Abstract
Histologic transformation has been described as an acquired mechanism of resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). We herein report the case of a woman with stage IV lung adenocarcinoma harboring EGFR exon 19 deletions who was initially treated with EGFR-TKIs; several cytotoxic chemotherapeutic regimens were used when resistance developed. A lymph node re-biopsy revealed histologic transformation of the tumor to combined small-cell lung cancer and squamous cell carcinoma with retained EGFR exon 19 deletions. Following sequential chemotherapy appropriate for transformed histology, a clinical response was achieved.
Keywords: non-small-cell lung cancer, EGFR mutation, acquired resistance, histologic transformation
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are extremely effective agents against non-small-cell lung cancer that harbors activating EGFR mutations. However, disease progression because of acquired resistance to the drugs is inevitable. For patients treated with first- or second-generation EGFR-TKIs, progression occurs about a year after treatment initiation.
Acquired EGFR exon 20 T790M mutation accounts for >50% of cases that become resistant to initial treatment with EGFR-TKIs (1). Other resistance mechanisms include the compensatory contribution of other receptor tyrosine kinases (e.g., c-MET amplification) (2, 3), activation of compensatory signaling pathways (e.g., PI3K-AKT pathway) (4, 5), and morphologic evolution of the tumor [e.g., epithelial-to-mesenchymal transition (6) or its transformation to small-cell lung cancer (SCLC) (7, 8)].
We herein report a rare case of EGFR-mutated lung adenocarcinoma that showed histologic transformation to combined SCLC and squamous cell carcinoma (SCC).
Case Report
A 70-year-old Japanese woman presented with back pain. She had never been a smoker, and her medical history was unremarkable. Her Eastern Cooperative Oncology Group performance status was zero. Chest computed tomography (CT) revealed a pulmonary mass in the right lower lobe and pleural effusion. She underwent bronchoscopy for the primary tumor; cytology revealed an adenocarcinoma harboring EGFR exon 19 deletions (Fig. 1). A further evaluation led to the diagnosis of adenocarcinoma of the right lower lobe with cT4N2M1b stage IV (UICC classification, 7th edition). She was sequentially treated with first-generation EGFR-TKIs and cytotoxic agents, including gefitinib, erlotinib, carboplatin (CBDCA) + pemetrexed + bevacizumab, docetaxel, and gemcitabine, all of which failed to halt disease progression. The patient underwent a re-biopsy of the primary lesion twice during the course, but adequate specimens for a pathological evaluation could not be obtained.
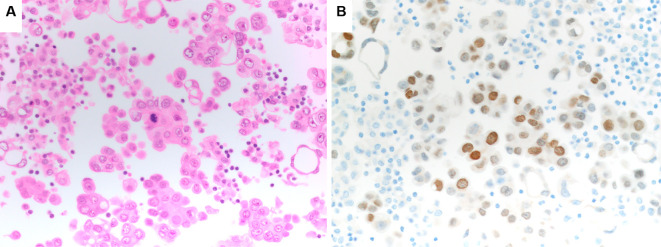
Figure 1.
A cytology specimen from a primary tumor confirmed the diagnosis of adenocarcinoma. A repeated evaluation did not reveal any component of small-cell or squamous cell lung carcinoma. (A) Hematoxylin and Eosin staining (200×). (B) Thyroid transcription factor-1 (TTF-1) (200×).
After four cycles of gemcitabine as fifth-line therapy and two years after the last EGFR-TKI treatment, disease progression with right hilar lymph node enlargement was observed (Fig. 2A-C). Endobronchial ultrasound-guided transbronchial needle aspiration of the lymph node revealed a combined tumor comprising SCLC, characterized by small cells arranged in a prominent nesting pattern with positive staining for synaptophysin; and SCC, characterized by keratin pearl formation with positive staining for p40 and cytokeratin AE1/AE3, with no evidence of adenocarcinoma histology (Fig. 3). The level of pro-gastrin-releasing peptide (ProGRP) was 83.9 pg/mL that of neuron-specific enolase (NSE) was 32.6 ng/mL, and that of carcinoembryonic antigen (CEA) was 26.1 ng/mL. A genetic analysis of the specimen showed that the exon 19 deletion was preserved, but there were no additional EGFR mutations, including none in exon 20 T790M. The PD-L1 tumor proportion score (assessed using Dako PD-L1 immunohistochemistry 22C3 pharmDx; Agilent Technologies, Santa Clara, USA) was 0%.
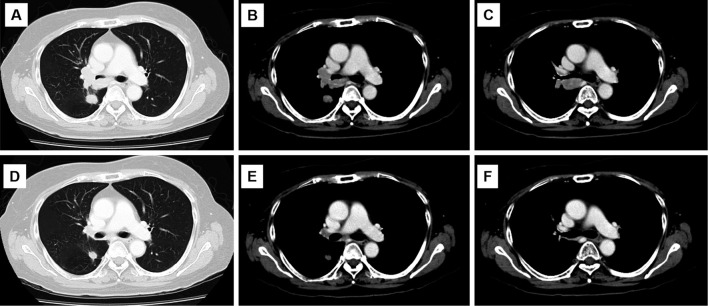
Figure 2.
Chest computed tomography at the time small-cell and squamous cell transformation was detected (A-C). After four cycles of carboplatin+etoposide (D-E).
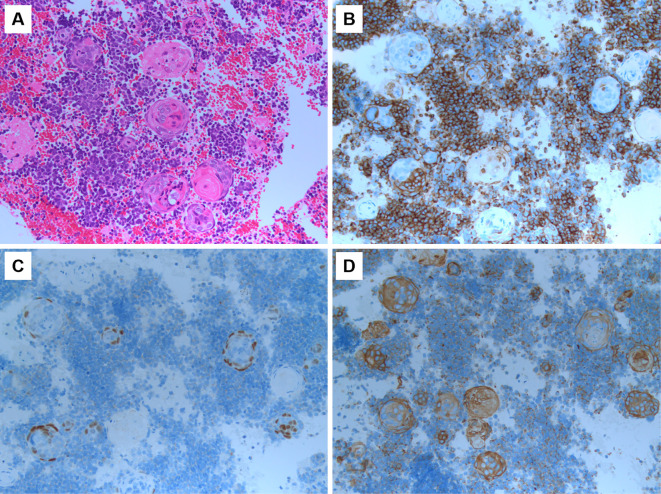
Figure 3.
Morphologic features and immunohistochemical staining of a biopsy specimen from a right hilar lymph node obtained by endobronchial ultrasound transbronchial needle aspiration confirming histologic transformation to both small-cell and squamous cell lung carcinoma. (A) Hematoxylin and Eosin staining (200×). (B) Synaptophysin (200×). (C) p40 (200×). (D) Cytokeratin AE1/AE3.
The patient was subsequently was treated with CBDCA and etoposide as sixth-line therapy; after four cycles, CT showed a partial response (Fig. 2D-F). The level of ProGRP and NSE were decreased to 67.8 pg/mL and 10.7 ng/mL, respectively. However, despite one month of watchful waiting thereafter, disease progression was noted with enlargement of brain metastases. The patient was then treated with CBDCA and nanoparticle albumin-bound paclitaxel as the seventh-line therapy; after three cycles, disease progression was noted with enlargement of brain metastases. At this time, the level of CEA rose to 147.6 ng/mL without concurrent ProGRP and NSE elevations. The patient was subsequently treated with vinorelbine as the eighth-line therapy; after four months, an ongoing response is now being observed, with shrinkage of the brain metastases, and the CEA level has decreased to 6 ng/mL.
Discussion
This patient's case suggests two important clinical points. First, EGFR-mutated lung adenocarcinoma can exhibit histologic transformation to combined SCLC and SCC as an acquired resistance mechanism. Second, cytotoxic chemotherapy appropriate for the transformed histology may be an effective salvage therapy for patients with EGFR-mutated lung adenocarcinoma who acquire resistance to EGFR-TKIs.
Histologic transformation of EGFR-mutated lung adenocarcinoma to SCLC has previously been identified as a mechanism of resistance to EGFR-TKIs (7, 8). Combined SCLC and adenocarcinoma transformation has been also detected in some tissue samples when adenocarcinoma progression was noted. Genomic sequencing of tumor samples from repeated biopsies of adenocarcinomas that progress on EGFR-TKIs has indicated that most such transformed SCLCs retain the same EGFR mutation of the primary adenocarcinoma (8). This suggests that SCLC evolves directly from the initial tumor. Recent studies have implied that adenocarcinoma may be predisposed at an early stage to transform to SCLC by inactivation of tumor suppressor genes, such as Rb and p53 (8). This is currently the only explanation of the molecular pathogenesis that has been posited. Histologic transformation to SCC has been also reported as another resistance mechanism for EGFR-TKIs (9), but its frequency is much lower than that of transformation to SCLC. At present, 13 cases of EGFR-mutated lung adenocarcinoma that transformed to SCC with or without a concomitant acquired T790M resistance mutation are listed in PubMed. Because of its rarity, little is known about the pathogenesis of transformation to SCC.
To our knowledge, this is the first case of EGFR-mutated lung adenocarcinoma that had histologic transformation to combined SCLC and SCC. Only one case of sequential occurrence of SCLC and SCC, without a detailed clinical course, has been reported (10). Possible explanations for such a finding include metaplastic transformation, the coexistence of both SCC and adenocarcinoma in the original tumor, and the development of a second primary cancer. Given the original EGFR mutation (exon 19 deletions) that was preserved even after transformation, the primary adenocarcinoma and the subsequent SCLC and SCC may have had the same origin. A metachronous second primary cancer seems less likely. The patient's course may suggest the presence of pluripotent cancer stem cells, and her history of treatment with multiple EGFR-TKIs and cytotoxic agents might have predisposed the primary adenocarcinoma to this rare histologic transformation. Intriguingly, a case of histologic transformation to SCC without prior exposure to EGFR-TKIs has been reported (11). In our case, the histologic transformation was detected some time after the last EGFR-TKI administration. This may also suggest that the underlying mechanism of resistance was distinct from EGFR inhibition. However, further interpretation is difficult due to the lack of a pathological evaluation at earlier points in the clinical course of this case.
The ideal therapeutic strategy for patients with histologic transformation as an acquired mechanism of resistance to EGFR-TKIs has not been established. For those with transformation to SCC with the concomitantly acquired T790M resistance mutation, osimertinib might be promising according to a limited number of case reports (9, 12). For those without acquired T790M, cytotoxic chemotherapy based on the new histology (SCLC or SCC) is currently the treatment option with the most experience available. In our case, salvage cytotoxic agents used to treat SCLC and non-small-cell lung cancer (NSCLC) yielded a clinical response. This suggests that cytotoxic chemotherapy may be effective for EGFR-mutated tumors that develop histologic transformation. The present findings also suggest that the level of tumor markers might help guide the treatment by implying the dominant histology. However, a systematic review indicated that current treatment strategies for primary SCLC have been largely ineffective for transformed SCLC (13). One possible interpretation of the response in our case is that the combination of transformed SCLC and SCC may behave differently from transformed SCLC alone. Of course, the future accumulation of similar cases is needed in order to reinforce this hypothesis.
In conclusion, EGFR-mutated lung adenocarcinoma might undergo histologic transformation to combined SCLC and SCC as an acquired mechanism of resistance to EGFR-TKIs. Cytotoxic chemotherapy appropriate for this transformed histology might be an effective salvage therapy. However, further investigations are warranted to better characterize this resistance mechanism and establish the optimal therapy.
Ethics approval is not applicable. Written informed consent was obtained from the patients for the publication of this case report.
Author's disclosure of potential Conflicts of Interest (COI).
Mikio Takamori: Honoraria, AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Boehringer Ingelheim Japan, Merck Sharp & Dohme, Kyorin Pharmaceutical, Kyowa Hakko Kirin, Novartis, Astellas and Qiagen.
References
- 1.Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer 84: 295-300, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Brugger W, Thomas M. EGFR-TKI resistant non-small cell lung cancer (NSCLC): new developments and implications for future treatment. Lung Cancer 77: 2-8, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Cretella D, Saccani F, Quaini F, et al. Trastuzumab emtansine is active on HER-2 overexpressing NSCLC cell lines and overcomes gefitinib resistance. Mol Cancer 13: 143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Yang Z, Li W, et al. TOPK promotes lung cancer resistance to EGFR tyrosine kinase inhibitors by phosphorylating and activating c-Jun. Oncotarget 7: 6748-6764, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res 4: 411-435, 2014. [PMC free article] [PubMed] [Google Scholar]
- 6.Sesumi Y, Suda K, Mizuuchi H, et al. Effect of dasatinib on EMT-mediated-mechanism of resistance against EGFR inhibitors in lung cancer cells. Lung Cancer 104: 85-90, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Lee JK, Lee J, Kim S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol 35: 3065-3074, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 8: 1265-1271, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Izumi H, Yamasaki A, Ueda Y, et al. Squamous cell carcinoma transformation from EGFR-mutated lung adenocarcinoma: a case report and literature review. Clin Lung Cancer 19: e63-e66, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Yao Y, Zhu Z, Wu Y, Chai Y. Histologic transformation from adenocarcinoma to both small cell lung cancer and squamous cell carcinoma after treatment with gefitinib: a case report. Medicine (Baltimore) 97: e0650, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le T, Sailors J, Oliver DH, Mayer M, Hoskin S, Gerber DE. Histologic transformation of EGFR mutant lung adenocarcinoma without exposure to EGFR inhibition. Lung Cancer 105: 14-16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno R, Proietti A, Ali G, et al. Squamous cell transformation and EGFR T790M mutation as acquired resistance mechanisms in a patient with lung adenocarcinoma treated with a tyrosine kinase inhibitor: a case report. Oncol Lett 14: 5947-5951, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca E, Pozzari M, Vermi W, et al. Outcome of EGFR-mutated adenocarcinoma NSCLC patients with changed phenotype to squamous cell carcinoma after tyrosine kinase inhibitors: A pooled analysis with an additional case. Lung Cancer 127: 12-18, 2019. [DOI] [PubMed] [Google Scholar]