Abstract
Purpose: Our study aimed to investigate the structural valve deterioration (SVD) after transcatheter aortic valve implantation (TAVI) using J-Valve.
Methods: In all, 14 patients with aortic stenosis (AS) and 4 patients with pure aortic regurgitation (PAR) were available in the study. Four-year follow-up was performed in all patients, and the clinical data and echocardiographic findings were recorded and analyzed.
Results: All patients survived at the 4-year follow-up. There was no evidence of morphological SVD or prosthetic valve thrombosis in enrolled patients. None of the hemodynamic SVD occurred in patients with PAR. Mean gradients decreased from 61.93 ± 15.42 mm Hg (pre-TAVI) to 19.64 ± 9.16 mm Hg (discharge) in patients with AS (p <0.001); subsequently, a slight increase was observed in the mean trans-aortic gradient throughout follow-up (p = 0.967). Overall, in patients with AS, six individuals suffered moderate (3/14, 21.4%) or severe (3/14, 21.4%) hemodynamic SVD at 4-year follow-up.
Conclusions: The limited number of cases provides a preliminary indication of the long-term efficacy of TAVI using J-Valve in patients with PAR. In patients with AS, although the higher rate of SVD was observed, the overall transcatheter heart valve (THV) hemodynamics remained stable over time after prosthetic valve implantation and the long-term durability of J-Valve was convincing.
Keywords: transcatheter aortic valve implantation, structural valve deterioration, aortic stenosis, pure aortic regurgitation, bicuspid aortic valve
Introduction
Transcatheter aortic valve implantation (TAVI) is a common alternative to surgical aortic valve replacement for severe aortic stenosis (AS) in patients with high surgical risk1) and the procedure exhibited a convincing long-term outcome for these patients.2,3) However, pure aortic regurgitation (PAR) was once recognized as a relative contraindication for TAVI, and the inoperable patients with PAR who were recommended to accept medical management followed with high morbidity and unfavorable outcomes. With the development of transcatheter heart valve (THV) devices and the technique of TAVI procedure, the second-generation THV has revolutionized the interventional treatment for PAR.4,5)
There are multiple reports presenting the feasibility of transcatheter approach for the treatment of PAR,4,6–11) and second-generation devices associated with an excellent procedural success rate and favorable perioperative outcomes compared with first-generation THV. In second-generation devices, the advantage is more pronounced on the “on-label” THVs (J-Valve and JenaValve) than “off-label” THVs.12) The J-Valve (Jie Cheng Medical Technologies, Suzhou, China) is a self-expandable TAVI device featuring three U-shaped anatomically oriented “graspers” for the accurate positioning to the Valsalva sinus.6) In previous studies, we have described J-Valve performed satisfactory early outcomes in high-risk surgical patients with AS or PAR.7) However, it was not reported that the long-term validity of the THV over time.
In this study, we evaluated the structural valve deterioration (SVD) of J-Valve through the periodically continuous long-term follow-up, which aimed to assess the long-term durability of J-Valve.
Materials and Methods
Study design and participants
This study was approved by the local ethical committee and all patients signed informed consent before enrollment. This single-center, prospective, randomized trial was conducted at Fuwai hospital (Beijing, China). The patients with severe aortic valve dysfunction were consecutively enrolled between July 2014 and June 2015, and the indication for TAVI, inclusion criteria, and other detail information were described in the methods of our previous study.7) In all, 18 patients, including 14 patients with AS and 4 with PAR, were performed echocardiographic examination during the periodic follow-up.
TAVI devices and procedures
The J-Valve system is a second-generation THV, which comprises a set of porcine aortic valve attached to a cylindrical nitinol stent featuring three U-shaped graspers encircled and attached with the THV stent. Before the THV deployment, the released graspers can anatomically be oriented to Valsalva sinus and serve as a land marker. The detail information of J-Valve system has been well presented in previous studies.6) All patients received transapical TAVI using the J-Valve system under fluoroscopic guidance and general anesthesia. The procedure of implantation has been described in detail previously.7,13,14)
Definitions and follow-up
Aortic valve was evaluated by two-dimensional echocardiography that performed by the same cardiologist to ensure comparability in terms of morphological structure, paravalvular leakage, trans-aortic mean gradient, effective orifice area, and intra-prosthetic aortic regurgitation (graded from 0 to 4, with higher grades indicating greater severity). Multi-detector computed tomography was performed for the patients suspected with valve thrombosis based on two-dimensional echocardiography. The hemodynamic SVD and morphological SVD were determined according to the standardized definitions proposed by EAPCI/ESC/EACTS.15) Morphological SVD was detected based on imaging examination, including the assessment of leaflet integrity, leaflet structure, leaflet function, and strut/frame. Hemodynamic SVD was classified as two degrees: (1) moderate SVD was defined as followings: (i) mean trans-aortic pressure gradient ≥20 and <40 mm Hg and/or (ii) ≥10 and <20 mm Hg change from discharge baseline, and/or (iii) moderate intra-prosthetic aortic regurgitation (new or worsening from discharge baseline, >1+/4+). (2) Severe SVD was defined as followings: (i) mean trans-aortic pressure gradient ≥40 mm Hg and/or (ii) ≥20 mm Hg change from discharge baseline, and/or (iii) severe new or worsening (>2+/4+) central aortic regurgitation.
Clinical status and echocardiographic follow-up data were collected at discharge, 1 month, 6 months, and 1, 2, 3, and 4 years. Data on adverse events, survival, and New York Heart Association (NYHA) class were obtained through periodically outpatient visits and telephone interviews.
Statistical analysis
The normality of continuous variables was evaluated by Shapiro–Wilk test. Based on data normality or not, the data were presented as the mean ± standard deviation or median (interquartile range). Categorical variables are presented as number (n) and percentage (%). Student’s t-test (paired or unpaired) was used for two-groups comparisons of values. One-way analysis of variance with LSD post hoc test was performed to compare the difference of mean aortic valve gradient and aortic valve orifice area among the time points reported. All analyses used SPSS software, version 25 (IBM Inc., Armonk, NY, USA).
Results
Four-year follow-up was available in all enrolled patients. During follow-up, no death reported in these patients. The baseline and procedural characteristics are shown in detail in Table 1. The mean age of the patients was 74.50 ± 5.22 years, and 72.2% (n = 13) were men. In these patients, 11 individuals had tricuspid aortic valve, while the remaining 7 had bicuspid aortic valve. Among them, the majority of the individuals (88.9%) had NYHA functional class III/IV preoperatively. One patient underwent prior coronary artery bypass grafting surgery.
Table 1. Baseline and procedural characteristics.
| Characteristics | AS (n = 14) | PAR (n = 4) | Total (n = 18) |
|---|---|---|---|
| Demographics | |||
| Male sex, n (%) | 10 (71.4) | 3 (75.0) | 13 (72.2) |
| Age, year | 74.07 ± 4.87 | 76.00 ± 6.88 | 74.50 ± 5.22 |
| Height, cm | 160.29 ± 7.30 | 159.25 ± 8.69 | 160.06 ± 7.37 |
| Weight, kg | 64.58 ± 14.91 | 63.25 ± 4.86 | 64.28 ± 13.21 |
| Aortic valve phenotypes, n (%) | |||
| BAV | 7 (50.0) | 0 (0) | 7 (38.9) |
| TAV | 7 (50.0) | 4 (100.0) | 11 (61.1) |
| Medical history, n (%) | |||
| Prior heart surgery | 1 (7.1) | 0 (0) | 1 (5.6) |
| Prior stroke | 6 (42.9) | 1 (25.0) | 7 (38.9) |
| Cardiovascular risk factors, n (%) | |||
| Hypertension | 11 (78.6) | 4 (100.0) | 15 (83.3) |
| Diabetes mellitus | 4 (28.6) | 1 (25.0) | 5 (27.8) |
| Atrial fibrillation | 1 (7.1) | 1 (25.0) | 2 (11.1) |
| Coronary artery disease | 8 (57.1) | 1 (25.0) | 9 (50.0) |
| Risk scores (median; IQR) | |||
| EuroSCORE II | 11.00 (10.00–11.25) | 12.00 (11.25–12.00) | 11.00 (10.75–12.00) |
| Logistic EuroSCORE II | 28.17 (23.92–31.73) | 31.73 (28.10–35.55) | 28.70 (24.77–32.32) |
| Echo parameters | |||
| Peak velocity, m/s | 4.93 ± 0.68 | 1.98 ± 0.21 | 4.28 ± 1.40 |
| Peak pressure gradient, mm Hg | 99.01 ± 27.81 | 15.70 ± 3.28 | 80.50 ± 43.17 |
| Mean pressure gradient, mm Hg | 61.93 ± 15.42 | 5.50 ± 1.29 | 49.39 ± 27.65 |
| Effective orifice area, cm2 | 0.63 ± 0.17 | 2.53 ± 0.22 | 1.05 ± 0.83 |
| Aortic annular diameter, mm | 21.00 ± 1.92 | 24.25 ± 0.96 | 21.72 ± 2.22 |
| LVDd, mm | 50.43 ± 8.36 | 61.50 ± 2.08 | 52.89 ± 8.75 |
| LVEF, % | 62.64 ± 13.73 | 64.10 ± 4.43 | 62.97 ± 12.16 |
| Functional status, n (%) | |||
| NYHA functional class III/IV | 13 (92.9) | 3 (75.0) | 16 (88.9) |
| Procedural characteristics, n (%) | |||
| Valve size | |||
| 21 mm | 5 (35.7) | 0 (0) | 5 (27.8) |
| 23 mm | 6 (42.9) | 0 (0) | 6 (33.3) |
| 25 mm | 2 (14.3) | 1 (25.0) | 3 (16.7) |
| 27 mm | 1 (7.1) | 3 (75.0) | 4 (22.2) |
| Device embolization | 1 (7.1) | 0 (0) | 1 (5.6) |
| Perivalvular leakage | |||
| None/trivial | 6 (42.9) | 4 (100.0) | 10 (55.6) |
| Mild | 8 (57.1) | 0 (0) | 8 (44.4) |
| Moderate and severe | 0 (0) | 0 (0) | 0 (0) |
Based on data normality, continuous variables were reported as mean ± standard deviation or median (interquartile range); categorical variables were presented as number (n) and percentage (%).
BAV: bicuspid aortic valve; EuroSCORE: European system for cardiac operative risk evaluation; LVDd: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; TAV: tricuspid aortic valve
Preoperative echocardiographic assessment
The mean preoperative left ventricular ejection fractions of the patients with AS and PAR were 62.64% ± 13.73% and 64.10% ± 4.43% (p = 0.840), respectively. The mean left ventricular end-diastolic dimension was 50.43 ± 8.36 mm and 61.50 ± 2.08 mm (p <0.001), respectively. Patients with AS had a mean aortic gradient of 61.93 ± 15.42 mm Hg and a mean effective orifice area of 0.63 ± 0.17 cm2. Patients with PAR had a mean effective orifice area of 2.53 ± 0.22 cm2. The mean aortic annulus diameter of patients with AS and PAR was 21.00 ± 1.92 mm and 24.25 ± 0.96 mm (p = 0.005), respectively.
Clinical follow-up outcomes
Significant clinical improvement was observed in the majority of enrolled patients. At 4-year follow-up, 77.8% of patients were in NYHA functional class I/II, and the left ventricular end-diastolic dimension had a decrease in patients with AS (46.14 ± 7.53 mm, p = 0.066) and PAR (43.75 ± 8.73 mm, p = 0.029) when compared to preoperation, respectively. At the end of the 4-year follow-up, four patients reported major adverse cardiovascular events, of which two suffered stroke that required hospitalization, one reported myocardial infarction, and one required hospitalization due to paroxysmal atrial fibrillation that was converted to sinus rhythm by drug therapy. The clinical results and echocardiographic findings are presented in Tables 2 and 3.
Table 2. Major adverse cardiovascular events and periodic echocardiography results in patients with aortic stenosis.
| Characteristics | Preoperation | Discharge | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| 1 month | 6 months | 1 year | 2 years | 3 years | 4 years | |||
| Peak velocity, m/s | 4.93 ± 0.68 | 2.90 ± 0.69 | 2.78 ± 0.74 | 2.76 ± 0.65 | 2.70 ± 0.85 | 2.77 ± 0.76 | 2.70 ± 0.76 | 2.88 ± 0.91 |
| Peak pressure gradient, mm Hg | 99.01 ± 27.81 | 35.45 ± 16.50 | 33.02 ± 16.15 | 32.06 ± 14.36 | 34.79 ± 15.92 | 32.73 ± 16.66 | 28.50 ± 17.07 | 36.31 ± 21.94 |
| Mean pressure gradient, mm Hg | 61.93 ± 15.42 | 19.64 ± 9.16 | 18.43 ± 8.74 | 18.50 ± 8.80 | 20.29 ± 8.51 | 18.71 ± 8.52 | 18.71 ± 9.68 | 21.71 ± 12.66 |
| Effective orifice area, cm2 | 0.63 ± 0.17 | 1.39 ± 0.22 | 1.50 ± 0.36 | 1.48 ± 0.33 | 1.47 ± 0.34 | 1.52 ± 0.40 | 1.52 ± 0.36 | 1.39 ± 0.35 |
| Perivalvular leakage n (%) | ||||||||
| None/trivial | – | 9 (64.3) | 9 (64.3) | 9 (64.3) | 9 (64.3) | 9 (64.3) | 9 (64.3) | 9 (64.3) |
| Mild | – | 5 (35.7) | 5 (35.7) | 5 (35.7) | 5 (35.7) | 3 (21.4) | 3 (21.4) | 3 (21.4) |
| Moderate/severe | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 2 (14.3) | 2 (14.3) |
| Intra-prosthetic AR n (%) | ||||||||
| None/trivial | – | 14 (100.0) | 14 (100.0) | 14 (100.0) | 13 (92.9) | 13 (92.9) | 13 (92.9) | 13 (92.9) |
| Mild | – | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) |
| Moderate and severe | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (7.1) | 1 (7.1) |
| LVDd, mm | 50.43 ± 8.36 | 48.50 ± 6.91 | 49.21 ± 6.75 | 47.93 ± 6.23 | 46.57 ± 5.56 | 46.50 ± 5.42 | 46.64 ± 7.44 | 46.14 ± 7.53 |
| LVEF, % | 62.64 ± 13.73 | 65.51 ± 9.16 | 62.36 ± 5.82 | 62.68 ± 7.24 | 65.26 ± 5.79 | 61.79 ± 9.71 | 60.86 ± 4.99 | 63.71 ± 4.68 |
| Major adverse cardiovascular events, n (%) | – | 0 | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) | 1 (7.1) |
Continuous variables were performed as mean ± standard deviation. Categorical variables were reported as number (percentage). The number of major adverse cardiovascular events was reported on the time point that the events occurred.
LVDd: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction
Table 3. Major adverse cardiovascular events and periodic echocardiography results in patients with pure aortic regurgitation.
| Characteristics | Preoperation | Discharge | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| 1 month | 6 months | 1 year | 2 years | 3 years | 4 years | |||
| Peak velocity, m/s | 1.98 ± 0.21 | 1.91 ± 0.40 | 1.53 ± 0.27 | 1.81 ± 0.25 | 1.76 ± 0.25 | 1.63 ± 0.19 | 1.70 ± 0.14 | 1.68 ± 0.15 |
| Peak pressure gradient, mm Hg | 15.70 ± 3.28 | 14.93 ± 6.40 | 9.60 ± 3.39 | 13.33 ± 3.62 | 12.55 ± 3.43 | 10.65 ± 2.56 | 11.60 ± 1.98 | 11.78 ± 2.01 |
| Mean pressure gradient, mm Hg | 5.50 ± 1.29 | 8.50 ± 3.32 | 5.75 ± 2.22 | 7.75 ± 1.50 | 7.00 ± 2.16 | 6.25 ± 1.89 | 6.00 ± 0.82 | 5.50 ± 0.58 |
| Effective orifice area, cm2 | 2.53 ± 0.22 | 2.33 ± 0.39 | 2.08 ± 0.17 | 2.05 ± 0.25 | 2.53 ± 0.37 | 2.67 ± 0.41 | 2.68 ± 0.69 | 2.55 ± 0.62 |
| Perivalvular leakage, n (%) | ||||||||
| None/trivial | – | 4 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) |
| Mild | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderate/severe | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intra-prosthetic AR, n (%) | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LVDd, mm | 61.50 ± 2.08 | 53.00 ± 3.74 | 53.50 ± 3.87 | 50.25 ± 5.25 | 45.00 ± 3.37 | 41.50 ± 4.12 | 44.00 ± 6.68 | 43.75 ± 8.73 |
| LVEF, % | 64.10 ± 4.43 | 59.73 ± 2.66 | 52.90 ± 3.33 | 59.70 ± 6.33 | 60.53 ± 2.67 | 60.75 ± 3.10 | 60.00 ± 4.69 | 61.00 ± 5.35 |
| Major adverse cardiovascular events, n (%) | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Continuous variables were performed as mean ± standard deviation. Categorical variables were reported as number (percentage). The number of major adverse cardiovascular events was reported on the time point that the events occurred.
LVDd: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction
Hemodynamic performance and durability
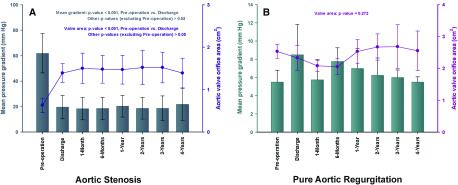
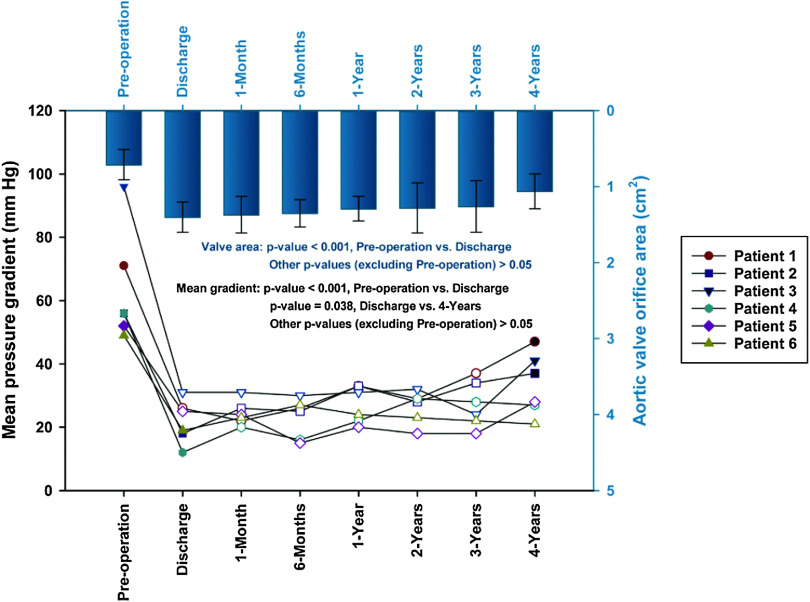
The echocardiographic assessment was available for 18 patients. There was no evidence of morphological SVD or prosthetic valve thrombosis in these patients. Aortic mean pressure gradients decreased from 61.93 ± 15.42 mm Hg (pre-TAVI) to 19.64 ± 9.16 mm Hg (discharge) in patients with AS (p < 0.001); subsequently, a slight increase was observed in the mean trans-aortic gradient throughout follow-up (p = 0.967). Four-year post surgeries, aortic valve gradients in patients with AS was 21.71 ± 12.66 mm Hg, and the mean effective orifice area was 1.39 ± 0.35 cm2 (Fig. 1A). In patients with PAR, there was no statistical difference in the change of mean aortic valve orifice area over time (p=0.272), and the aortic valve gradients and mean effective orifice area were 5.50 ± 0.58 mm Hg and 2.55 ± 0.62 cm2 at 4-year follow-up, respectively (Fig. 1B). According to the standardized definitions of SVD and valve failure from EAPCI/ESC/EACTS,15) three patients with AS had moderate hemodynamic SVD at discharge, and three more AS patients suffered moderate hemodynamic SVD at 1-year follow-up. Overall, six patients with AS were determined as hemodynamic SVD at 4-year follow-up, among which, three were moderate degree and three were severe (Fig. 2). In patients with AS, mild paravalvular regurgitation was observed in five patients (35.7%) at discharge, and two of them progressed to moderate degree at 2-year follow-up. Mild or more than mild paravalvular regurgitation was not found in patients with PAR during the whole period of follow-up visits.
Fig. 1. The data of mean trans-aortic pressure gradient and aortic valve orifice area were showed at all time points reported. The mean gradient was presented with box and whiskers, and valve area with dots in the plot. (A) Aortic stenosis and (B) pure aortic regurgitation.
Fig. 2. The mean aortic pressure gradient for each patient (n = 6) who diagnosed with SVD was presented in the plot. A slight reduction in mean aortic valve orifice area over time was maintained after discharge in these patients. The data of mean aortic valve orifice area was presented with box and whiskers and aortic mean pressure gradient of the six patients was presented with symbols of different shapes. The symbols that filled with white color represented the occurrence of moderate SVD at the time point, and these filled with black color represented the occurrence of severe SVD. SVD: structural valve deterioration.
Discussion
Our results demonstrated the sustained 4-year clinical benefit of TAVI using a second-generation THV (J-valve) in patients with AS and PAR at high-risk for surgery. None of morphological or hemodynamic SVD was observed in patients with PAR. After discharge, overall THV hemodynamics remained stable over time in patients with AS. According to the definitions of SVD from EAPCI/ESC/EACTS,15) three patients with AS were determined as hemodynamic SVD at discharge, and three more patients were diagnosed during 4-year follow-up. None of morphological SVD was reported in patients with AS. J-Valve presented a favorable performance of hemodynamics and durability in long-term follow-up.
The treatment of PAR with TAVI was considered as a relative contraindication due to the increased risk for paravalvular regurgitation and THV embolization and migration in the absence of aortic valve leaflets calcification. The inoperable patients with PAR were recommended to accept medical management; however, these patients suffered an annual mortality risk of 20%.16) Therefore, an unmet need highlighted to treat this patient population with TAVI. With the THV and the technique advanced, the second-generation devices for TAVI provide credible treatment for patients with PAR.12) J-Valve and Jenavalve17) have been certified for PAR and had a significantly higher procedural success rate compared to other “off- label” second-generation devices.12) In addition, the feasibility of TAVI for patients with PAR was proven in several second-generation THV, including Evolut-R,18) SAPIEN 3,8) ACURATE neo,9) Direct Flow Medical,10) Lotus11) et al. The 1-year outcome of TAVI using a second-generation THV (J-Valve and Jenavalve) for PAR has been reported, and it presented a satisfactory outcome.4,5,7) However, the long-term durability and efficiency of these devices were not demonstrated. Our study first reported the long-term follow-up of TAVI using J-Valve for PAR, and the dependable durability and excellent hemodynamic performance were observed in patients with PAR at 4-year follow-up.
In our study, hemodynamic SVD occurred in 21.4% at discharge and 42.8% at 4 years follow-up in patients with AS. In Didier’s study, the incidence of SVD was 8.3% at 1 year and 15.3% between 4- and 5-year follow-up2). Total SVD was observed in 10.8% surviving patients19) in Durand’s study. The incidence of SVD at discharge and 1 year were significantly higher in our study than others.2,19,20) We hypothesized the reasons may be concluded as followed: First, every THV has its unique design of strut and frame, such as J-Valve was designed as a short cylindrically self-expandable stent with three U-shaped graspers encircling the prosthetic valve, which is benefit for the surgical procedure in patients with PAR. The shape of THV stent and its expanding patterns (self-expanding or balloon-expanding) may determine the incidence of short-term postoperative SVD, and affect the long-term durability of the THV thereby. Second, all cohort patients in our study survived during the whole follow-up; however, there are patient deaths during the follow-up in other studies.2,19) A competing risk may be exerted by patient’s death against the risk of THV’s degeneration over time. It cannot be precisely predicted how long the normal function of THV would have lasted if the patient dies before the end point. That means the true durability of the THV cannot be determined because death conceals the chance for that THV to become deteriorative at a later time point.15) Furthermore, the higher incidence of SVD may attribute to the limited number of enrolled patients in our study; thus, the confirmation of the long-term safety and validity of the THV was less powerful.
Although our long-term clinical outcomes were promising, more extensive prospective studies are required to confirm the long-term durability of the J-Valve. To the best of our knowledge, the present report is the first long-term follow-up results of the performance and durability of the second-generation THV in patients with PAR. Our study provided further evidence of the sustained and long-term durability of J-Valve in patients with AS and PAR.
Conclusion
Although the limited number of patients enrolled, the favorable preliminary results of the long-term durability and excellent THV hemodynamics of J-Valve still could provide evidence for the extensive applications of TAVI in patients with PAR. In patients with AS, J-Valve had a higher aortic valve mean gradient than other second-generation THVs at discharge, but it still had a non-inferior long-term durability outcome in 4-year follow-up and the THV hemodynamics remained stable over time after implantation.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1).Miura M, Shirai S, Uemura Y, et al. Early safety and efficacy of transcatheter aortic valve implantation for asian nonagenarians (from KMH registry). Int Heart J 2017; 58: 900–7. [DOI] [PubMed] [Google Scholar]
- 2).Didier R, Eltchaninoff H, Donzeau-Gouge P, et al. Five-year clinical outcome and valve durability after transcatheter aortic valve replacement in high-risk patients. Circulation 2018; 138: 2597–607. [DOI] [PubMed] [Google Scholar]
- 3).de Freitas Campos Guimarães L, Urena M, Wijeysundera HC, et al. Long-term outcomes after transcatheter aortic valve-in-valve replacement. Circ Cardiovasc Interv 2018; 11: e007038. [DOI] [PubMed] [Google Scholar]
- 4).Silaschi M, Conradi L, Wendler O, et al. The JUPITER registry: one-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv 2018; 91: 1345–51. [DOI] [PubMed] [Google Scholar]
- 5).Tung M, Wang X, Li F, et al. A versatile transapical device for aortic valvular disease: One-year outcomes of a multicenter study on the J-Valve system. J Cardiol 2018; 72: 377–84. [DOI] [PubMed] [Google Scholar]
- 6).Liu X, Tang Y, Luo F, et al. Transapical implantation of a self-expandable aortic valve prosthesis utilizing a novel designed positioning element. Catheter Cardiovasc Interv 2017; 89: E30–E37. [DOI] [PubMed] [Google Scholar]
- 7).Luo X, Wang X, Li X, et al. Transapical transcatheter aortic valve implantation using the J-Valve system: a 1-year follow-up study. J Thorac Cardiovasc Surg 2017; 154: 46–55. [DOI] [PubMed] [Google Scholar]
- 8).Minol JP, Veulemans V, Zeus T, et al. Implantation of a SAPIEN 3 valve in a patient with pure aortic regurgitation. J Heart Valve Dis 2016; 25: 498–500. [PubMed] [Google Scholar]
- 9).Cerillo AG, Griese D, Berti S. Successful percutaneous implantation of symetis ACURATE neo transcatheter aortic bioprosthesis for the treatment of pure aortic regurgitation. Catheter Cardiovasc Interv 2016; 88: 319–23. [DOI] [PubMed] [Google Scholar]
- 10).Schofer J, Nietlispach F, Bijuklic K, et al. Transfemoral implantation of a fully repositionable and retrievable transcatheter valve for noncalcified pure aortic regurgitation. JACC Cardiovasc Interv 2015; 8: 1842–9. [DOI] [PubMed] [Google Scholar]
- 11).Wöhrle J, Rodewald C, Rottbauer W. Transfemoral aortic valve implantation in pure native aortic valve insufficiency using the repositionable and retrievable lotus valve. Catheter Cardiovasc Interv 2016; 87: 993–5. [DOI] [PubMed] [Google Scholar]
- 12).Wernly B, Eder S, Navarese EP, et al. Transcatheter aortic valve replacement for pure aortic valve regurgitation: “on-label” versus “off-label” use of TAVR devices. Clin Res Cardiol 2019; 108: 921–30. [DOI] [PubMed] [Google Scholar]
- 13).Cheng J, Chen M, Zhu D, et al. Successful trans- apical aortic valve implantation for a high risk patient with aortic stenosis using a new second-generation TAVI device - J-Valve system. J Cardiothorac Surg 2015; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Zhu D, Hu J, Meng W, et al. Successful transcatheter aortic valve implantation for pure aortic regurgitation using a new second generation self-expanding J-Valve(TM) system - the first in-man implantation. Heart Lung Circ 2015; 24: 411–4. [DOI] [PubMed] [Google Scholar]
- 15).Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017; 38: 3382–90. [DOI] [PubMed] [Google Scholar]
- 16).Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24: 1231–43. [DOI] [PubMed] [Google Scholar]
- 17).Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second- generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014; 7: 1168–74. [DOI] [PubMed] [Google Scholar]
- 18).Spina R, Khalique O, Kodali S, et al. Urgent transcatheter aortic valve replacement for severe acute aortic regurgitation following open mitral valve surgery. Catheter Cardiovasc Interv 2019; 93: 996–1001. [DOI] [PubMed] [Google Scholar]
- 19).Durand E, Sokoloff A, Urena-Alcazar M, et al. Assessment of long-term structural deterioration of transcatheter aortic bioprosthetic valves using the New European definition. Circ Cardiovasc Interv 2019; 12: e007597. [DOI] [PubMed] [Google Scholar]
- 20).Holy EW, Kebernik J, Abdelghani M, et al. Long-term durability and haemodynamic performance of a self-expanding transcatheter heart valve beyond five years after implantation: a prospective observational study applying the standardised definitions of structural deterioration and valve failure. EuroIntervention 2018; 14: e390–e396. [DOI] [PubMed] [Google Scholar]