Abstract
Objective
With the aging of society, both osteoporosis and fatty liver disease (FLD) are becoming important issues. However, the relationship between osteoporosis and FLD remains controversial. We investigated the association between bone metabolism and FLD in a Japanese community in a cross-sectional study.
Methods
A total of 1,020 participants were enrolled in a health survey. FLD was diagnosed by ultrasonography. Bone metabolism was evaluated based on bone mineral density (BMD), which was assessed using dual-energy X-ray absorptiometry, and with the bone formation index (total type I procollagen N-terminal propeptide/bone-alkaline phosphatase ratio; P1NP/BAP ratio) and the bone resorption index (crosslinked N-telopeptide of type I collagen/tartrate-resistant acid phosphatase-5b ratio; NTx/TRACP-5b ratio) calculated from serum bone turnover markers.
Results
The BMD (percentage of the young adult mean) was the same level in both male and female participants with and without FLD. Both men and women showed an age-dependent decrease in their bone formation index and bone resorption index values. Men of ≥70 years of age and women of 60-69 years of age with FLD had significantly lower bone formation index values and higher bone resorption index values. However, similar findings were not seen in women of ≥70 years of age.
Conclusion
Although the BMD levels were the same, regardless of the presence or absence of FLD, elderly participants with FLD showed decreased bone formation and increased bone resorption, with sex differences. Because our results suggest that FLD in elderly individuals is detrimental for bone metabolism, and that it leads to bone loss and osteoporosis, further studies using a cohort population are warranted.
Keywords: bone mineral density, bone turnover markers, bone formation index, bone resorption index, fatty liver disease
Introduction
Osteoporosis, a condition characterized by decreased bone strength, leads to an increased risk of fracture in association with aging or underlying conditions or major risk factors associated with bone demineralization (1). Numerous epidemiological studies have proven that osteoporosis is prevalent all over the world (2-4), but only a small proportion of people with osteoporosis are diagnosed and treated (5,6). Osteoporosis is considered to be becoming a major health problem, and the current number of osteoporotic fracture cases will significantly increase because of the aging of populations all over the world (7,8). In addition to the reduction of bone mineral density (BMD) with advancing age, there are a number of risk factors for fracture, including female sex, current cigarette smoking, and poor nutrition (1). Furthermore, various chronic diseases, such as diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, rheumatoid arthritis, and severe liver disease are associated with an increase in the risk of osteoporosis (1). Regarding the association with liver disease, it is well known that increased bone resorption and decreased bone formation accelerate bone loss in patients with primary biliary cholangitis (9). However, the relationship between osteoporosis and fatty liver disease (FLD) remains controversial (10-13).
FLD, which includes both alcoholic and nonalcoholic variations, encompasses a spectrum of clinical features from simple steatosis to steatohepatitis, which can progress to fibrosis and cirrhosis, liver failure, and hepatocellular carcinoma (14). FLD is one of the most common liver disorders worldwide (15,16), and is associated with a global economic burden as well as poor health-related quality of life (17,18). The prevalence of nonalcoholic FLD (NAFLD) has risen in all regions, with an overall prevalence worldwide of approximately 25% (19). Obesity, diabetes mellitus, and metabolic syndrome, which partially overlap with osteoporosis, are major risk factors for NAFLD (1,20,21). In addition, similar to the clinical manifestations observed in patients with osteoporosis, patients with FLD often show no remarkable clinical signs or symptoms until abnormal liver aminotransferases or features of fatty liver are noted during evaluations performed for other reasons (15,22).
Japan has the highest proportion of older adults and is the fastest aging society in the world (23). Due to the aging and Westernization of lifestyles in Japan, in addition to the increased prevalence of osteoporosis, the prevalence of metabolic disorders, such as obesity, diabetes mellitus, and FLD, has increased (24,25). However, few studies have investigated the relationship between osteoporosis and FLD (10-13). Thus, we investigated the association between bone metabolism, as evaluated by BMD and bone turnover marker levels, and FLD diagnosed by ultrasonography, in a Japanese community study.
Materials and Methods
Study population
The Iwaki Health Promotion Project is an ongoing community-based health promotion study of Japanese people of ≥20 years of age that was designed to prevent lifestyle-related diseases and prolong their lifespan. This program has been carried out annually since 2005 with approximately 1,000 participants in the Iwaki region of Hirosaki City in Aomori Prefecture, which is located in northern Japan (26-29). All study participants participated voluntarily in response to a public announcement, and approximately 600 data points were collected from each participant, including their demographic information, medical history, lifestyle data, and microbiota and blood chemical analysis data. Our research on the association between FLD and bone turnover markers is one part of this project. In 2016, 1,148 individuals were enrolled in this project. Of these, 45 participants whose clinical data were incomplete were excluded. Furthermore, we excluded participants from the analysis based on the following criteria: 1) hepatitis B surface antigen or hepatitis C virus antibody positivity (n=43); and 2) taking medication for osteoporosis (n=40). After applying the exclusion criteria, 1,020 participants (male, n=424; female, n=596) were included in the current study. This study was approved by the Ethics Committee of the Hirosaki University School of Medicine, and written informed consent was obtained from all participants.
Clinical and laboratory assessment
The following clinical characteristics were measured: height, body weight, and body composition. Body mass index (BMI) was calculated as body weight divided by height squared and expressed in kg/m2. Body fat percentage (BFP) was measured by a body composition analyzer (MC-190; Tanita, Tokyo, Japan). Alcohol intake, smoking, and exercise habits were determined from a questionnaire. High-risk drinking was defined as 30 g of alcohol per day in men and 20 g of alcohol per day in women. Whole blood samples were obtained after overnight fasting, and laboratory tests included the platelet count, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl-transpeptidase (GGT), total bilirubin, glucose, hemoglobin A1c (HbA1c), total cholesterol, triglyceride, calcium, phosphate, collagen type IV, and serum turnover markers.
Measurements of BMD and serum bone turnover markers
The BMD of the forearm was measured by dual-energy X-ray absorptiometry (DXA) using a DSC-600EXV (Hitachi Aloka Medical, Tokyo, Japan), as in a previous report (30). The results were calculated and presented as a percentage of the young adult mean (%YAM) values.
As bone turnover markers, serum total type I procollagen N-terminal propeptide (P1NP) and bone-alkaline phosphatase (BAP) were determined using an electro-chemiluminescence immunoassay (ECLIA) and a chemiluminescent enzyme immunoassay (CLEIA), respectively, and the serum levels of crosslinked N-telopeptide of type I collagen (NTx) and tartrate-resistant acid phosphatase-5b (TRACP-5b) were determined using an enzyme immunoassay (EIA) in a commercial laboratory (LSI Medience, Tokyo, Japan). To evaluate bone metabolism, indexes of bone formation and resorption were calculated as ratios of P1NP-to-BAP and NTx-to-TRACP-5b, respectively (31-33).
Assessment of FLD
FLD was diagnosed based on abdominal ultrasound findings using a Prosound F37 (Hitachi Aloka Medical). Ultrasound examinations were performed by one of five well-trained hepatology specialists, each with more than 5 years of experience, without detailed knowledge of the participant's data. Images were stored and reevaluated by a single hepatologist with more than 20 years of experience. Echogenicity that indicated FLD was as follows: a diffuse increase in fine echoes in liver parenchyma; impaired visualization of the hepatic vessel border, diaphragm, and posterior right lobe of the liver (34). More specifically, FLD was diagnosed when a bright liver and/or hepatorenal echo contrast were observed on B-mode ultrasonography.
Statistical analysis
All statistical analyses of collected data were performed using the Excel statistical software package for Macintosh (Ekuseru-Toukei 2016; Esumi, Tokyo, Japan). Categorical variables were compared using the chi-squared test. Bone formation index and bone resorption index were compared between participants with or without FLD using the Mann-Whitney U test. Items that showed significant differences in a univariate analysis for the comparison of participants with and without FLD were included in a multivariate analysis. Spearman's correlation coefficient was used to calculate the correlation between age and BMD, bone formation index, or bone resorption index. P values of <0.05 were considered to indicate statistical significance.
Results
Characteristics of the participants
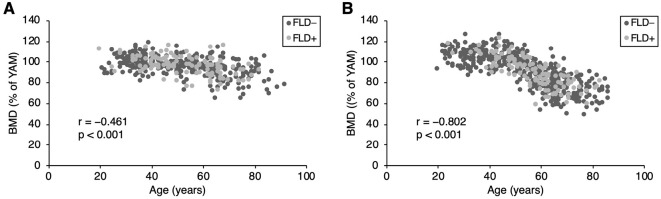
The characteristics of the study participants are shown in Table 1. The average age of the participants was 53.1±15.5 years, and there were more women than men (596/424). Among the 1,020 participants, 117 men (27.6%) and 118 women (19.8%) had FLD. There were no participants with chronic liver disease or liver cirrhosis on ultrasonography. The mean alcohol consumption, the percentages of high-risk drinkers and current smokers, and BMI values in men were higher than those in women. On the other hand, the BFP in women was higher than that in men. Most biochemical data, including the albumin, AST, ALT, GGT, total bilirubin, glucose, calcium, and triglyceride levels, were higher in men. Only the platelet count and phosphate level were higher in women. With respect to bone metabolism, BMD decreased in an age-dependent manner (men: r=-0.461, p<0.001; women: r=-0.802, p<0.001, respectively), and women over approximately 50 years of age showed a remarkable decrease (Fig. 1). Women showed a significantly lower BMD (91.6±16.0% vs. 96.0±9.1%, p<0.001) and a higher percentage of low BMD (<70% of the YAM) participants (0.7% vs. 10.5%, p<0.001) in comparison to men (Table 1). The P1NP level and the P1NP/BAP ratio were higher in women. There were no differences between men and women in the levels of BAP, NTx, TRACP-5b, or the NTx/TRACP-5b ratio (Table 1).
Table 1.
Baseline Characteristics of Study Participants.
| Characteristics | Total (n=1,020) |
Men (n=424) |
Women (n=596) |
p value |
|---|---|---|---|---|
| Age (years) | 53.1 (15.5) | 52.4 (15.8) | 53.7 (15.2) | 0.168 |
| FLD | 235 (23.0) | 117 (27.6) | 118 (19.8) | 0.004 |
| Alcohol consumption (g/day) | 13.2 (22.4) | 25.0 (28.3) | 4.8 (11.2) | <0.001 |
| High-risk drinkers* | 186 (18.2) | 114 (26.8) | 42 (7.0) | <0.001 |
| Current smoker | 190 (18.6) | 125 (29.4) | 65 (10.9) | <0.001 |
| Habitual exerciser | 310 (30.4) | 137 (32.3) | 173 (29.0) | 0.261 |
| BMI (kg/m2) | 22.9 (3.3) | 23.6 (3.0) | 22.4 (3.5) | <0.001 |
| BFP (%) | 26.1 (8.1) | 20.5 (5.7) | 30.1 (7.2) | <0.001 |
| BMD YAM (%) | 93.4 (13.7) | 96.0 (9.1) | 91.6 (16.0) | <0.001 |
| BMD <70% YAM | 66 (6.4) | 3 (0.7) | 63 (10.5) | <0.001 |
| Platelet count (104/μL) | 24.7 (5.5) | 24.1 (5.1) | 25.1 (5.8) | 0.015 |
| Albumin (g/dL) | 4.49 (0.29) | 4.55 (0.28) | 4.45 (0.29) | <0.001 |
| AST (U/L) | 23.2 (9.5) | 25.5 (9.6) | 21.6 (9.1) | <0.001 |
| ALT (U/L) | 22.1 (14.8) | 26.9 (16.3) | 18.7 (12.7) | <0.001 |
| GGT (U/L) | 33.9 (42.5) | 49.8 (59.4) | 22.6 (15.4) | <0.001 |
| Total bilirubin (mg/dL) | 0.79 (0.31) | 0.83 (0.34) | 0.77 (0.28) | 0.010 |
| Glucose (mg/dL) | 92.0 (20.2) | 93.8 (16.8) | 90.6 (22.3) | <0.001 |
| HbA1c (%) | 5.85 (0.65) | 5.84 (0.58) | 5.87 (0.69) | 0.350 |
| Total cholesterol (mg/dL) | 205.0 (35.7) | 202.5 (32.6) | 207.4 (37.7) | 0.090 |
| Triglyceride (mg/dL) | 97.2 (66.7) | 114.7 (73.8) | 84.8 (58.0) | <0.001 |
| Calcium (mg/dL) | 9.50 (0.32) | 9.54 (0.32) | 9.47 (0.31) | 0.003 |
| Phosphate (mg/dL) | 3.50 (0.44) | 3.32 (0.41) | 3.63 (0.41) | <0.001 |
| Collagen type IV (ng/mL) | 127.1 (46.8) | 126.8 (44.4) | 127.3 (48.5) | 0.671 |
| P1NP (μg/L) | 47.6 (21.4) | 44.2 (21.1) | 50.0 (21.3) | <0.001 |
| BAP (μg/L) | 13.3 (5.7) | 13.0 (5.2) | 13.5 (6.0) | 0.707 |
| NTx (nM/BCE/L) | 15.3 (4.8) | 15.0 (4.7) | 15.4 (4.9) | 0.202 |
| TRACP-5b (mU/dL) | 412.7 (186.0) | 397.7 (169.8) | 423.4 (196.2) | 0.073 |
| P1NP/BAP ratio | 3.74 (1.33) | 3.51 (1.16) | 3.91 (1.42) | <0.001 |
| NTx/TRACP-5b ratio | 0.042 (0.019) | 0.041 (0.015) | 0.042 (0.021) | 0.389 |
Data are n (%) or means (±SD). Boldface type indicates a significant p value (p<0.05).
*High-risk drinkers were classified as men and women who drink more than 30 or 20 g/day of alcohol, respectively.
FLD: fatty liver disease, BMI: body mass index, BFP: body fat percentage, BMD: bone mineral density, YAM: young adult mean, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GGT: gamma-glutamyl-transpeptidase, HbA1c: hemoglobin A1c, P1NP: procollagen type I N-terminal peptide, BAP: bone-specific alkaline phosphatase, NTx: N-terminal telopeptide of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase-5b
Figure 1.
Bone mineral density (BMD) in men and women. Regardless of the presence or absence of fatty liver, BMD decreased in an age-dependent manner in both men (A) and women (B). Women over approximately 50 years of age showed a remarkable decrease in BMD (B).
The characteristics of male and female participants with and without FLD are shown in Tables 2 and 3, respectively. In both sexes, the participants with FLD predominantly had higher BMI and BFP values, and showed higher platelet counts and AST, ALT, GGT, glucose, HbA1c, total cholesterol, and triglyceride levels in comparison to those without FLD. On the other hand, in both male and female participants, there were no differences in the prevalence of high-risk drinkers, current smoking, and low habitual exercise, BMD, or the serum levels of phosphate, in participants with and without FLD (Table 2, 3). In men, the participants with FLD showed lower serum levels of type IV collagen (Table 2), while in women, the participants with FLD were older and showed lower alcohol consumption, lower serum levels of total bilirubin, and higher serum levels of calcium in comparison to the participants without FLD (Table 3). As for bone metabolism, in male and female participants, there were no differences in the mean BMD or the percentage of participants with low BMD (<70% of YAM) between the participants with and without FLD (Table 2, 3, Fig. 1). In men, the serum levels of P1NP, NTx, and TRACP-5b in the participants with FLD were significantly lower than those in participants without FLD (Table 2). In women, the serum levels of BAP in participants with FLD were significantly higher than those in participants without FLD (Table 3). In both male and female participants, the P1NP/BAP ratio in participants with FLD was significantly lower than that in participants without FLD (Table 2, 3). On the other hand, only in men, the NTx/TRACP-5b ratio was significantly higher in participants with FLD than in those without FLD (Table 2). Furthermore, in a multivariate analysis that included statistically significant factors that were identified in a univariate analysis, BMI and the serum ALT and triglyceride levels were found to be significantly higher in male participants with FLD than in male participants without FLD (Table 2). On the other hand, the multivariate analysis showed that female participants with FLD had significantly higher platelet counts, serum levels of AST, ALT, and triglyceride, and lower P1NP/BAP ratios in comparison to those without FLD (Table 3).
Table 2.
Univariate and Multivariate Analysis of Factors Associated with FLD in Men.
| FLD (-) (n=307) |
FLD (+) (n=117) |
Univariate p value |
Multivariate | ||
|---|---|---|---|---|---|
| OR (95% CI) | p value | ||||
| Age (years) | 52.0 (16.6) | 53.3 (13.6) | 0.320 | ||
| Alcohol consumption (g/day) | 24.1 (27.0) | 27.2 (31.4) | 0.761 | ||
| High-risk drinkers* | 107 (34.9) | 37 (31.6) | 0.530 | ||
| Current smoker | 94 (30.6) | 31 (26.5) | 0.405 | ||
| Habitual exerciser | 102 (33.2) | 35 (29.9) | 0.515 | ||
| BMI (kg/m2) | 22.8 (2.5) | 25.7 (3.1) | <0.001 | 1.21 (1.04-1.40) | 0.014 |
| BFP (%) | 19.3 (5.5) | 23.8 (5.2) | <0.001 | 0.265 | |
| BMD YAM (%) | 95.9 (9.2) | 96.4 (8.9) | 0.814 | ||
| BMD <70% YAM | 3 (1.0) | 0 (0.0) | 0.283 | ||
| Platelet count (104/μL) | 23.7 (5.2) | 24.9 (4.8) | 0.013 | 0.073 | |
| Albumin (g/dL) | 4.54 (0.30) | 4.58 (0.23) | 0.252 | ||
| AST (U/L) | 24.4 (8.5) | 28.2 (11.5) | <0.001 | 0.088 | |
| ALT (U/L) | 23.0 (13.0) | 37.4 (19.2) | <0.001 | 1.07 (1.04-1.10) | <0.001 |
| GGT (U/L) | 43.9 (57.9) | 65.2 (60.9) | <0.001 | 0.619 | |
| Total bilirubin (mg/dL) | 0.82 (0.34) | 0.84 (0.36) | 0.802 | ||
| Glucose (mg/dL) | 91.9 (15.4) | 98.9 (19.0) | <0.001 | 0.198 | |
| HbA1c (%) | 5.77 (0.50) | 6.01 (0.74) | <0.001 | 0.640 | |
| Total cholesterol (mg/dL) | 200.6 (32.8) | 207.7 (31.8) | 0.046 | 0.257 | |
| Triglyceride (mg/dL) | 103.0 (62.5) | 145.2 (90.8) | <0.001 | 1.00 (1.00-1.01) | 0.041 |
| Calcium (mg/dL) | 9.55 (0.33) | 9.52(0.32) | 0.426 | ||
| Phosphate (mg/dL) | 3.32 (0.40) | 3.31 (0.45) | 0.932 | ||
| Collagen type IV (ng/mL) | 129.7 (37.3) | 124.1 (61.4) | 0.006 | 0.753 | |
| P1NP (μg/L) | 46.0 (22.8) | 39.5 (15.2) | 0.001 | 0.408 | |
| BAP (μg/L) | 13.1 (5.0) | 12.6 (5.6) | 0.229 | ||
| NTx (nM/BCE/L) | 15.4 (5.0) | 14.0 (3.3) | 0.001 | 0.815 | |
| TRACP-5b (mU/dL) | 423.0 (185.8) | 331.6 (89.3) | <0.001 | 0.058 | |
| P1NP/BAP ratio | 3.59 (1.18) | 3.29 (1.09) | 0.014 | 0.761 | |
| NTx/TRACP-5b ratio | 0.040 (0.015) | 0.044 (0.013) | <0.001 | 0.187 | |
Data are n (%) or means (±SD). Boldface type indicates a significant p value (p<0.05).
*High-risk drinkers were classified as men and women who drink more than 30 or 20 g/day of alcohol, respectively.
FLD: fatty liver disease, BMI: body mass index, BFP: body fat percentage, BMD: bone mineral density, YAM: young adult mean, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GGT: gamma-glutamyl-transpeptidase, HbA1c: hemoglobin A1c, P1NP: procollagen type I N-terminal peptide, BAP: bone-specific alkaline phosphatase, NTx: N-terminal telopeptide of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase-5b
Table 3.
Univariate and Multivariate Analysis of Factors Associated with FLD in Women.
| FLD (-) (n=478) |
FLD (+) (n=118) |
Univariate p value |
Multivariate | ||
|---|---|---|---|---|---|
| OR (95% CI) | p value | ||||
| Age (years) | 53.0 (15.8) | 56.5 (12.2) | 0.037 | 0.930 | |
| Alcohol consumption (g/day) | 5.2 (11.8) | 3.0 (8.1) | 0.014 | 0.265 | |
| High-risk drinkers* | 36 (7.5) | 6 (5.1) | 0.352 | ||
| Current smoker | 51 (10.7) | 14 (11.9) | 0.709 | ||
| Habitual exerciser | 140 (29.3) | 33 (28.0) | 0.777 | ||
| BMI (kg/m2) | 21.6 (2.9) | 25.7 (3.8) | <0.001 | 0.112 | |
| BFP (%) | 28.6 (6.6) | 36.3 (6.3) | <0.001 | 0.181 | |
| BMD YAM (%) | 91.9 (16.4) | 90.3 (14.5) | 0.227 | ||
| BMD <70% YAM | 51 (10.7) | 12 (10.2) | 0.874 | ||
| Platelet count (104/μL) | 24.6 (5.4) | 27.2 (6.9) | <0.001 | 1.08 (1.03-1.13) | 0.001 |
| Albumin (g/dL) | 4.45 (0.29) | 4.45 (0.28) | 0.712 | ||
| AST (U/L) | 20.6 (8.0) | 25.4 (12.0) | <0.001 | 0.93 (0.88-0.99) | 0.028 |
| ALT (U/L) | 16.3 (8.9) | 28.0 (19.6) | <0.001 | 1.10 (1.05-1.15) | <0.001 |
| GGT (U/L) | 20.9 (14.3) | 29.3 (17.5) | <0.001 | 0.905 | |
| Total bilirubin (mg/dL) | 0.78 (0.28) | 0.72 (0.27) | 0.008 | 0.151 | |
| Glucose (mg/dL) | 88.8 (22.3) | 97.7 (20.5) | <0.001 | 0.132 | |
| HbA1c (%) | 5.78 (0.63) | 6.21 (0.82) | <0.001 | 0.071 | |
| Total cholesterol (mg/dL) | 205.0 (38.0) | 217.0 (34.9) | 0.001 | 0.217 | |
| Triglyceride (mg/dL) | 75.3 (40.3) | 123.0 (93.0) | <0.001 | 1.01 (1.00-1.02) | <0.001 |
| Calcium (mg/dL) | 9.45 (0.31) | 9.53 (0.33) | 0.038 | 0.858 | |
| Phosphate (mg/dL) | 3.64 (0.42) | 3.63 (0.40) | 0.971 | ||
| Collagen type IV (ng/mL) | 127.9 (51.0) | 124.7 (36.8) | 0.178 | ||
| P1NP (μg/L) | 49.8 (20.6) | 50.9 (24.0) | 0.665 | ||
| BAP (μg/L) | 13.1 (5.9) | 14.9 (6.2) | <0.001 | 0.724 | |
| NTx (nM/BCE/L) | 15.5 (4.9) | 15.2 (4.5) | 0.874 | ||
| TRACP-5b (mU/dL) | 427.2 (202.9) | 407.9 (166.6) | 0.609 | ||
| P1NP/BAP ratio | 4.00 (1.48) | 3.54 (1.08) | 0.002 | 0.82 (0.67-1.00) | 0.049 |
| NTx/TRACP-5b ratio | 0.042 (0.023) | 0.041 (0.013) | 0.531 | ||
Data are n (%) or means (±SD). Boldface type indicates a significant p value (p<0.05).
*High-risk drinkers were classified as men and women who drink more than 30 or 20 g/day of alcohol, respectively.
FLD: fatty liver disease, BMI: body mass index, BFP: body fat percentage, BMD: bone mineral density, YAM: young adult mean, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GGT: gamma-glutamyl-transpeptidase, HbA1c: hemoglobin A1c, P1NP: procollagen type I N-terminal peptide, BAP: bone-specific alkaline phosphatase, NTx: N-terminal telopeptide of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase-5b
Comparison of bone turnover indices in participants with and without FLD according to age group
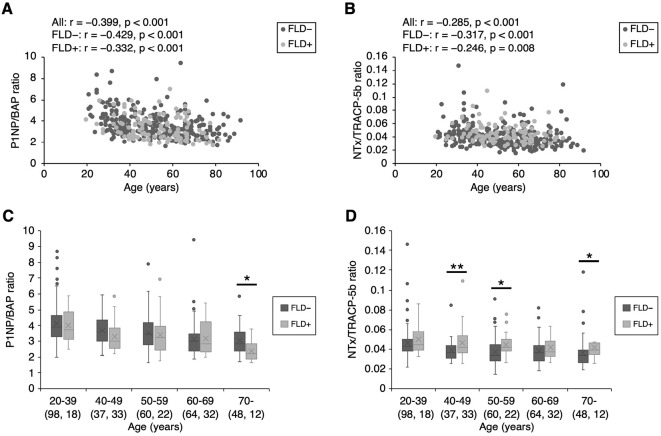
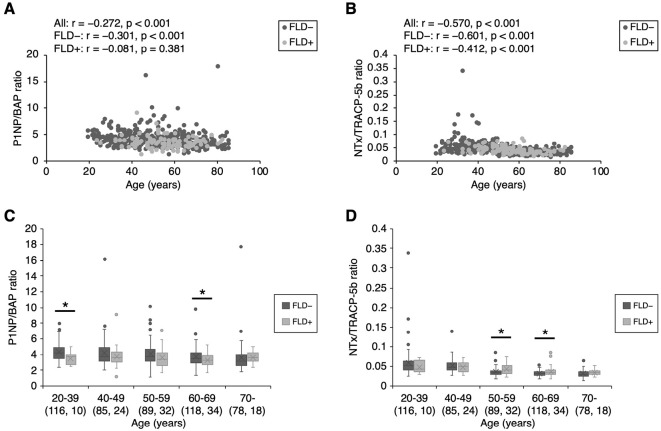
Figs. 2, 3 show the bone formation index (P1NP/BAP ratio) and bone resorption index (NTx/TRACP-5b ratio) values in men and women, respectively. In men, both indices were decreased in an age-dependent manner, regardless of the presence or absence of FLD [bone formation index: all participants: r=-0.399; participants without FLD, r=-0.429; participants with FLD, r=-0.332 (all p<0.001); bone resorption index: all participants, r=-0.285 (p<0.001); participants without FLD, r=-0.317 (p<0.001); participants with FLD, r=-0.246 (p=0.008)] (Fig. 2A, B). On the other hand, in women, the bone resorption index was decreased in an age-dependent manner [all participants, r=-0.570; participants without FLD, r=-0.601; participants with FLD, r=-0.412 (all p<0.001)] (Fig. 3B). However, there was no correlation between the bone formation index and age in female participants with FLD, although in female participants without FLD the bone formation was decreased in an age-dependent manner [all participants, r=-0.272 (p<0.001); participants without FLD, r=-0.301 (p<0.001), participants with FLD, r=-0.081 (p=0.381)] (Fig. 3A). In the analysis by age group, the bone formation index values observed in participants with FLD were lower than those in participants without FLD in male participants of ≥70 years of age (Fig. 2C) and female participants of 20-39 and 60-69 years of age (Fig. 3C). In addition, the bone resorption index values of participants with FLD were higher than those in participants without FLD in male participants of 40-59 and ≥70 years of age (Fig. 2D), and female participants of 50-69 years of age (Fig. 3D). Consequently, men of ≥70 years of age and women of 60-69 years of age with FLD simultaneously had lower bone formation index values (p=0.012 and p=0.020, respectively) (Fig. 2C, 3C) and higher bone resorption index values (p=0.034 and p=0.017, respectively) in comparison to participants without FLD (Fig. 2D, 3D).
Figure 2.
Bone formation index and bone resorption index values in men. Bone formation index and bone resorption index values decreased in an age-dependent manner in men, regardless of the presence of FLD [bone formation index: all participants, r=-0.399; participants without FLD, r=-0.429; participants with FLD, r=-0.332 (all p<0.001); bone resorption index, all participants: r=-0.285 and p<0.001, participants without FLD: r=-0.317 and p<0.001, participants with FLD: r=-0.246 and p=0.008] (A, B). Among men of ≥70 years of age, lower bone formation index values were observed in participants with fatty liver in comparison to those without fatty liver (C). Among men of 40-59 and ≥70 years of age, higher bone resorption index values were observed in participants with fatty liver in comparison to those without fatty liver (D). The numbers in parentheses indicate the number of participants. *p<0.05. **p<0.01. FLD: fatty liver disease
Figure 3.
Bone formation index and bone resorption index values in women. Bone formation index values decreased in an age-dependent manner in women without FLD. However, there was no correlation between the bone formation index and age in women with FLD [all participants, r=-0.272 (p<0.001); participants without FLD, r=-0.301 (p<0.001); participants with FLD, r=-0.081 (p=0.381)] (A). Bone resorption index values decreased in an age-dependent manner in women [all participants, r=-0.570; participants without FLD, r=-0.601; participants with FLD, r=-0.412 (all p<0.001)] (B). Among women of 20-39 and 60-69 years of age, lower bone formation index values were observed in the participants with FLD in comparison to those without FLD (C). Among women of 50-69 years of age, higher bone resorption index values were observed in participants with FLD in comparison to those without FLD (D). The numbers in parentheses indicate the number of participants. *p<0.05. FLD: fatty liver disease
Discussion
The present study investigated the relationship between FLD and bone metabolism in an unselected community-dwelling population using a cross-sectional study design. In this study, the prevalence of FLD and participants with low BMD was similar to that in previous reports (1-4,19,22), and the BMD did not differ between participants with and without FLD. However, the bone formation index (P1NP/BAP ratio) and bone resorption index (NTx/TRACP-5b ratio) were not balanced in elderly participants. In other words, the bone turnover in the elderly participants with FLD simultaneously showed the attenuation of bone formation and promotion of bone resorption, indicating a risk of developing osteoporosis.
The relationship between bone metabolism and FLD is still controversial. Some researchers have reported that people with FLD show a lower BMD, which leads to the development of osteoporosis (10,11), while other researchers reported that the BMD did not differ between people with FLD and controls (12,13). In addition, the relationship between bone turnover markers and FLD is controversial (35,36). In our study, the BMD did not differ between the participants with and without FLD, in either sex (Table 2, 3). As for turnover markers, the bone formation index values in female participants with FLD were lower than those observed in female participants without FLD (Table 3, p=0.049), but this difference was not observed in men. However, according to the analysis by age group, among both men and women, elderly participants with FLD showed lower bone formation index values and higher bone resorption index values in comparison to elderly participants without FLD (Fig. 2, 3). These conflicting results, including previous reports, might be partially attributable to differences in age, gender, and race. Of course, various other factors, such as lifestyle - including smoking, nutrition, alcohol consumption, and physical activity - might affect bone metabolism. These risk factors are classified as non-modifiable factors, modifiable factors, or secondary factors. Prior fracture, female sex, advanced age, and white race have been pointed out as major nonmodifiable factors, while current cigarette smoking, poor nutrition, low body weight, alcoholism, recurrent falls, and inadequate physical activity have been pointed out as major modifiable factors (1,36). Thus, the controversial conclusions might be caused by many of these risk factors when evaluating the association between FLD and osteoporosis.
Chronic diseases, including chronic liver disease, chronic kidney disease, cardiovascular disease, diabetes mellitus, and dementia, are secondary causes that increase the number of osteoporotic fractures (1,37). Although chronic liver diseases have various etiologies, including hepatitis virus, cholestasis, and alcohol consumption, patients with chronic liver disease or cirrhosis show bone loss and a high prevalence of osteoporosis, which is known as hepatic osteodystrophy (38,39). Several mechanisms of hepatic osteodystrophy have been proposed, including cytokines (e.g., transforming growth factor β, tumor necrosis factor α, and interleukin-6), vitamin D metabolism, and sex hormones (40,41). However, much is still unknown about hepatic osteodystrophy in NAFLD and nonalcoholic steatohepatitis (NASH) (40). In our study, FLD affected bone turnover in the elderly population; however, further studies are needed to clarify the mechanisms involved in the association between FLD and bone metabolism.
In our study, men of ≥70 years of age with FLD showed lower bone formation index values and higher bone resorption index values in comparison to men without FLD (Fig. 2C, D); however, women of ≥70 years of age had the same bone turnover index values, regardless of the presence or absence of FLD (Fig. 3C, D). Regarding this sex difference in bone metabolism in the elderly, previous reports have indicated that the collagen turnover of the extracellular matrix and muscle strength, which affect bone metabolism, are age- and sex-dependent (42,43). Thus, we analyzed the bone formation index and bone resorption index according to sex and age group. In fact, the bone formation index values in men and the bone resorption index values in both sexes decreased in an age-dependent manner, regardless of the presence or absence of FLD (Fig. 2A, B, 3B). However, in women, there was no correlation between the bone formation index and age in the participants with FLD, although the bone formation index decreased in an age-dependent manner in the participants without FLD (Fig. 3A). Unfortunately muscle strength was not evaluated in this study. On the other hand, in women, the participants with FLD showed higher BMI values, in comparison to the participants without FLD (Table 2, 3). Obesity represents a major public health issue that is present worldwide and which is associated with the increased incidence of diseases such as cardiovascular and metabolic diseases, cancer, and osteoporosis (44,45). However, despite these causative effects of obesity, the overweight or obese population frequently shows a more favorable prognosis, commonly referred to as the “obesity paradox” (46-48). Although the factors contributing to our study results were not clear, some factors, such as the collagen turnover and the obesity paradox might partially explain why-among women of ≥70 years of age-there was no difference in bone metabolism between the participants with without FLD.
Study limitations
The present study was associated with some limitations. First, because of the cross-sectional design, we could not determine whether FLD is a risk factor for the future onset of osteoporosis. Thus, longitudinal studies are required to investigate the association between FLD and BMD. Second, although BMD measurements are performed at the lumbar spine and hip to best detect the fracture risk, these measurements are costly and require space and frequent calibration (49-51). Due to these limitations, we evaluated the BMD in the forearm during general medical examinations in this study, on the basis that the BMD in the forearm is correlated with the BMD in the lumbar spine (30,52). Third, the diagnosis of FLD was made based on ultrasonography without a liver biopsy due to the invasive nature of a liver biopsy. Instead, a common ultrasonographic definition of FLD was established and used as a noninvasive modality (34,53). Fourth, our study was a health promotion study, which differs from an ordinary health check-up survey; thus, the participants who participated in our study were interested in their health and may have been healthier in comparison to the general population, resulting in a possible selection bias. Despite these limitations, this general population-based study revealed an association between bone turnover indices and FLD in old age. Given this result, the BMD may be expected to decrease in the elderly population with FLD.
Conclusion
Elderly individuals with FLD had lower bone formation index values and higher bone resorption index values, with sex differences, although the BMD remained the same regardless of the presence or absence of FLD. Our results suggest that FLD in the elderly is detrimental for bone metabolism, which leads to bone loss; thus, further studies using a cohort population are warranted.
This study was approved by the Ethics Committee of the Hirosaki University School of Medicine, and written informed consent was obtained from all participants.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by JST COI Grant Number JPMJCE1302.
Acknowledgement
We are extremely grateful to all the participants in the Iwaki Health Promotion Project and the entire staff of the project who conducted the interviews and collected the data. We appreciate Mr. Jeffery G. Stocker's contribution to the proofreading.
References
- 1.Lindsay R, Cosman F. Osteoporosis. In: Harrison's Principal of Internal Medicine. 20th ed Jameson JL, Fauci A, Kasper D, Hauser S, Longo D, Loscalzo J, Eds. McGraw Hill, Toronto, 2018: 2942-2959. [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8: 136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29: 2520-2526, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung EYN, Tan KCB, Cheung CL, Kung AWC. Osteoporosis in East Asia: current issues in assessment and management. Osteoporos Sarcopenia 2: 118-133, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum 35: 293-305, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int 15: 767-778, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Odén A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int 26: 2243-2248, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17: 1726-1733, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Solaymani-Dodaran M, Card TR, Aithal GP, West J. Fracture risk in people with primary biliary cirrhosis: a population-based cohort study. Gastroenterology 131: 1752-1757, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Chen HJ, Yang HY, Hsueh KC, et al. Increased risk of osteoporosis in patients with nonalcoholic fatty liver disease: a population-based retrospective cohort study. Medicine (Baltimore) 97: e12835, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HJ, Shim SG, Ma BO, Kwak JY. Association of nonalcoholic fatty liver disease with bone mineral density and serum osteocalcin levels in Korean men. Eur J Gastroenterol Hepatol 28: 338-344, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Dauriz M, Gatti D, et al. Systematic review with meta-analysis: non-alcoholic fatty liver disease is associated with a history of osteoporotic fractures but not with low bone mineral density. Aliment Pharmacol Ther 49: 375-388, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Upala S, Jaruvongvanich V, Wijarnpreecha K, Sanguankeo A. Nonalcoholic fatty liver disease and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab 35: 685-693, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116: 1413-1419, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142: 1592-1609, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Mills SJ, Harrison SA. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr Gastroenterol Rep 7: 32-36, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology 150: 1778-1785, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 70: 531-544, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15: 11-20, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Wani K, Yakout SM, Ansari MGA, et al. Metabolic syndrome in Arab adults with low bone mineral density. Nutrients 11: E1405, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckstein N, Buchmann N, Demuth I, et al. Association between metabolic syndrome and bone mineral density-data from the Berlin Aging Study II (BASE-II). Gerontology 62: 337-344, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Abdelmalek MA, Diehl AM. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. In: Harrison's Principal of Internal Medicine. 20th ed Jameson JL, Fauci A, Kasper D, Hauser S, Longo D, Loscalzo J, Eds. McGraw Hill, Toronto, 2018: 2401-2405. [Google Scholar]
- 23.Muramatsu N, Akiyama H. Japan: super-aging society preparing for the future. Gerontologist 51: 425-432, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Ninomiya T. Japanese legacy cohort studies: The Hisayama Study. J Epidemiol 28: 444-451, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimura N, Muraki S, Oka H, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 27: 620-628, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Hatakeyama S, Imai A, et al. Relationship between oxidative stress and lower urinary tract symptoms: results from a community health survey in Japan. BJU Int 123: 877-884, 2019. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki E, Sasaki S, Chiba D, et al. Age-related reduction of trunk muscle torque and prevalence of trunk sarcopenia in community-dwelling elderly: validity of a portable trunk muscle torque measurement instrument and its application to a large sample cohort study. PLoS One 13: e0192687, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satake R, Sugawara N, Sato K, et al. Prevalence and predictive factors of irritable bowel syndrome in a community-dwelling Population in Japan. Intern Med 54: 3105-3112, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki E, Ishibashi Y, Tsuda E, et al. Evaluation of locomotive disability using loco-check: a cross-sectional study in the Japanese general population. J Orthop Sci 18: 121-129, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Ota S, Chiba D, Sasaki E, et al. Symptomatic bone marrow lesions induced by reduced bone mineral density in middle-aged women: a cross-sectional Japanese population study. Arthritis Res Ther 21: 113, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viljakainen H, Ivaska KK, Paldánius P, et al. Suppressed bone turnover in obesity: a link to energy metabolism? A case-control study. J Clin Endocrinol Metab 99: 2155-2163, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Alatalo SL, Ivaska KK, Waguespack SG, Econs MJ, Väänänen HK, Halleen JM. Osteoclast-derived serum tartrate-resistant acid phosphatase 5b in Albers-Schonberg disease (type II autosomal dominant osteopetrosis). Clin Chem 50: 883-890, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int 82: 108-115, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123: 745-750, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Maagensen H, Junker AE, Jørgensen NR, Gluud LL, Knop FK, Vilsbøll T. Bone turnover markers in patients with nonalcoholic fatty liver disease and/or type 2 diabetes during oral glucose and isoglycemic intravenous glucose. J Clin Endocrinol Metab 103: 2042-2049, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Díez Rodríguez R, Ballesteros Pomar MD, Calleja Fernández A, et al. Vitamin D levels and bone turnover markers are not related to non-alcoholic fatty liver disease in severely obese patients. Nutr Hosp 30: 1256-1262, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag 14: 2029-2049, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karoli Y, Karoli R, Fatima J, Manhar M. Study of hepatic osteodystrophy in patients with chronic liver disease. J Clin Diagn Res 10: OC31-OC34, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal RK, Kumar M, Sachdeva PR, Kumar A. Prospective study of profile of hepatic osteodystrophy in patients with non-choleastatic liver cirrhosis and impact of bisphosphonate supplementation. United European Gastroenterol J 4: 77-83, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehnert S, Aspera-Werz RH, Ruoß M, et al. Hepatic osteodystrophy-molecular mechanisms proposed to favor its development. Int J Mol Sci 20: E2555, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bihari C, Lal D, Thakur M, et al. Suboptimal level of bone-forming cells in advanced cirrhosis are associated with hepatic osteodystrophy. Hepatol Commun 2: 1095-1110, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kehlet SN, Willumsen N, Armbrecht G, et al. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS One 13: e0194458, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KM, Lim S, Oh TJ, et al. Longitudinal changes in muscle mass and strength, and bone mass in older adults: gender-specific associations between muscle and bone losses. J Gerontol A Biol Sci Med Sci 73: 1062-1069, 2018. [DOI] [PubMed] [Google Scholar]
- 44.Kushner RF. Evaluation and management of obesity. In: Harrison's Principal of Internal Medicine. 20th ed Jameson JL, Fauci A, Kasper D, Hauser S, Longo D, Loscalzo J, Eds. McGraw Hill, Toronto, 2018: 2843-2850. [Google Scholar]
- 45.Shapses SA, Pop LC, Wang Y. Obesity is a concern for bone health with aging. Nutr Res 39: 1-13, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modig K, Erdefelt A, Mellner C, Cederholm T, Talbäck M, Hedström M. “Obesity Paradox” holds true for patients with hip fracture: a registry-based cohort study. J Bone Joint Surg Am 101: 888-895, 2019. [DOI] [PubMed] [Google Scholar]
- 47.Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eat Weight Disord 23: 293-302, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Dimitri P, Bishop N, Walsh JS, Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone 50: 457-466, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 332: 767-773, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Torgerson DJ, Campbell MK, Thomas RE, Reid DM. Prediction of perimenopausal fractures by bone mineral density and other risk factors. J Bone Miner Res 11: 293-297, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 12: 989-995, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Faulkner KG, von Stetten E, Miller P. Discordance in patient classification using T-scores. J Clin Densitom 2: 343-350, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Tobari M, Hashimoto E, Yatsuji S, Torii N, Shiratori K. Imaging of nonalcoholic steatohepatitis: advantages and pitfalls of ultrasonography and computed tomography. Intern Med 48: 739-746, 2009. [DOI] [PubMed] [Google Scholar]