Graphical abstract

Keywords: Transesophageal echocardiography, Three-dimensional echocardiography, Percutaneous valve repair, Tricuspid regurgitation, Mitral regurgitation
Highlights
-
•
TEE is critical in evaluation and guidance of mitral and tricuspid interventions.
-
•
3D MPR allows simultaneous valve views in 2 long-axis, 1 short-axis, and 3D planes.
-
•
3D MPR enables accurate spatial delineation of MR/TR location and clip positioning.
-
•
Adequate mitral and tricuspid views are achievable in most patients with 3D MPR.
Introduction
Severe tricuspid regurgitation (TR) often occurs as a result of or concurrently with left heart disease, and concomitant intervention is recommended.1,2 Both conservative and surgical management approaches to severe TR continue to have high mortality rates.3,4 Transcatheter tricuspid intervention has therefore become an attractive option of growing popularity. The heterogenous anatomy of the tricuspid valve, difficulties in imaging, current relative lack of procedural experience, and use of technology (such as the off-label use of the MitraClip device for TR) not originally designed for the tricuspid valve have brought about challenges to this procedure. Consequently, lower success rates have been reported by the international TriValve registry compared with other valvular interventions.5 We present a successful case of percutaneous mitral and tricuspid valve repairs using the MitraClip and highlight the importance of guidance using three-dimensional (3D) multiplanar reconstruction (MPR) transesophageal echocardiography (TEE).
Case Presentation
An 82-year-old man with known mitral regurgitation (MR) under surveillance presented to the cardiology clinic with 3 months of progressive dyspnea on minimal exertion, orthopnea, and fatigue. His background also included atrial fibrillation, hypertension, diabetes, dyslipidemia, hypothyroidism, obstructive sleep apnea, chronic kidney disease, obesity, and ataxia. Transthoracic echocardiography at an outside center showed severe MR and TR, normal left and right ventricular systolic function, biatrial dilation, and significant pulmonary hypertension (estimated systolic pressure 75 mm Hg). A nuclear stress test did not demonstrate resting or inducible ischemia. Following multidisciplinary heart team review, the patient was thought to be a high-risk candidate for open heart surgery and therefore was referred to us for percutaneous mitral valve repair.
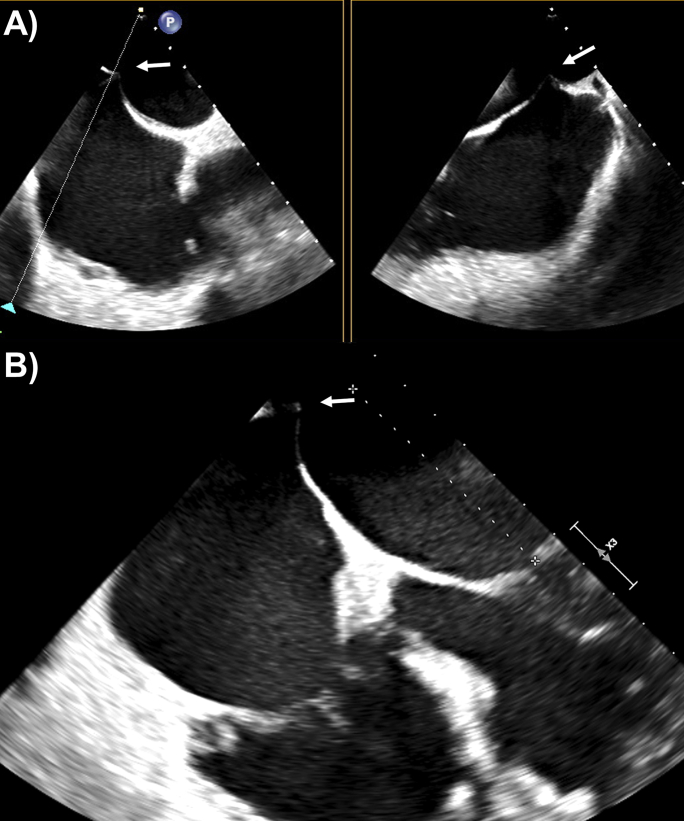
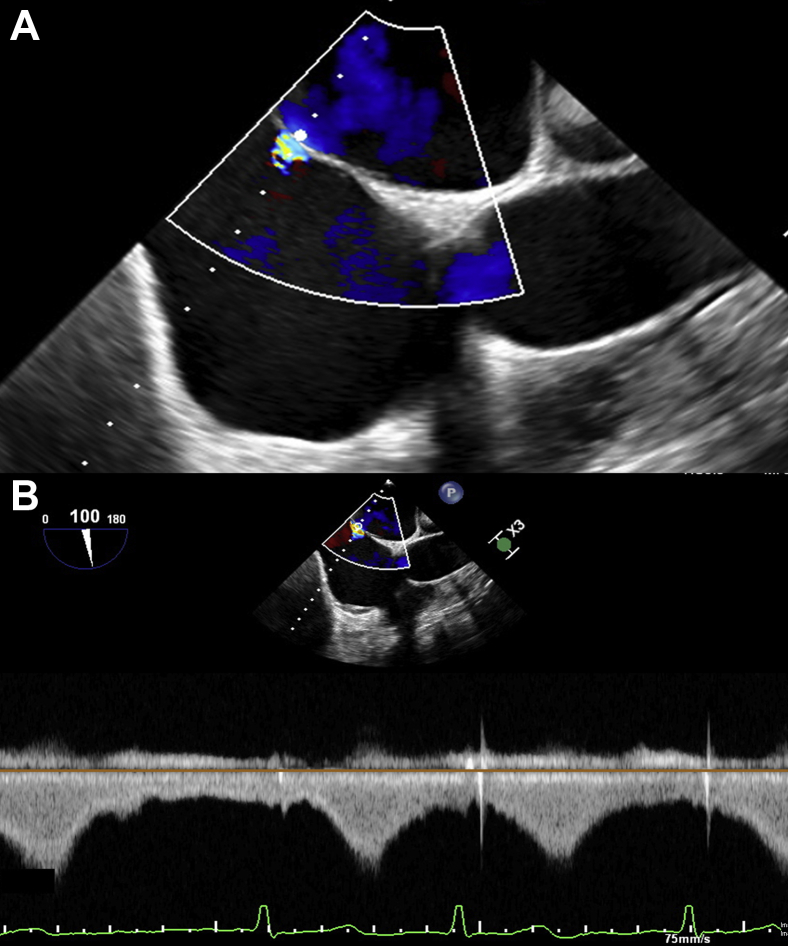
In the hybrid operating theater, preprocedural TEE performed using the Philips Epic machine and X8-2t probe (Philips Medical Systems, Andover, MA) confirmed degenerative mitral valve P2 prolapse with severe MR and a mean gradient of 2 mm Hg (Figures 1A and 1B). Additionally, there was severe functional TR between the septal and predominantly anterior leaflets and posterior leaflet from annular dilation, with a mean gradient of 1 mm Hg (Figures 2A and 2B). There was a normal left ventricular ejection fraction of 60%. Right ventricular size and systolic function were also normal. The decision was made to percutaneously repair both the mitral and tricuspid valves.
Figure 1.
Preprocedural 3D TEE, mitral valve en face view from the left atrium, with severe MR (A) without and (B) with color Doppler illustrating P2 prolapse.
Figure 2.
Preprocedural 3D TEE of the tricuspid valve from the right atrium with severe TR (A) without and (B) with color Doppler. AL, Anterior leaflet; P2, posterior second scallop of mitral valve; PL, posterior leaflet; SL, septal leaflet.
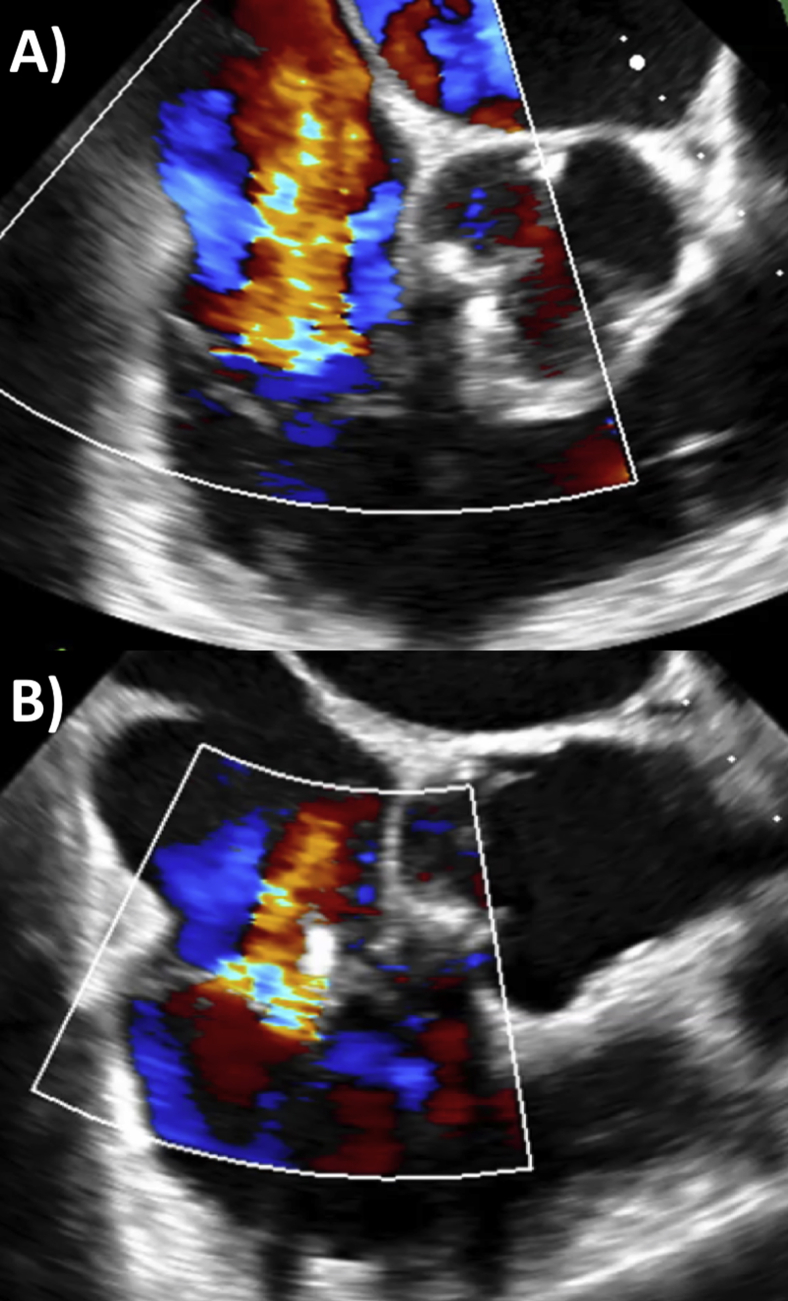
The MR was tackled first. Transseptal puncture was performed using a Brockenbrough needle under fluoroscopy with guidance on TEE from the bicaval view. Using biplane imaging, the interatrial septum was crossed posteriorly to gain adequate distance from the mitral valve (measured at 5.8 cm). This location allowed enough space for the device catheter to direct toward the mitral valve (Figures 3A and 3B). With the use of live Philips 3D MPR (MultiVue) software and a midesophageal long-axis view as the primary view (120°–140°), a MitraClip XTR device was steered to the left ventricular side of the mitral valve, orthogonal to leaflet coaptation of the P2 and A2 scallops. After deployment, there remained moderate residual MR medial to the clip and a mean transmitral gradient of 3 mm Hg (Figure 4A). A second MitraClip NTR device was similar deployed just medial to the first clip (Figure 4B, Video 1), resulting in only mild residual MR. Pulmonary vein flow went from systolic reversal to normal flow (Figures 5A and 5B) and a mean transmitral gradient of 4 mm Hg. Blood pressure remained unchanged at about 100/60 mm Hg throughout the procedure, while the left atrial V wave halved from 50 to 25 mm Hg after the clips were placed. After removing the sheath to the left atrium, there was a small iatrogenic atrial septal defect, with unidirectional left-to-right shunting, which was left alone (Figures 6A and 6B).
Figure 3.
Transseptal puncture under transesophageal echocardiographic guidance: (A) biplane imaging from bicaval view (midtransesophageal 100°) and (B) height of puncture from mitral valve 5.8 cm (midtransesophageal 10° view); white arrows indicate transseptal puncture site.
Figure 4.
Three-dimensional MPR TEE: (A) residual moderate MR after first clip and (B) imaging guidance of second clip; the midesophageal long-axis view is set at the primary image (top left), bicommissural view (top right), mitral valve short-axis view (bottom left), and 3D view from the left atrium (bottom right) in each panel. White arrows represent clips.
Figure 5.
Pulmonary venous flow (A) systolic reversal (white arrow) before mitral intervention and (B) normal flow after second MitraClip.
Figure 6.
Small iatrogenic atrial septal defect following transseptal puncture on bicaval view (midtransesophageal 100°; A) by color Doppler and (B) on continuous-wave Doppler showing unidirectional left-to-right shunting.
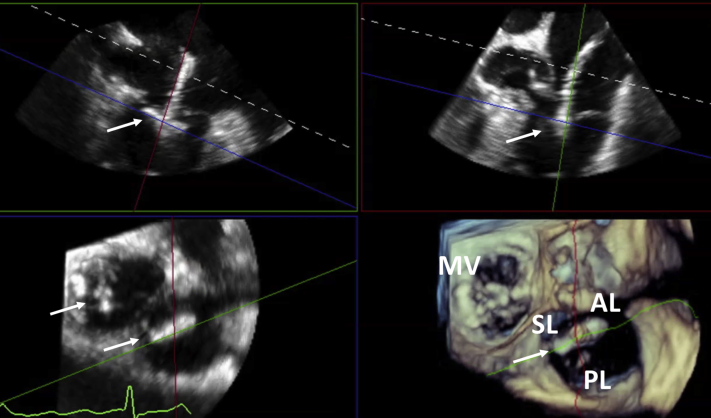
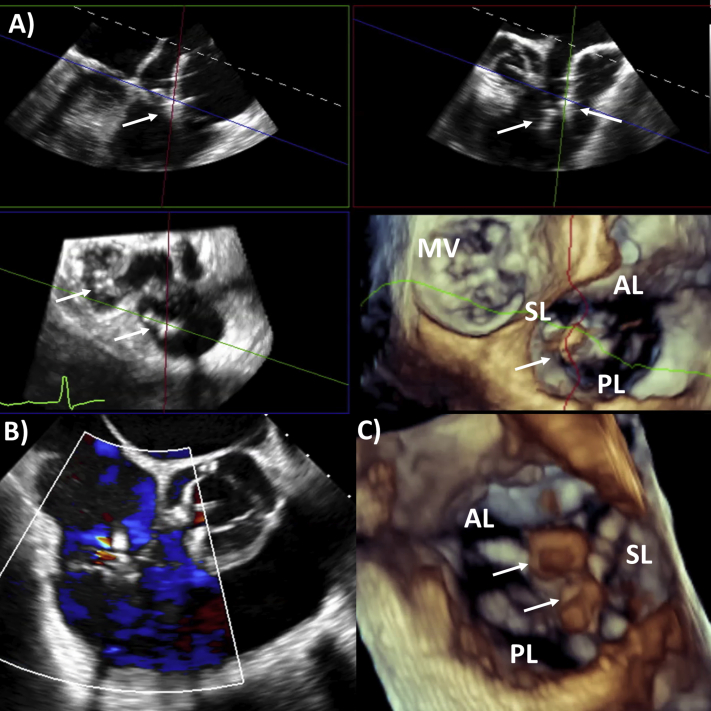
Attention was then turned to the TR. The 3D MPR was set using the midesophageal reverse four-chamber view as the primary view (170°–180°). A third MitraClip XTR device was delivered with live 3D MPR guidance, grasping the septal and anterior tricuspid leaflets (Figure 7, Video 2). However, the TR only partially decreased from the original severe to moderate (Figures 8A and 8B). A fourth and final MitraClip XTR device was added between the septal and posterior tricuspid valve leaflets (Figure 9A), with resultant trivial TR remaining and an unchanged final mean transtricuspid gradient of 1 mm Hg (Figures 9B and 9C). Video 3 illustrates the final results with two MitraClips each in the mitral and tricuspid valves.
Figure 7.
Three-dimensional MPR guidance of percutaneous tricuspid repair: first clip between septal and anterior leaflet (reverse four-chamber view as primary image, top left; reverse right ventricular inflow-outflow view, top right; valvular short-axis view, bottom left; and 3D tricuspid valve view from right atrium, bottom right). AL, Anterior leaflet; MV, mitral valve; PL, posterior leaflet; SL, septal leaflet. White arrows represent clips.
Figure 8.
(A) Midtransesophageal 100° right ventricular inflow-outflow view showing severe TR before the procedure and (B) moderate TR after the first clip.
Figure 9.
(A) Three-dimensional MPR guidance of percutaneous tricuspid repair: second clip between septal and posterior leaflet (same orientations for the four images as in Figure 5A), (B) midtransesophageal 100° right ventricular inflow-outflow view showing trivial TR after the second clip, and (C) 3D image of the two tricuspid valve clips from the right ventricular side. AL, Anterior leaflet; MV, mitral valve; PL, posterior leaflet; SL, septal leaflet; TV, tricuspid valve. White arrows represent clips.
Postprocedural transthoracic echocardiography the following day continued to show trivial MR and TR (Figures 10A–10D), with mean gradients of 3 and 1 mm Hg, respectively, normal left ventricular (ejection fraction 55%) and right ventricular systolic function, and no complications. The patient's dyspnea was significantly alleviated, and orthopnea and fatigue had resolved by the 1-month follow-up appointment.
Figure 10.
Color Doppler on transthoracic echocardiography of MR (apical four-chamber view) before (A) and after (B) percutaneous repair and TR (reverse apical four-chamber view) before (C) and after (D) percutaneous repair. White arrows represent clips.
Discussion
Percutaneous valvular interventions have advanced significantly over the past decade, with emerging roles for mitral and tricuspid interventions in high-risk surgical candidates and secondary valve disease.6 Compared with aortic and mitral valve interventions, tricuspid valve procedures are in their infancy but rapidly growing. The success rate of tricuspid interventions is currently lower than that of mitral and aortic interventions. Tricuspid intervention needs to be improved through increased understanding and experience, multimodality imaging assessment, technological advances in devices, and identifying predictors of procedural failure and pitfalls to overcome.5, 6 The off-label use of the MitraClip is currently the most popular approach for treating TR percutaneously. Despite the challenges to date, the TriValve registry reports low mortality and significant clinical improvement for tricuspid valve interventions to date over 18 months, and compared with matched control subjects who are medically managed, tricuspid intervention was associated with increased survival and decreased heart failure rehospitalizations.7 The TRILUMINATE single-arm prospective international trial has recently reported initial favorable efficacy and safety outcomes till 6 months.8 This shows great promise for the use of the transcatheter tricuspid valve TriClip system reduce TR.
Evaluation of MR and TR severity, mechanism, and etiology should be routinely performed using TEE and contributes to decision-making for valvular interventions.9 Unexpected mechanisms and etiologies may arise that influence the treatment strategy. It is also immensely valuable in the guidance of percutaneous mitral and tricuspid interventions.6,10,11 In brief, the transseptal puncture is performed using biplane imaging at the bicaval view to determine the superior-to-inferior and anterior-to-posterior axes. The idea is to position the catheter along the interatrial septum, with a posterior and superior puncture to obtain ≥4 cm height from the mitral annulus.10 The sheath with the MitraClip device is visualized entering the left atrium and turning down toward the mitral valve. With real-time 3D guidance, the MitraClip orientation is adjusted, entering the left ventricle and maneuvered to grasp the leaflets at the location of MR. Residual MR is assessed using color Doppler, gradients, and pulmonary vein flow to determine if additional clips are necessary.
For tricuspid valve edge-to-edge repair, a recent comprehensive review suggested the following steps.11 Using the bicaval view on biplane imaging, the device is directed within the right atrium toward the tricuspid valve. The clip delivery system is then aligned using the midesophageal commissural view (60°–80°) with biplane imaging, as well as the transgastric short-axis view (10°–40°) views to visualize the location of TR and orientation of the device, followed by grasping of leaflets. Residual TR is assessed in a similar way to MR, mainly at the commissural view. Transthoracic echocardiography remains mandatory in the follow-up of these patients.
Our case demonstrates the added value of live 3D MPR MultiVue software, particularly for tricuspid valve procedural guidance. Use of 3D MPR allows simultaneous visualization of the clip with the valve leaflets in two midesophageal planes (such as an anterior-to-posterior view for grasping and a medial-to-lateral view for positioning along the mitral valve), as well as the short-axis view and 3D en face view, without and with color Doppler. Therefore, the need to change views and probe position (such as between midesophageal and transgastric) for complete assessment is avoided, although to improve resolution and frame rates, a smaller area of interrogation for 3D imaging can be subsequently performed. The errors of parallax associated with 3D echocardiography can be minimized with visualization of the valve and device concurrently with two-dimensional and 3D images in 3D MPR. Use of MPR allows accurate assessment of imaging planes crossing the 3D en face image so that correct location can be identified.
After the elimination of parallax, accurate measurements can be obtained from the two-dimensional images in MPR. Measurements taken from 3D en face views alone are prone to error.12 The short-axis views can be adjusted to visualize the valve from either the atria or the ventricle. We prefer using the midtransesophageal long-axis and reverse four-chamber views as the starting point to set the 3D MPR imaging for mitral and tricuspid valve interventions, respectively. However, in theory any standard mitral or tricuspid valve views can depict the same information accurately in 3D MPR. The imaging planes can be adjusted to follow where the device is being directed and positioned, accurately delineate the spatial location where valvular regurgitation is greatest, guiding the device to that position with optimal orientation relative to the valve. In addition, live 3D MPR is valuable when more than one clip is used, allowing ease of differentiation and orientation between the previously placed and the clip about to be placed. Adequate and frequently high-quality views of the mitral and tricuspid valve to precisely guide intervention can be achieved in the vast majority of patients. We now use 3D MPR routinely in all percutaneous mitral and tricuspid valve repairs involving TEE.
Conclusion
TEE continues to play a central role in the assessment and guidance of MR and TR interventions. We recommend using live 3D MPR or equivalent technology for percutaneous valvular interventions because of the simultaneous visualization of device positioning, leaflet grasping, and short-axis views in two and three dimensions, allowing accurate spatial delineation of valve pathology and intervention. In our experience, adequate tricuspid valve views in addition to the mitral valve can be obtained in the vast majority of patients to guide percutaneous interventions with high precision.
Footnotes
Conflicts of interest: T.K.M.W. is supported by the National Heart Foundation of New Zealand Overseas Clinical and Research Fellowship Grant number 1775. The other authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.01.002.
Supplementary Data
Three-dimensional MPR views of mitral valve grasping by the second MitraClip placed; midesophageal long-axis view as primary view (top left), bicommissural view (top right), short-axis view (bottom left), and 3D en face view from left atrium (bottom right).
Three-dimensional MPR views of tricuspid valve and aligning the first clip along septal and anterior leaflet direction; midesophageal reverse four-chamber as primary view (top left), reverse right ventricular inflow-outflow view (top right), short-axis view (bottom left), and 3D view of tricuspid valve from right atrium (bottom right; gain turned down to visualize clip orientation rather than leaflets).
Final results on 3D view of the mitral (right) and tricuspid (left) valves with two clips each from the ventricular aspect.
References
- 1.Tam D.Y., Tran A., Mazine A., Tang G.H.L., Gaudino M.F.L., Calafiore A.M. Tricuspid valve intervention at the time of mitral valve surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. 2020 doi: 10.1093/icvts/ivz036. In press. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., III, Guyton R.A. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 3.Kadri A.N., Menon V., Sammour Y.M., Gajulapalli R.D., Meenakshisundaram C., Nusairat L. Outcomes of patients with severe tricuspid regurgitation and congestive heart failure. Heart. 2019;105:1813–1817. doi: 10.1136/heartjnl-2019-315004. [DOI] [PubMed] [Google Scholar]
- 4.Axtell A.L., Bhambhani V., Moonsamy P., Healy E.W., Picard M.H., Sundt T.M., III Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:715–725. doi: 10.1016/j.jacc.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taramasso M., Alessandrini H., Latib A., Asami M., Attinger-Toller A., Biasco L. Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the international TriValve registry. JACC Cardiovasc Interv. 2019;12:155–165. doi: 10.1016/j.jcin.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Taramasso M., Gavazzoni M., Pozzoli A., Dreyfus G.D., Bolling S.F., George I. Tricuspid regurgitation: predicting the need for intervention, procedural success, and recurrence of disease. JACC Cardiovasc Imaging. 2019;12:605–621. doi: 10.1016/j.jcmg.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Taramasso M., Benfari G., van der Bijl P., Alessandrini H., Attinger-Toller A., Biasco L. Transcatheter versus medical treatment of symptomatic severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:2998–3008. doi: 10.1016/j.jacc.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Nickenig G., Weber M., Lurz P., von Bardeleben R.S., Sitges M., Sorajja P. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–2011. doi: 10.1016/S0140-6736(19)32600-5. [DOI] [PubMed] [Google Scholar]
- 9.Zoghbi W.A., Adams D., Bonow R.O., Enriquez-Sarano M., Foster E., Grayburn P.A. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Katz W.E., Conrad Smith A.J., Crock F.W., Cavalcante J.L. Echocardiographic evaluation and guidance for MitraClip procedure. Cardiovasc Diagn Ther. 2017;7:616–632. doi: 10.21037/cdt.2017.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn R.T., Nabauer M., Zuber M., Nazif T.M., Hausleiter J., Taramasso M. Intraprocedural imaging of transcatheter tricuspid valve interventions. JACC Cardiovasc Imaging. 2019;12:532–553. doi: 10.1016/j.jcmg.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Drake D.H., Zimmerman K.G., Hepner A.M., Nichols C.D. Echo-guided mitral repair. Circ Cardiovasc Imaging. 2014;7:132–141. doi: 10.1161/CIRCIMAGING.112.000458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional MPR views of mitral valve grasping by the second MitraClip placed; midesophageal long-axis view as primary view (top left), bicommissural view (top right), short-axis view (bottom left), and 3D en face view from left atrium (bottom right).
Three-dimensional MPR views of tricuspid valve and aligning the first clip along septal and anterior leaflet direction; midesophageal reverse four-chamber as primary view (top left), reverse right ventricular inflow-outflow view (top right), short-axis view (bottom left), and 3D view of tricuspid valve from right atrium (bottom right; gain turned down to visualize clip orientation rather than leaflets).
Final results on 3D view of the mitral (right) and tricuspid (left) valves with two clips each from the ventricular aspect.