a staged reconstruction for the functional single ventricle. Am J Cardiol. 1993;71:959–962. doi: 10.1016/0002-9149(93)90914-x. [DOI] [PubMed] [Google Scholar]
- 13.Ilbawi M.N., Grieco J., DeLeon S.Y., Idriss F.S., Muster A.J., Berry T.E. Modified Blalock-Taussig shunt in new-born infants. J Thorac Cardiovasc Surg. 1984;88:770–775. [PubMed] [Google Scholar]