Abstract
Crystalline arthropathies are well-known pathologies in a native knee; however, the literature is scarce with regards to crystalline arthropathies in a total knee arthroplasty (TKA). The presentation of crystalline arthropathy in a TKA can be similar to a periprosthetic joint infection (PJI), making it difficult to distinguish between the 2 diagnoses. We present 1 case highlighting the similarity between crystalline arthropathy and PJI. A 71-year-old man with a history of bilateral TKAs presented with bilateral painful knee effusions and was initially presumed to have PJIs; however, he was later diagnosed with gout and successfully treated medically. A complete review of the literature demonstrates that crystalline arthropathies after TKA are infrequently reported and can be difficult to decipher from PJIs, and there is a lack of standardized treatment.
Keywords: Gout, Pseudogout, Crystalline arthropathy, TKA, Periprosthetic joint infection
Introduction
Crystalline arthropathies are characterized by deposition of crystals within a synovial membrane or joint cavity. The most common crystalline arthropathies are gout and pseudogout, caused by deposition of monosodium urate and calcium pyrophosphate crystals, respectively. The typical presentation of gout and pseudogout is nearly identical, manifesting warmth, swelling, erythema, and pain within a joint [1]. Studies have reported the overall prevalence of gout to be 0.94% [2], whereas pseudogout is more prevalent, affecting up to 8.1% of people in the United States [3].
The symptoms of an acute flare of crystalline arthropathy can very closely mimic those of septic arthritis. This is of particular importance in discerning infection after total knee arthroplasty (TKA). The prevalence of periprosthetic joint infection (PJI) after primary TKA is 1%-2% [4]; however, the prevalence of crystalline arthropathy after TKA is not well documented. To our knowledge, there are only 17 cases of gout and 12 cases of pseudogout in the setting of TKA reported in the literature over the past 26 years [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. The following is a case report of an acute gouty flare after primary TKA and a review of the existing literature on crystalline arthropathy in the setting of TKA.
Case history
A 71-year-old man, who provided informed consent to this case report, presented to an outside hospital with fever, malaise, and bilateral knee pain of a few days' duration. He had undergone right and left TKA 13 and 14 years earlier, respectively. His medical history was significant for chronic kidney disease, diabetes mellitus type 2, congestive heart failure, spinal stenosis, and chronic obstructive pulmonary disease. He had no known history of gout and denied using alcohol, tobacco, or illicit drugs. He denied history of significant knee pain after placement of his TKA until his current symptoms.
He received 4 days of intravenous vancomycin and ciprofloxacin for presumed health care–associated pneumonia, as he had an infiltrate on a chest radiograph. The presence of pneumonia was later questioned as advanced imaging did not show the infiltrate. Aspirations of both knees were performed 1 day after his last dose of antibiotics, revealing a leukocyte count of 10,694/mm³ in the right knee and 1138/mm³ in the left knee. Gram stains and cultures remained negative; however, the fluid was not analyzed for crystals at that time. Because of his systemic symptoms and bilateral knee pain, swelling, and erythema, there was concern for bilateral periprosthetic knee infection, and the patient was transferred to our facility, a tertiary care hospital, for further management.
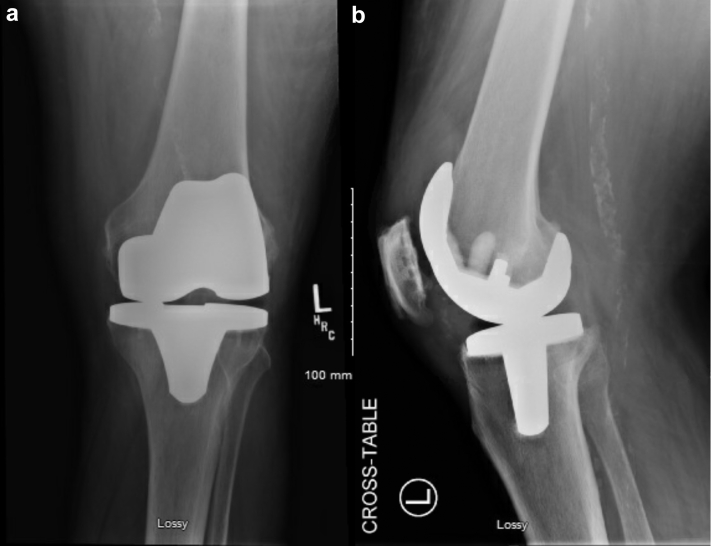
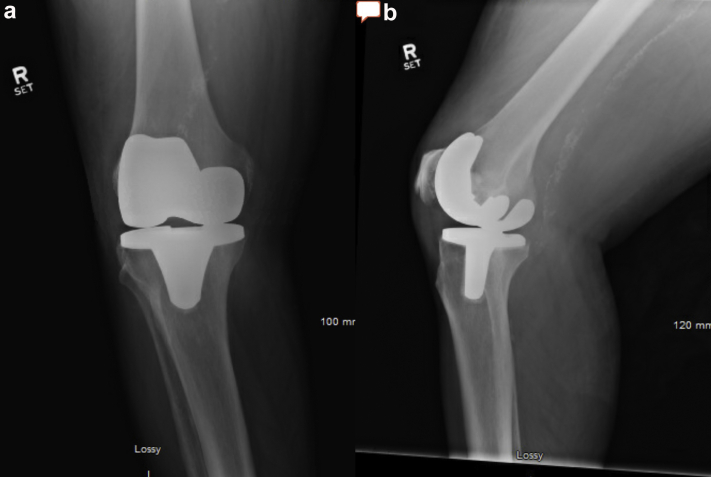
On examination, after transfer to the current hospital, he required admission to the ICU owing to frequent desaturations and altered mental status. He required supplemental oxygen and continuous positive airway pressure to maintain his oxygenation. His bilateral knees were erythematous, diffusely tender to palpation, and warm to touch. His TKA incisions were well healed bilaterally. There were effusions present bilaterally. Repeat joint aspirates (now 4 days after the last dose of antibiotics) yielded approximately 15 cc of yellow turbid fluid from each knee. The cell count was 22,594/mm³ (84% polymorphonuclear leukocytes) in the right knee and 36,016/mm³ (92% polymorphonuclear leukocytes) in the left knee. Both fluid samples were positive for monosodium urate crystals, and again cultures did not show any growth. Aerobic, anaerobic, acid-fast bacilus, and fungal cultures were incubated for a total of 4 days, 4 days, 6 weeks, and 4 weeks, respectively. The patient's erythrocyte sedimentation rate was 96 mm/h and his C-reactive protein (CRP) was >270 mg/L. He had a normal serum white blood cell (WBC) count of 8.02 × 10⁹/L. Blood and urine cultures were also drawn at the time of presentation to the hospital, and both were negative. Radiographs, seen in Figure 1, Figure 2, demonstrated well-aligned bilateral TKAs without any sign of osteolysis or loosening. There are eccentric gaps seen in the anteroposterior radiographs, which could indicate possible polyethylene wear.
Figure 1.
(a) anteroposterior and (b) lateral radiographs of the left knee at the time of presentation.
Figure 2.
(a) anteroposterior and (b) lateral radiographs of the right knee at the time of presentation.
Based on these results, he was felt to have bilateral knee gouty arthritis with or without infection. He was started on colchicine, but after 3 doses, he had a 1.3-point increase in his creatinine and was subsequently switched to prednisone 40 mg daily for a total of 10 days. After 1 day of treatment, he also was transferred out of the ICU to the floor as his respiratory status improved. He was later diagnosed with an acute COPD exacerbation. By the fourth day of gout treatment, he stated that his bilateral knee pain was mostly resolved and he was ambulating with physical therapy. Two weeks after his treatment concluded, he reported no knee pain bilaterally, thereby favoring a diagnosis of aseptic gout. This was reconfirmed at 15 months when he reported no pain at his right or left knee. He was able to ambulate with a walker for up to 1 block but would have to rest due to back pain and leg weakness.
Discussion
There are 17 reported cases of gout and 12 reported cases of pseudogout after TKA in the literature, totaling 28 patients. Thirteen patients were male and 15 were female. The average patient age was 68 years (range, 39 to 88 years), and the interval time between TKA and presenting symptoms of crystalline arthropathy flare varied widely, ranging from 3 days to 20 years. Twenty patients had a documented history of the presence or absence of crystalline arthropathy: 11 reporting a prior history and 9 no prior history of crystalline arthropathy. There were 4 cases of newly diagnosed crystalline arthropathy that also had a coincident PJI in the same knee. These patients had varying presentations, with a wide spectrum of mild to severe illness on presentation. Eleven patients were treated surgically with a debridement and polyethylene exchange, including all 4 with coexisting septic arthritis. The remaining 17 patients were treated medically without surgery. All 24 patients without coexisting septic arthritis had resolution of their symptoms, with 1 patient experiencing occasional flares while off his gout medication. The mean follow-up was 54 weeks, ranging from 2 days to 8 years [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. See Table 1.
Table 1.
Characteristics and treatment in all reported patients with crystalline arthropathy in TKA.
| Author | Age (yr) | Sex | Time after TKA | Affected side | History of gout/pseudogout | Diagnosis | Associated infection | Surgical treatment? | Medical treatment | Final follow-up | Successful outcome?a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Williamson et al. [5] | 86 | F | 10 y | Left | N/a | Gout | No | Y | Colchicine, allopurinol | 1 y | Y |
| Blyth et al. [6] | 58 | M | 4 y | Right | Y | Gout | Staphylococcus aureus | Y | Antibiotics | N/a | N |
| 52 | M | 3 d | Right | Y | Gout | No | N | NSAIDs, colchicine, allopurinol | 1 y | Y | |
| Archibeck et al. [7] | 66 | M | 10 and 12 y | Bilateral | N | Gout | No | Y | Colchicine, allopurinol | 5 mo | Y |
| 88 | F | 9 y | Left | Y | Gout | No | Y | Colchicine | 4 mo | Y | |
| Crawford et al. [8] | 59 | F | 3 mo | Left | N | Gout | No | N | NSAIDs, allopurinol | 1 y | Y |
| Salin et al. [9] | 50s | F | 4 and 4 y | Bilateral | N | Gout | No | Y | Allopurinol, steroids | 1 mo | Y |
| 80s | F | 11 y | Left | Y | Gout | Escherichia coli | Y | Antibiotics, NSAIDs | N/a | Y | |
| Berger et al. [10] | 39 | M | 4 y | Right | Y | Gout | No | Y | NSAIDs, colchicine | N/a | Y |
| Fokter et al. [11] | 68 | M | 11 mo | Left | N/a | Gout | No | N | NSAIDs, allopurinol | 1 wk | Y |
| Freehill et al. [12] | 77 | F | 10 y | Right | Y | Gout | Escherichia coli | Y | Antibiotics | 8 wk | Y |
| Partridge et al. [15] | 66 | F | 8 y | Right | Y | Gout | No | Y | Antibiotics, steroids | 1 y | Y |
| Chen et al. [14] | 43 | M | 7 y | Left | Y | Gout | No | N | Colchicine, allopurinol, steroids | 1 y | Y |
| 72 | M | 10 y | Right | Y | Gout | No | N | Steroids | 5 mo | Y | |
| Yahia et al. [19] | 72 | F | 31 d | Left | N/a | Gout | No | N | Colchicine | N/a | N/a |
| Zadaka et al. [13] | 58 | F | 7 y | Right | N | Gout | No | N | Steroids, allopurinol | 2 d | Y |
| 77 | M | 13 and 20 y | Bilateral | N | Gout/Pseudogout | No | N | Anitibiotics | "several days" | Y | |
| Levi et al. [16] | 70 | F | 8 y | Bilateral | N/a | Pseudogout | No | Y | Antibiotics, steroids | 16 mo | Y |
| Koyama et al. [17] | 64 | F | 13 d | Right | N | Pseudogout | No | N | NSAIDs | 1 y | Y |
| 81 | M | 2 wk | Left | N | Pseudogout | No | N | NSAIDs | 1 y | Y | |
| Harato et al. [18] | 85 | M | 15 d | Right | Y | Pseudogout | No | N | NSAIDs | 1 y | Y |
| Yahia et al. [19] | 79 | M | 7 d | Right | N/a | Pseudogout | No | N | Colchicine | N/a | N/a |
| 73 | F | 3 y | Right | N/a | Pseudogout | No | N | NSAIDs | N/a | N/a | |
| 67 | M | 9 y | Right | N/a | Pseudogout | No | N | Steroids, colchicine | N/a | N/a | |
| 77 | F | 15 d | Right | N/a | Pseudogout | Campylobacter fetus | N | Antibiotics | N/a | N/a | |
| Holt et al. [20] | 72 | M | 2 y | Left | N | Pseudogout | No | Y | NSAIDs | 1 wk | Y |
| Hirose et al. [21] | 59 | F | 7 d | Right | Y | Pseudogout | No | N | NSAIDs | 1 y | Y |
| Sonsale et al. [22] | 69 | F | 10 y | Right | N | Pseudogout | No | N | NSAIDs | 8 y | Y |
NSAIDs, nonsteroidal anti-inflammatory drugs.
Successfwl outcome defined as no recurrence of presenting symptoms.
Distinguishing between crystalline arthropathy and PJI in a patient who presents with a warm, painful, erythematous prosthetic knee can be challenging. They share many clinical features, including clinical presentation, serum laboratory values, and synovial fluid characteristics.
Routinely obtaining microscopic crystal analysis from knee joint aspiration is a simple measure to diagnose crystalline arthropathy in a TKA. Upon review of previously published cases, 4 of 4 patients who did not undergo preoperative crystal analysis were taken to the operating room for a debridement, though all 4 patients demonstrated crystals on intraoperative tissue analysis and were ultimately thought to be aseptic. Another potential problem in not identifying crystals, when actually present, is that of disease recurrence, which may confuse the diagnostician at the time of representation, raising suspicion for recurrence of infection. Rothenbacher et al. [23] demonstrated that 37% of patients who had a gout flare in a native joint had at least one episode of recurrence. As 1 case report highlights, recurrent flares are also possible in TKA [14].
Parvizi and colleagues recently published validated, evidence-based criteria for the diagnosis of PJI. They outline major and minor criteria to diagnose PJI. The major criteria include the presence of a sinus tract communicating with the prosthesis, and a pathogen isolated by culture from 2 separate samples from the affected joint. The minor criteria incorporate serum markers such as erythrocyte sedimentation rate, D-dimer, and CRP. It also includes synovial markers such as WBC, leukocyte esterase, alpha-defensin, polymorphonuclear leukocyte percentage, and CRP. The minor criteria each have a point value assigned to them, and a combined score of ≥6 means infected, 2-5 is possibly infected and requires further investigation, and <2 is not infected. However, the authors reported likely inaccuracy in the presence of crystalline arthropathy, and the focus was on chronic infections, defined as infections occurring greater than 3 months after index arthroplasty, with symptoms lasting longer than 6 weeks [24]. Acute hematogenous PJIs, as what was suspected in the current case, have also been studied, often demonstrating synovial WBC levels >10,000 (in contrast to the 3000 cutoff in chronic PJI) [25], although validated diagnostic criteria are not yet available.
The current patient was initially presumed to have an acute PJI and transferred to a tertiary care facility for treatment. His knee aspiration cultures remained negative, although multiple authors have described negative cultures in the presence of other clear markers of infection, such as intraoperative purulence, a sinus tract, or acute inflammation on histopathologic analysis of periprosthetic tissue samples [[26], [27], [28], [29], [30], [31]]. Shanmugasundaram et al. [28] reported a false negative rate of 46% of preoperative aspiration cultures relative to intraoperative cultures in periprosthetic knee infections. It has been recommended that antibiotics be stopped for a minimum of 2 weeks before obtaining culture in the workup of PJI [4]. In the current case, antibiotics had been given 1 day before his first knee aspiration, potentially confounding the negative result. Trampuz et al. [32] found that culture sensitivities decreased to 47.8% in patients who received antibiotics within 2 weeks of culture acquisition.
There are newer tests that are potential solutions in helping to discern between PJI and crystalline arthropathy. Next-generation sequencing and polymerase chain reaction can identify specific or broad ranges of pathogens in culture-negative PJIs [33,34]. Other recently described diagnostic tools for PJI include alpha-defensin and leukocyte esterase; however, these tests may not clearly distinguish between crystalline arthropathy and PJI, as they are proteins secreted by neutrophils, which are present in both conditions [[35], [36], [37]]. This is highlighted by Partridge et al. [15], who reported a case of gout after TKA, found to have positive alpha-defensin on workup, but no evidence of infection. There is also increasing utilization of other biomarkers in diagnosing PJIs, such as interleukin-6 and tumor necrosis factor-α [38]. We are hopeful that newer tests, such as next-generation sequencing, polymerase chain reaction, and other biomarkers, may prove effective and become more widespread to distinguish PJI and crystalline arthropathy.
If a diagnosis of crystalline arthropathy is suspected in a knee that has previously undergone TKA, there is no published consensus regarding the best method of treatment. The current patient was treated initially with colchicine; however, this likely led to acute kidney injury, necessitating a change in therapy to prednisone, days after which his symptoms resolved. The existing literature demonstrates multiple cases of crystalline arthropathy after TKA that have been successfully treated medically with colchicine, allopurinol, nonsteroidal anti-inflammatory drugs, or corticosteroids [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. As the reported cases of crystalline arthropathy in TKA are so infrequent, especially when compared to periprosthetic knee infections, the authors would recommend caution before excluding the diagnosis of infection. Patients with TKA and crystalline arthropathy should be monitored closely during treatment to ensure their symptoms resolve.
Given the known sequelae of PJI, the astute orthopaedic surgeon might ask if distinguishing between PJI and crystalline arthropathy is futile, favoring a low threshold to surgically treat any red, painful, and warm prosthetic knee. Is there harm in treating all potential crystalline arthropathies after TKA as if they were infected? Potential risks of unnecessary operative intervention include increased cost [39], exposure to anesthetic, particularly if comorbidities are present [40], the psychosocial burden to the patient [41], and the risk of introducing infection into an aseptic knee, as revision surgery has been shown to carry increased infection risk [42,43]. On the contrary, delaying operative debridement of PJI may result in a missed window of opportunity for retention of nonmodular components, resulting in a more invasive 1- or 2-stage exchange.
The limitations of this study include the small series of reported cases. With small series of patients, all from case reports, it is difficult to draw significant conclusions in regards to diagnosis and treatment. We also acknowledge the limitation in regards to timing of antibiotics in relation to the cultures obtained in our case report. This could potentially confound the results of the culture and lead to a false-negative result.
Summary
Crystalline arthropathy in TKA is infrequently reported in the literature, and it can present in a very similar manner to a PJI. We present 1 case where a patient was originally presumed to have a knee PJI; however, on further workup, he was diagnosed with bilateral gouty arthropathy in his TKA and treated medically with success. There is a need for additional research and creation of guidelines in the diagnosis and treatment of periprosthetic crystalline arthropathy. In patients presenting with a warm, painful, erythematous prosthetic knee, we recommend routine analysis of knee joint aspiration for crystals, and if positive, treating medically under close observation until complete resolution of symptoms. To avoid missing a coexisting PJI, as these far outnumber the reported cases of crystalline arthropathies in TKA, cultures should be obtained before antibiotic therapy and monitored closely for at least 2 weeks [4]. Clinicians should have a high index of suspicion for a PJI, but if there is an absence of infection, the patient can be treated medically with good results.
Conflict of interest
The authors declare there are no conflicts of interest.
Appendix A. Supplementary data
References
- 1.Singh N., Vogelgesang S.A. Monoarticular arthritis. Med Clin North Am. 2017;101(3):607. doi: 10.1016/j.mcna.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Roddy E., Choi H.K. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155. doi: 10.1016/j.rdc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abhishek A., Doherty M. Epidemiology of calcium pyrophosphate crystal arthritis and basic calcium phosphate crystal arthropathy. Rheum Dis Clin North Am. 2014;40:177. doi: 10.1016/j.rdc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J., Erkocak O.F., Della Valle C.J. Culture-negative periprosthetic joint infection. J Bone Joint Surg. 2014;96:430. doi: 10.2106/JBJS.L.01793. [DOI] [PubMed] [Google Scholar]
- 5.Williamson S.C., Roger D.J., Petrera P., Glockner F. Acute gouty arthropathy after total knee arthroplasty. A case report. J Bone Joint Surg Am. 1994;76:126. doi: 10.2106/00004623-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Blyth P., Pai V.S. Recurrence of gout after total knee arthroplasty. J Arthroplasty. 1999;14:380. doi: 10.1016/s0883-5403(99)90067-0. [DOI] [PubMed] [Google Scholar]
- 7.Archibeck M.J., Rosenberg A.G., Sheinkop M.B., Berger R.A., Jacobs J.J. Gout-induced arthropathy after total knee arthroplasty: a report of two cases. Clin Orthop Relat Res. 2001;(392):377. doi: 10.1097/00003086-200111000-00049. [DOI] [PubMed] [Google Scholar]
- 8.Crawford L., Kumar A., Shepard G.J. Gouty synovitis after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong) 2007;15(3):384. doi: 10.1177/230949900701500330. [DOI] [PubMed] [Google Scholar]
- 9.Salin J.W., Lombardi A.V.J., Berend K.R., Chonko D.J. Acute gouty arthropathy after total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2008;37(8):420. [PubMed] [Google Scholar]
- 10.Berger J.S., Weinik M.M. Acute gouty arthropathy mimicking infection after total knee arthroplasty. PM R. 2009;1(3):284. doi: 10.1016/j.pmrj.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Fokter S.K., Repse-Fokter A. Acute gouty arthritis in a patient after total knee arthroplasty. Wien Klin Wochenschr. 2010;122(11–12):366. doi: 10.1007/s00508-010-1384-3. [DOI] [PubMed] [Google Scholar]
- 12.Freehill M.T., McCarthy E.F., Khanuja H.S. Total knee arthroplasty failure and gouty arthropathy. J Arthroplasty. 2010;25(4):658.e7. doi: 10.1016/j.arth.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Zadaka A., Gioe T., Gertner E. Acute crystal-induced arthritis following arthroplasty. J Knee Surg. 2010;23(1):17. doi: 10.1055/s-0030-1262903. [DOI] [PubMed] [Google Scholar]
- 14.Chen F., Glezos C., Blum Y., Hossack M., Schwechter E.M. Nonsurgical treatment of aseptic periprosthetic gout flare of the knee: a report of 2 cases. JBJS Case Connect. 2016;6(4):e93. doi: 10.2106/JBJS.CC.16.00076. [DOI] [PubMed] [Google Scholar]
- 15.Partridge D.G., Gordon A., Townsend R. False-positive synovial fluid alpha-defensin test in a patient with acute gout affecting a prosthetic knee. Eur J Orthop Surg Traumatol. 2017;27(4):549. doi: 10.1007/s00590-017-1942-8. [DOI] [PubMed] [Google Scholar]
- 16.Levi G.S., Sadr K., Scuderi G.R. Bilateral pseudogout 8 years after bilateral total knee arthroplasty. Orthop Clin North Am. 2012;43(5):e59. doi: 10.1016/j.ocl.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Koyama K., Ohba T., Sato H., Haro H. Pseudogout mimicking infection following total knee arthroplasty: a report of two cases. JBJS Case Connect. 2012;2(1):e3. doi: 10.2106/JBJS.CC.K.00049. [DOI] [PubMed] [Google Scholar]
- 18.Harato K., Yoshida H. Pseudogout in the early postoperative period after total knee arthroplasty. J Arthroplasty. 2013;28(2):374.e9. doi: 10.1016/j.arth.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Yahia S.A., Zeller V., Desplaces N. Crystal-induced arthritis after arthroplasty: 7 cases. Joint Bone Spine. 2016;83(5):559. doi: 10.1016/j.jbspin.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Holt G., Vass C., Kumar C.S. Acute crystal arthritis mimicking infection after total knee arthroplasty. BMJ. 2005;331(7528):1322. doi: 10.1136/bmj.331.7528.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirose C.B., Wright R.W. Calcium pyrophosphate dihydrate deposition disease (pseudogout) after total knee arthroplasty. J Arthroplasty. 2007;22(2):273. doi: 10.1016/j.arth.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Sonsale P.D., Philipson M.R. Pseudogout after total knee arthroplasty. J Arthroplasty. 2007;22(2):271. doi: 10.1016/j.arth.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Rothenbacher D., Primatesta P., Ferreira A., Cea-Soriano L., Rodriguez L.A.G. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology (Oxford) 2011;50(5):973. doi: 10.1093/rheumatology/keq363. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J., Tan T.L., Goswami K. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 25.Konigsberg B.S., Della Valle C.J., Ting N.T., Qiu F., Sporer S.M. Acute hematogenous infection following total hip and knee arthroplasty. J Arthroplasty. 2014;29(3):469. doi: 10.1016/j.arth.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Gallo J., Kolar M., Dendis M. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97. [PubMed] [Google Scholar]
- 27.Gomez E., Cazanave C., Cunningham S.A. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50(11):3501. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanmugasundaram S., Ricciardi B.F., Briggs T.W.R., Sussmann P.S., Bostrom M.P. Evaluation and management of periprosthetic joint infection-an international, multicenter study. HSS J. 2014;10(1):36. doi: 10.1007/s11420-013-9366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berbari E.F., Marculescu C., Sia I. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007 Nov;45(9):1113. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 30.Font-Vizcarra L., Garcia S., Martinez-Pastor J.C., Sierra J.M., Soriano A. Blood culture flasks for culturing synovial fluid in prosthetic joint infections. Clin Orthop Relat Res. 2010;468(8):2238. doi: 10.1007/s11999-010-1254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghanem E., Parvizi J., Clohisy J., Burnett S., Sharkey P.F., Barrack R. Perioperative antibiotics should not be withheld in proven cases of periprosthetic infection. Clin Orthop Relat Res. 2007;461:44. doi: 10.1097/BLO.0b013e318065b780. [DOI] [PubMed] [Google Scholar]
- 32.Trampuz A., Piper K.E., Jacobson M.J. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 33.Tarabichi M., Shohat N., Goswami K. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am. 2018;100:147. doi: 10.2106/JBJS.17.00434. [DOI] [PubMed] [Google Scholar]
- 34.Melendez D.P., Greenwood-Quaintance K.E., Berbari E.F. Evaluation of a genus-and group-specific rapid PCR assay panel on synovial fluid for diagnosis of prosthetic knee infection. J Clin Microbiol. 2016;54:120. doi: 10.1128/JCM.02302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone W.Z., Gray C.F., Parvataneni H.K. Clinical evaluation of synovial alpha defensin and synovial C-reactive protein in the diagnosis of periprosthetic joint infection. J Bone Joint Surg Am. 2018;100(14):1184. doi: 10.2106/JBJS.17.00556. [DOI] [PubMed] [Google Scholar]
- 36.Kelly M.P., Darrith B., Hannon C.P., Nam D., Courtney P.M., Della Valle C.J. Synovial fluid alpha-defensin is an adjunctive tool in the equivocal diagnosis of periprosthetic joint infection. J Arthroplasty. 2018;33(11):3537. doi: 10.1016/j.arth.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y.S., Koo K.-H., Kim H.J. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99(24):2077. doi: 10.2106/JBJS.17.00123. [DOI] [PubMed] [Google Scholar]
- 38.Gollwitzer H., Dombrowski Y., Prodinger P.M. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95:644. doi: 10.2106/JBJS.L.00205. [DOI] [PubMed] [Google Scholar]
- 39.Kamath A.F., Ong K.L., Lau E. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492. doi: 10.1016/j.arth.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Bilimoria K.Y., Liu Y., Paruch J.L. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahill J.L., Shadbolt B., Scarvell J.M., Smith P.N. Quality of life after infection in total joint replacement. J Orthop Surg (Hong Kong) 2008;16(1):58. doi: 10.1177/230949900801600115. [DOI] [PubMed] [Google Scholar]
- 42.Nikolaus O.B., McLendon P.B., Hanssen A.D., Mabry T.M., Berbari E.F., Sierra R.J. Factors associated with 20-year cumulative risk of infection after aseptic index revision total knee arthroplasty. J Arthroplasty. 2016;31(4):872. doi: 10.1016/j.arth.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Peersman G., Laskin R., Davis J., Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.