Abstract
Osteoarthritis (OA) is a chronic joint disorder that causes degeneration of cartilage, synovial inflammation, and formation of osteophytes. Aging, obesity, and sex are considered the main risk factors of OA. Recent studies have suggested that metabolic syndrome (MetS) disorders, such as hypertension, hyperlipidemia, diabetes mellitus, and obesity, may be involved in the pathogenesis and progression of OA. MetS disorders are common diseases that also result in atherosclerosis. Researchers believe that OA and atherosclerosis have underlying similar molecular mechanisms because the prevalence of both diseases increases with age. Oxidation of low-density lipoprotein (ox-LDL) is believed to play a role in the pathogenesis of atherosclerosis. Recent reports have shown that ox-LDL and low-density lipoprotein receptor 1 (LOX-1) are involved in the pathogenesis of OA. The purpose of this narrative review is to summarize the current understanding of the role of the LOX-1/ox-LDL system in the pathogenesis of OA and to reveal common underlying molecular pathways that are shared by MetS in OA and the LOX-1/ox-LDL system.
Keywords: Osteoarthritis, low-density lipoprotein, oxidation, pathogenesis, atherosclerosis, metabolic syndrome
Introduction
Osteoarthritis (OA) is a major and common disease with a prevalence that is continuously increasing because of global aging.1 The number of patients with knee OA in Japan is estimated to be approximately 25 million (ROAD study).2 Generally, treatment of end-stage OA requires surgery, such as total knee arthroplasty. The medical costs of arthroplasty have been increasing worldwide.3,4 Until recently, conservative and curative treatments for OA have not been determined. Currently, clarifying the cause of OA and developing fundamental treatment strategies are critical.
OA was originally considered to be caused by mechanical stress due to obesity.5 Therefore, the cause of OA was believed to be mainly wear and tear of chondrocytes by mechanical stress. Interestingly, recent studies have shown that metabolic syndrome (MetS) disorders are involved in the pathogenesis and progression of OA.6 Some studies have also suggested involvement of lipid peroxidation in the pathogenesis of degeneration of articular cartilage.7–9 Hypercholesterolemia, which causes atherosclerosis (AS), has been reported to be associated with the risk of knee OA10 independently of obesity. Investigations to clarify the factors and mechanisms underlying AS and OA are currently underway. In this narrative review, we address the evidence underlying the molecular mechanisms of hyperlipidemia and AS in the pathogenesis of OA in detail.
Epidemiology of the association of dyslipidemia and OA
Epidemiological studies have shown that hypercholesterolemia, which is a representative disease of dyslipidemia, is a risk factor of knee and hand OA.11,12 A cross-sectional study also showed a correlation between hypercholesterolemia and knee OA.13 A case–control study suggested that serum cholesterol was an independent systemic risk factor for OA.14 Furthermore, a cohort study showed a correlation between high serum cholesterol levels and generalized OA.15 Oliviero et al.16 demonstrated that patients with OA have the highest concentrations of apolipoprotein (APO) A-I and total cholesterol compared with controls. Interestingly, Zhang et al.17 suggested that dyslipidemia is associated with a higher risk of lumbar disc herniation. These authors also suggested that serum lipid levels could be useful predictors of intervertebral disc degradation in a Chinese population. These studies highlight the relationship between dyslipidemia and OA.
Possible involvement of cholesterol in the pathogenesis of OA
Serum levels of low-density lipoprotein (LDL) are elevated in hypercholesterolemia. LDL is a major group of lipoproteins that transport molecules with cholesterol esters in a hydrophilic shell of phospholipids, triglycerides, APO, and free cholesterol.18
A recent study that used ApoE knockout mice showed that increased synovial inflammation and accelerated ectopic bone spur formation during experimental OA were derived from high LDL levels.19 Another study showed that elevated LDL levels induced OA-like changes in an inflammatory OA model in mice with a cholesterol-rich diet or LDL receptor deficiency.20 This study demonstrated that synovial production of pro-inflammatory cytokines was increased in mice with high LDL levels. Furthermore, this study showed that synovial activation was significantly increased in response to high LDL levels. A further study in ApoE knockout mice showed the effects of hypercholesterolemia on progression of OA.21 Degradative OA symptoms were reported in rats with destabilized knees that were fed a high-cholesterol diet compared with controls.7 Taken together, these results emphasize the importance of cholesterol in the pathogenesis of OA. The effects of statins on progression of knee or hip OA have been investigated, but the results are conflicting.22,23 Therefore, while there appears to be a relationship between cholesterol and OA, the effectiveness of statin treatment in OA has not been fully determined.
Association between AS and OA
Recent studies have shown that cardiovascular disease and OA also have a clinical relationship.24 Belen et al.25,26 showed a significant relationship between femoral plaques, carotid intima-media thickness, and hand OA. Cemeroglu et al.27 also reported a significant relationship between severe coronary stenosis and hand OA. An association between knee OA and the severity of AS was also suggested by Ekim et al.28 Few cohort studies have demonstrated that hand, knee, and hip OA are associated with AS.29–31 An association between progression of OA and AS has been previously reported.32,33 Some studies have reported that the correlation between OA and AS is more pronounced in female than in male patients.26,29 Recent studies have demonstrated that there is a correlation between AS and cardiovascular disease,34 and that cardiovascular disease and OA have some common risk factors.24,34
Chronic inflammation and aging are some of the common key factors between AS and OA.35 “Inflammaging” refers to combined inflammation and aging, and is defined as inflammation according to aging.36 Inflammaging also refers to chronic inflammation observed histologically.37 In fact, OA and AS have common molecular mediators that are induced by some inflammatory cytokines.24
Role of LDL receptor 1 and oxidation of LDL in the pathogenesis of AS
The most likely major contributing factor for the incidence of atherosclerotic lesions is an imbalance between cholesterol influx and efflux within tissues.38 Transport of cholesterol is regulated by several cell surface receptors, including CD36, ABCA1, and scavenger receptor class B type I.39 CD36 has been recognized as a binding and internalizing factor in oxidation of low-density lipoprotein (ox-LDL) and a wide range of ligands, such as long-chain fatty acids, apoptotic cells, anionic phospholipids, and other altered LDLs.40 CD36 also plays a role as a governing scavenger receptor in ox-LDL uptake and recognizes lipid moieties in ox-LDL.
Macrophages and phagocytes perform ox-LDL in the walls of blood vessels. Subsequently, they appear as characteristic foam cells.41 However, ox-LDL results in endothelial dysfunction.42,43
A receptor of ox-LDL—designated low-density lipoprotein receptor 1 (LOX-1)—was cloned from cultured bovine vascular endothelial cells.44 LOX-1 has a type II membrane protein structure with a long C-terminal extracellular domain and a short N-terminal cytosolic domain. LOX-1 is also distinct from the types 1 and 2 scavenger receptors, such as CD36 and CD68, respectively.45 Although the potential role of this receptor in atherogenesis is still unknown, ox-LDL uptake via this receptor, which is expressed in the vascular endothelium, has been suggested to be involved in endothelial dysfunction in atherogenesis.43 Therefore, LOX-1 and ox-LDL have a crucial role in the pathogenesis of AS caused by hypercholesterolemia.
Evidence for involvement of LOX-1/ox-LDL in OA
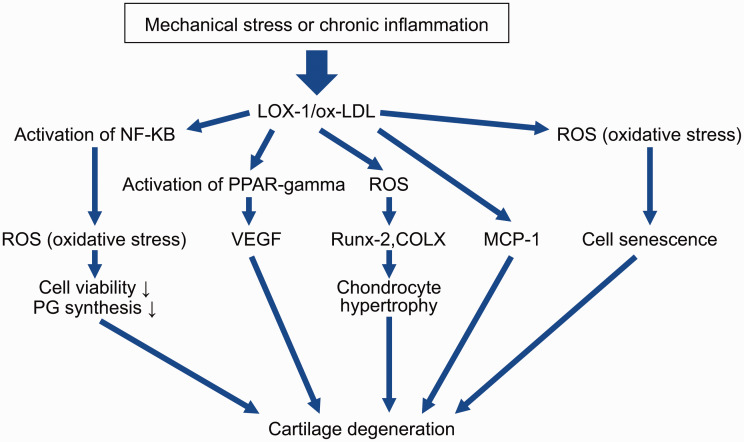
For more than 10 years, several studies have indicated possible involvement of LOX-1/ox-LDL in the pathogenesis of degeneration of cartilage. In their pioneer study, Nakagawa et al.46 reported LOX-1 expression in articular chondrocytes in a rat arthritis model and the presence of ox-LDL in articular cartilage. Furthermore, these authors found LOX-1 mRNA and protein expression in cultured rat chondrocytes in basal culture conditions and enhanced treatment with interleukin (IL)-1β and ox-LDL.47 Additionally, ox-LDL dose-dependently reduced viability of chondrocytes, which induced non-apoptotic cell death.47 These observations suggest that ox-LDL and its receptor LOX-1 are significant regulators of degradation of cartilage. Kakinuma et al.48 reported ox-LDL in human synovial fluid and expression of LOX-1 and ox-LDL in human rheumatoid arthritis cartilage. Their study was the first to indicate possible involvement of LOX-1 and ox-LDL in synovial fluid. Although these studies indicate possible involvement of the LOX-1/ox-LDL system in degeneration of cartilage, they mainly show that the LOX-1/ox-LDL system is involved in degeneration of cartilage in rheumatoid arthritis and not in OA. We found that ox-LDL binding to LOX-1 increased production of intracellular reactive oxygen species (ROS) in bovine articular chondrocytes (BACs) (Table 1).49 A novel finding of this study was that ROS induced by the LOX-1/ox-LDL system was involved in the pathogenesis of OA. Additionally, LOX-1/ox-LDL mRNA and protein expression induced by mechanical stress resulted in proteoglycan synthesis and decreased cell viability in BACs.50 Furthermore, we suggested that binding of ox-LDL with LOX-1 upregulates vascular endothelial growth factor (VEGF) mRNA and protein expression in BACs via activation of peroxisome proliferator-activated receptor (PPAR)-gamma.51 Our research team, along with Simopoulou et al.,52,53 showed LOX-1 and ox-LDL mRNA and protein expression in human articular chondrocytes. Our findings also suggested that ox-LDL enhanced monocyte chemotactic protein 1 (MCP-1) mRNA and protein expression in human articular chondrocytes and supported the hypothesis that ox-LDL is involved in degeneration of articular cartilage.54 We also attempted to clarify the role of the LOX-1/ox-LDL system in the pathogenesis of OA induced by aging, and we proposed that the LOX-1/ox-LDL system is involved in premature senescence in chondrocytes by suppressing telomerase activity.55 In a subsequent study, we clarified that the LOX-1/ox-LDL system promoted a hypertrophic chondrocyte-like phenotype through oxidative stress in cultured BACs.56 Interestingly, these findings indicate that oxidative stress induced by LOX-1/ox-LDL is involved in the endochondral ossification process in the pathogenesis of OA. However, these findings were based on an in vitro study using BACs or articular human chondrocytes and not on an in vivo study. As a next step, Ishikawa et al.57 attempted to confirm whether LOX-1 could be considered a biomarker of arthritis using human arthritis chondrocytes and a murine arthritis model. These authors suggested that serum LOX-1 could be a useful biomarker for diagnosis of arthritis and evaluation of disease activity in arthritis. Furthermore, their results indicated that LOX-1 may be a potent therapeutic target for arthritis. However, these findings have more implications for rheumatoid arthritis than for OA. We also recently attempted to clarify the role of the LOX-1/ox-LDL system in the pathogenesis of OA in an in vivo study in mice. We found that LOX-1 knockout mice showed resistance in a murine knee OA model by destabilizing the medial meniscus.58 This study suggests that the LOX-1/ox-LDL system is involved in the in vivo pathogenesis of OA induced by mechanical stress. In a subsequent in vivo study, we found that the LOX-1/ox-LDL system was involved in the pathogenesis of murine age-related OA via endochondral ossification.59 Recently, using a zymosan-induced murine arthritis model, we successfully demonstrated that the LOX-1/ox-LDL system was involved in the pathogenesis of inflammatory OA.60 Therefore, in vitro and in vivo studies indicate involvement of the LOX-1/ox-LDL system in OA. Recently, Xinhua et al.61 showed possible involvement of the LOX-1/ox-LDL system in pathogenesis and progression of human intervertebral disc degeneration or herniation. These results indicate that the LOX-1/ox-LDL system is strongly involved in the pathogenesis of OA (Table 1, Figure 1). Interestingly, these findings show that the LOX-1/ox-LDL system is involved in OA through oxidative stress.
Table 1.
Effects of LOX-1/ox-LDL interaction in articular chondrocytes.
| ref Author | Year | Material and methods (OA model) | Conclusion(s) | |
|---|---|---|---|---|
| 49 Nishimura S, et al. | 2004 | BACs | LOX-1/ox-LDL in BACs increase production of ROS and activate nuclear factor kappa B. | |
| 50 Akagi M, et al. | 2004 | Cyclic tensile stretch load of BACs | There are synergistic effects of cyclic tensile stretch load and ox-LDL on cell viability and proteoglycan synthesis in chondrocytes, which may be mediated through enhanced expression of LOX-1. | |
| 51 Kanata S, et al. | 2006 | BACs | The LOX-1/ox-LDL system upregulates VEGF expression in articular cartilage. | |
| 52 Akagi M, et al. | 2007 | Human cartilage in OA | ox-LDL significantly reduces chondrocyte viability and proteoglycan synthesis. | |
| 53 Simopoulou T, et al. | 2007 | Osteoarthritic articular cartilage | LOX-1 may be involved in the progression and pathogenesis of OA. | |
| 54 Akagi M, et al. | 2009 | HACs | ox-LDL is involved in degeneration of cartilage through MCP-1 expression. | |
| 55 Zushi S, et al. | 2009 | BACs | The LOX-1/ox-LDL system induces stress-induced premature senescence of chondrocytes and this results in suppression of telomerase activity. | |
| 56 Kishimoto H, et al. | 2010 | BACs | Binding of ox-LDL to LOX-1 induces a hypertrophic chondrocyte-like phenotype through oxidative stress, indicating that ox-LDL plays a role in degeneration of cartilage. | |
| 57 Ishikawa M, et al. | 2012 | Human chondrocytes and mice | The LOX-1 signal is a potent biomarker and therapeutic target for human arthritis. | |
| 58 Hashimoto K, et al. | 2016 | Joint instability-induced model of OA with LOX-1 KO mice | The LOX-1/ox-LDL system plays a role in the pathogenesis of instability-induced OA via endochondral ossification. | |
| 59 Hashimoto K, et al. | 2017 | Aging OA model with LOX-1 KO mice | The LOX-1/ox-LDL system in chondrocytes plays a role in the pathogenesis of age-related OA. | |
| 60 Li X, et al. | 2017 | Degenerated human IVDs | Increased accumulation of LOX-1/ox-LDL in IVDs induces a role of the receptor–ligand interaction in herniation or degeneration of IVDs. | |
| 61 Hashimoto K, et al. | 2018 | ZIA-induced arthritis with LOX-1 KO mice | The LOX-1/ox-LDL system is involved in murine development of ZIA. |
LOX-1, low-density lipoprotein receptor 1; ox-LDL, oxidation of low-density lipoprotein; OA, osteoarthritis; BACs, bovine articular chondrocytes; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; HACs, human articular chondrocytes; MCP-1, monocyte chemoattractant protein 1; KO, knockout; IVDs, intervertebral discs; ZIA, zymosan-induced arthritis.
Figure 1.
Effects of LOX-1/ox-LDL interaction in articular chondrocytes.
LOX-1, low-density lipoprotein receptor 1; ox-LDL, oxidation of low-density lipoprotein; NF-KB, nuclear factor kappa B; PPAR gamma, peroxisome proliferator-activated receptor γ; MCP-1, monocyte chemoattractant protein 1; PG, proteoglycan; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; COLX, type X collagen.
Common molecules between MetS in OA and LOX-1/ox-LDL
OA is associated with hypertension and LOX-1/ox-LDL
Generally, hypertension is a major common component of MetS. One role and mechanism of hypertension in MetS is considered to be impaired bone metabolism due to interrupted blood flow in the subchondral bone, while another mechanism involves apoptosis of osteoblasts.62,63 Recently, we showed that the renin–angiotensin system (RAS) component is expressed in mouse growth plates.64 We also showed that the local RAS was involved in differentiation of chondrocytes in the ataxia–telangiectasia group D complementing cell line.65 Furthermore, we found that the Tsukuba spontaneous hypertension mouse model had more severe OA than controlled wild type mice in the forced running model.66 These findings indicate that the RAS is involved in the pathogenesis of OA. Activation of LOX-1 contributes as a mediator of the RAS system.67 Angiotensin II mediates vasoconstriction through activation of the angiotensin 1 receptor. The LOX-1 receptor mediates ox-LDL internalization and upregulates angiotensin 1 receptor via the NADPH oxidase, mitogen-activated protein kinase, and nuclear factor kappa B pathways.68 As positive feedback, angiotensin II upregulates LOX-1 expression.69,70 Therefore, LOX-1 is responsible, at least in part, for physiological and pathological effects via angiotensin II, regulating important functions of this pathway as a normotensive factor, and initiating cardiovascular disease and OA. These findings indicate that angiotensin II (RAS component) is a common factor between OA and LOX-1/ox-LDL associated with hypertension (Table 2).
Table 2.
Common molecules between MetS components and the LOX-1/ox-LDL system.
| MetS components | LOX-1/ox-LDL system |
|---|---|
| HT | RAS |
| HL | LDL |
| Obesity | Adipokine (MCP-1) |
| DM | AGEs, ROS, PPARγ |
MeTs, metabolic syndrome; LOX-1, low-density lipoprotein receptor 1; ox-LDL, oxidation of low-density lipoprotein; HT, hypertension; RAS, renin–angiotensin system; HL, hyperlipidemia; LDL, low-density lipoprotein; MCP-1, monocyte chemotactic protein 1; DM, diabetes mellitus; AGEs, advanced glycation end-products; ROS, reactive oxygen species; PPAR-γ: peroxisome proliferator-activated receptor γ.
OA is associated with obesity and LOX-1/Ox-LDL
Although progression of OA is induced by mechanical stress with obesity, obesity also has other systemic effects. “Adipokine” is a generic term for bioactive proteins that are secreted from fat cells.71 Adipokines are released from adipose tissue subjected to mechanical stress and are inflammatory cytokines, which contribute to degeneration of cartilage.72 Adipokines include tumor necrosis factor-α, plasminogen activator inhibitor type 1, and heparin-binding epidermal growth factor, which act to promote arteriosclerosis, and leptin and adiponectin, which act prophylactically in arteriosclerosis.73 Tumor necrosis factor-α is also an inflammatory cytokine that is secreted by macrophages and from large fat cells, and has been reported to contribute to insulin resistance.74 In the state where visceral fat is accumulated, abnormal secretion of adipokines occurs, and insulin resistance is increased.75 In particular, MCP-1 is a common adipokine that is involved downstream in the LOX-1/ox-LDL system (Table 2).
OA is associated with diabetes mellitus and LOX-1/ox-LDL
The negative effects of diabetes on joints can be explained by induction of oxidative stress and pro-inflammatory cytokines.76 Because of the susceptibility to ROS-induced oxidative stress in hyperglycemic conditions,77 endothelial cells favor production of IL-6 and prostaglandin E2.78 Catabolism of hyperglycemia in human cartilage is neutralized by the PPAR-γ activator.79 However, the negative effects caused by diabetes can also be explained by accumulation of aging products in joint tissues that are exposed to chronic high glucose concentrations. Hyperglycemia results in accumulation of advanced glycan end-products (AGEs).80 AGEs release pro-inflammatory cytokines and destroy the cartilage matrix. Moreover, LOX-1 is thought to be a ligand of AGEs.67,69 Insulin resistance can also damage joint tissue not only because of local insulin resistance of the diabetic synovium, but also owing to systemic low-grade inflammatory conditions associated with obesity and conditions of insulin resistance.81 Therefore, ROS, AGEs, and PPAR-γ are common factors in the effects of the LOX-1/ox-LDL system and diabetes mellitus in OA (Table 2). Release of common inflammatory cytokines and maintenance of an ROS-induced oxidative status82 may contribute to progression of OA, which may then contribute to the oxidatively stressed microenvironment present in the articular joint (Figure 2).
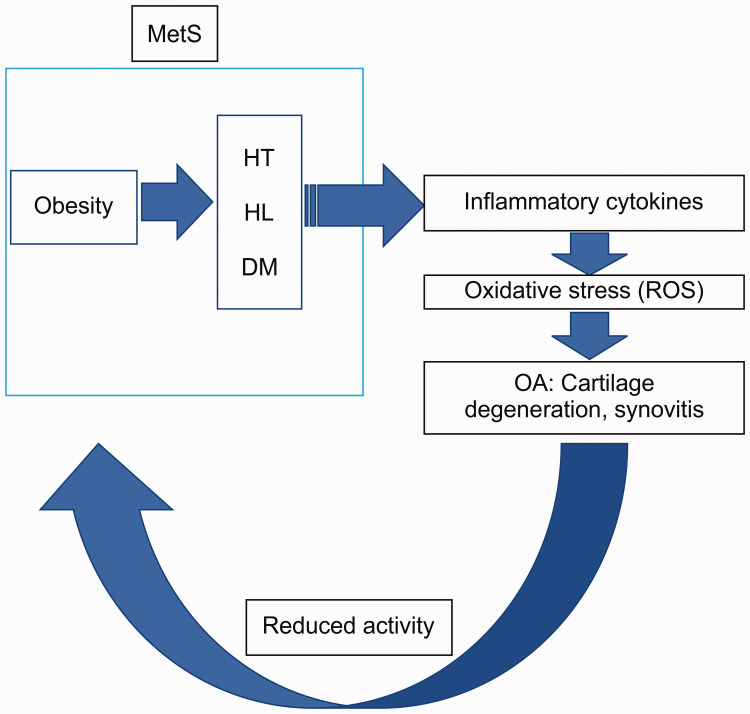
Figure 2.
Cycle of MetS in OA.
This figure shows the oxidative environment in OA. MetS disorders, such as HT, HL, and DM, induce the release of inflammatory cytokines. Oxidative substances, such as ROS or ox-LDL, are then released, leading to degeneration of cartilage and synovitis. A lack of exercise due to pain also leads to obesity and this in turn further induces MetS. Such a vicious cycle is presumably caused by the oxidative stress environment in OA. MetS, metabolic syndrome; OA, osteoarthritis; HT, hypertension; HL, hyperlipidemia; DM, diabetes mellitus; ROS, reactive oxygen species; OA, osteoarthritis.
Non-pharmacological treatment in OA
Physical activity
Generally, exercise is important for preventing MetS, such as obesity. Onset and continuation of fatty liver disease may be associated with other aspects of lifestyle.83 The body mass index, a sedentary lifestyle, loose-fitting clothing, sleep deprivation, and a lower frequency of food intake daily are associated with the presence of non-alcoholic fatty liver disease. Tailored and adapted physical activity can also be used in treatment of MetS and OA as part of a correct and healthy lifestyle to prevent diabetes mellitus.84 Furthermore, a lifestyle incorporating exercise is an important preventative strategy because glucose derivatives, AGEs, sorbitol, and diacylglycerol promote and involve activation of the inflammatory process.84 Moderate physical activity may act as a preventive measure for knee OA and synovitis. The knee joint of active people expresses lower inflammatory cytokines, such as Il-1β, IL-6, tumor necrosis factor-α, and matrix metalloproteinase-13, and it expresses higher levels of cytokines that are protective for the synovium, such as IL-4, IL-10, and lubricin.85 Therefore, moderate physical activity may rescue type B synoviocyte dysfunction at the early stage of OA.85
Nutrition
A healthy diet is important for preventing MetS. The Mediterranean diet has a protective effect on early OA.86 A diet enriched by olive tree compounds that are found in extra virgin olive oil and olive leaf extract has a recovery effect for cartilage in the early phase of OA. Vitamin D is also a recommended supplement.87 Healthy cartilage turnover depends on suitable accessibility of vitamin D.88 Adequate levels of vitamin D stimulate mature chondrocytes to synthesize the proteoglycan matrix.89 Deficiency in vitamin D is widely associated with several musculoskeletal diseases.90 In the young healthy sedentary rat model, vitamin D supplementation with the diet has a favorable effect on articular development of cartilage thickness, joint lubrication, and deposition of extracellular matrix fibers.91 Additionally, coenzyme Q10 suppresses ox-LDL-induced endothelial oxidative injury by modulation of LOX-1-mediated ROS generation via the AMP‐activated kinase/protein kinase C/NADPH oxidase signaling pathway.92 The authors speculate that coenzyme Q10 suppresses LOX-1-induced ROS, and then may suppress all reciprocally generated ROS related to MetS.
Future prospects
These studies mentioned above that suggest a relationship between the LOX-1/ox-LDL system and OA have some limitations. First, the direct mechanisms connecting mechanical stress and expression of the LOX-1/ox-LDL system have not been determined. Second, the origin of ox-LDL has not been clarified. Third, whether ox-LDL or LOX-1/ox-LDL double-knockout mice also demonstrate resistance against OA is unclear. Similarly, involvement of the LOX-1/ox-LDL system in the pathogenesis of OA in subchondral bone has not been clarified. The LOX-1 index or soluble LOX-1 has recently attracted attention as a clinical biomarker.92–94 The relationship between the prognosis of OA and the LOX-1 index needs to be investigated in the future.
Conclusions
We reviewed articles that investigated the relationship between OA and AS, especially studies that investigated the LOX-1/ox-LDL system in OA. We also clarified molecules that are common to the underlying pathological mechanisms of MetS and the LOX-1/ox-LDL system. We speculate that there is involvement of the oxidative environment of the joint in inducing OA. Evidence shows a correlation between MetS in OA and the LOX-1/ox-LDL system. Suppressing the environment of the cartilage joint that enhances oxidative stress involving the LOX-1/ox-LDL system, which contributes to MetS in OA, may lead to prevention and suppression of OA. In the future, a potential approach for treatment of OA may involve development of anti-oxidative agents and management of patients, promoting a healthy lifestyle free from factors affecting oxidative stress.
Acknowledgement
The authors would like to thank Editage (www.editage.jp) for English language editing.
Author contributions
KH: study concept and design; and KH and MA: analysis and interpretation of data. Both authors drafted the manuscript and approved the final version.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Kazuhiko Hashimoto https://orcid.org/0000-0002-8332-0063
References
- 1.Cram P, Lu X, Kates SL, et al. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2001. JAMA 2012; 308: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimura N, Muraki S, Oka H, et al. Cohort profile: research on osteoarthritis/osteoporosis against disability study. Int J Epidemiol 2010; 39: 988–995. [DOI] [PubMed] [Google Scholar]
- 3.Lernout T, Labalette C, Sedel L, et al. Cost analysis in total hip arthroplasty: experience of a teaching medical center located in Paris. Orthop Traumatol Surg Res 2010; 96: 113–123. [DOI] [PubMed] [Google Scholar]
- 4.Delanois RE, Mistry JB, Gwam CU, et al. Current epidemiology of revision total knee arthroplasty in the United States. J Arthroplast 2017; 32: 2663–2668. [DOI] [PubMed] [Google Scholar]
- 5.Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work 2015; 50: 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuo Q, Yang W, Chen J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012; 8: 729–737. [DOI] [PubMed] [Google Scholar]
- 7.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem 2000; 275: 20069–20076. [DOI] [PubMed] [Google Scholar]
- 8.Shah R Raska K JrandTiku ML.. The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: a pathogenic role in osteoarthritis. Arthritis Rheum 2005; 52: 2799–2807. [DOI] [PubMed] [Google Scholar]
- 9.Antony B, Venn A, Cicuttini F, et al. Correlates of knee bone marrow lesions in younger adults. Arthritis Res Ther 2016; 18: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farnaghi S, Crawford R, Xiao Y, et al. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int J Rheum Dis 2017; 20: 131–140. [DOI] [PubMed] [Google Scholar]
- 11.Addimanda O, Mancarella L, Dolzani P, et al. Clinical associations in patients with hand osteoarthritis. Scand J Rheumatol 2012; 41: 310–313. [DOI] [PubMed] [Google Scholar]
- 12.Afifi AEA, Shaat RM, Gharbia OM, et al. Osteoarthritis of knee joint in metabolic syndrome. Clin Rheumatol 2018; 37: 2855–2861. [DOI] [PubMed] [Google Scholar]
- 13.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995; 22: 1118–1123. [PubMed] [Google Scholar]
- 14.Stürmer T, Sun Y, Sauerland S, et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol 1998; 25: 1827–1832. [PubMed] [Google Scholar]
- 15.Al-Arfaj AS. Radiographic osteoarthritis and serum cholesterol. Saudi Med J 2003, 24: 745–747. [PubMed] [Google Scholar]
- 16.Oliviero F, Lo Nigro A, Bernardi D, et al. A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin Chim Acta 2012; 413: 303–307. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhao Y, Wang M, et al. Serum lipid levels are positively correlated with lumbar disc herniation–a retrospective study of 790 Chinese patients. Lipids Health Dis 2016; 15: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelsey DE, Toher JL, Foster MT, et al. Laboratory validation of a low density lipoprotein apolipoprotein-B assay. Clin Biochem 2014; 47: 211–215. [DOI] [PubMed] [Google Scholar]
- 19.De Munter W, Van Den Bosch MH, Slöetjes AW, et al. High LDL levels lead to increased synovial inflammation and accelerated ectopic bone formation during experimental osteoarthritis. Osteoarthr Cartil 2016; 24: 844–855. [DOI] [PubMed] [Google Scholar]
- 20.De Munter W, Blom AB, Helsen MM, et al. Cholesterol accumulation caused by low density lipoprotein receptor deficiency or a cholesterol-rich diet results in ectopic bone formation during experimental osteoarthritis. Arthritis Res Ther 2013; 15: R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farnaghi S, Prasadam I, Cai G, et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB J 2017; 31: 356–367. [DOI] [PubMed] [Google Scholar]
- 22.Veronese N, Koyanagi A, Stubbs B, et al. Statin use and knee osteoarthritis outcomes: a longitudinal cohort study. Arthritis Care Res (Hoboken) 2019; 71: 1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook MJ, Sorial AK, Lunt M, et al. Effect of timing and duration of statin exposure on risk of hip or knee revision arthroplasty: a population-based cohort study. J Rheumatol 2020; 47: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest 2015; 45: 405–414. [DOI] [PubMed] [Google Scholar]
- 25.Belen E, Karaman O, Caliskan G, et al. An indicator of subclinical cardiovascular disease in patients with primary osteoarthritis: epicardial fat thickness. Int J Clin Exp Med 2015; 8: 9491–9497. [PMC free article] [PubMed] [Google Scholar]
- 26.Belen E, Karaman O, Caliskan G, et al. Impaired aortic elastic properties in primary osteoarthritis. Vascular 2016; 24: 70–77. [DOI] [PubMed] [Google Scholar]
- 27.Cemeroglu O, Aydın HI, Yasar ZS, et al. Hand and heart, hand in hand: is radiological hand osteoarthritis associated with atherosclerosis? Int J Rheum Dis 2014; 17: 299–303. [DOI] [PubMed] [Google Scholar]
- 28.Ekim AA, İnal EE, Kaya DS, et al. Relationship between atherosclerosis and knee osteoarthritis as graded by radiography and ultrasonography in females. J Phys Ther Sci 2016; 28: 2991–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson H, Helgadottir GP, Aspelund T, et al. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik Study. Ann Rheum Dis 2009; 68: 1696–1700. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson H, Helgadottir GP, Aspelund T, et al. The presence of total knee or hip replacements due to osteoarthritis enhances the positive association between hand osteoarthritis and atherosclerosis in women: the AGES-Reykjavik Study. Ann Rheum Dis 2011; 70: 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson H, Helgadottir GP, Aspelund T, et al. Hand osteoarthritis severity is associated with total knee joint replacements independently of BMI. The Ages-Reykjavik Study. Open Rheumatol J 2011; 5: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Novera D, Wluka AE, et al. Association between popliteal artery wall thickness and knee structure in adults without clinical disease of the knee: a prospective cohort study. Arthritis Rheumatol 2015; 67: 414–422. [DOI] [PubMed] [Google Scholar]
- 33.Gielis WP, Welsing PMJ, Van Spil WE, et al. A sex-specific association between incident radiographic osteoarthritis of hip or knee and incident peripheral arterial calcifications: 8-year prospective data from Cohort Hip and Cohort Knee (CHECK). Osteoarthr Cartil 2017; 25: 1814–1821. [DOI] [PubMed] [Google Scholar]
- 34.Bierma-Zeinstra SMA, Waarsing JH. The role of atherosclerosis in osteoarthritis. Best Pract Res Clin Rheumatol 2017; 31: 613–633. [DOI] [PubMed] [Google Scholar]
- 35.Rezuș E, Cardoneanu A, Burlui A, et al. C The link between inflammaging and degenerative joint diseases. Int J Mol Sci 2019; 20: E614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: asystemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128: 92–105. [DOI] [PubMed] [Google Scholar]
- 37.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthritis!). Osteoarthr Cartil 2013; 21: 16–21. [DOI] [PubMed] [Google Scholar]
- 38.Shang W, Yu X, Wang H, et al. Fibroblast growth factor 21 enhances cholesterol efflux in THP-1 macrophage-derived foam cells. Mol Med Rep 2015; 11: 503–508. [DOI] [PubMed] [Google Scholar]
- 39.Yu XH, Fu YC, Zhang DW, et al. Foam cells in atherosclerosis. Clin Chim Acta 2013; 424: 245–252. [DOI] [PubMed] [Google Scholar]
- 40.Nomura M, Liu J, Yu ZX, et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J Mol Cell Cardiol 2019; 127: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Li X, Jiao K, et al. Albiflorin inhibits the formation of THP-1-derived foam cells through the LOX-1/NF-κB pathway. Minerva Med 2019; 110: 107–114. [DOI] [PubMed] [Google Scholar]
- 42.He D, Xu L, Wu Y, et al. Rac3, but not Rac1, promotes Ox-LDL induced endothelial dysfunction by downregulating autophagy. J Cell Physiol 2019; 235: 1531–1542. [DOI] [PubMed] [Google Scholar]
- 43.Kattoor AJ, Goel A, Mehta JL. LOX-1: regulation, signaling and its role in atherosclerosis. Antioxidants (Basel) 2019; 8: pii: E218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997; 386: 73–77. [DOI] [PubMed] [Google Scholar]
- 45.Ohki I, Ishigaki T, Oyama T, et al. Crystal structure of human lectin-like, oxidized low-density lipoprotein receptor 1 ligand binding domain and its ligand recognition mode to OxLDL. Structure 2005; 13: 905–917. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa T, Akagi M, Hoshikawa H, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates leukocyte infiltration and articular cartilage destruction in rat zymosan-induced arthritis. Arthritis Rheum 2002; 46: 2486–2494. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa T, Yasuda T, Hoshikawa H, et al. LOX-1 expressed in cultured rat chondrocytes mediates oxidized LDL-induced cell death-possible role of dephosphorylation of Akt. Biochem Biophys Res Commun 2002; 299: 91–97. [DOI] [PubMed] [Google Scholar]
- 48.Kakinuma T, Yasuda T, Nakagawa T, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates matrix metalloproteinase 3 synthesis enhanced by oxidized low-density lipoprotein in rheumatoid arthritis cartilage. Arthritis Rheum 2004; 50: 3495–3503. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura S, Akagi M, Yoshida K, et al. Oxidized low-density lipoprotein (Ox-LDL) binding to lectin-like Ox-LDL receptor-1 (LOX-1) in cultured bovine articular chondrocytes increases production of intracellular reactive oxygen species (ROS) resulting in the activation of NF-KappaB. Osteoarthr Cartil 2004; 12: 568–576. [DOI] [PubMed] [Google Scholar]
- 50.Akagi M, Nishimura S, Yoshida K, et al. Cyclic tensile stretch load and oxidized low density lipoprotein synergistically induce lectin-like oxidized Ldl receptor-1 in cultured bovine chondrocytes, resulting in decreased cell viability and proteoglycan synthesis. J Orthop Res 2006; 24: 1782–1790. [DOI] [PubMed] [Google Scholar]
- 51.Kanata S, Akagi M, Nishimura S, et al. Oxidized LDL binding to LOX-1 upregulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-gamma. Biochem Biophys Res Commun 2006; 348: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 52.Akagi M, Kanata S, Mori S, et al. Possible involvement of the oxidized low-density lipoprotein/lectin-like oxidized low-density lipoprotein receptor-1 system in pathogenesis and progression of human osteoarthritis. Osteoarthr Cartil 2007; 15: 281–290. [DOI] [PubMed] [Google Scholar]
- 53.Simopoulou T, Malizos KN, Tsezou A. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human articular chondrocytes. Clin Exp Rheumatol 2007; 25: 605–612. [PubMed] [Google Scholar]
- 54.Akagi M, Ueda A, Teramura T, et al. Oxidized LDL binding to LOX-1 enhances MCP-1 expression in cultured human articular chondrocytes. Osteoarthr Cartil 2009; 17: 271–275. [DOI] [PubMed] [Google Scholar]
- 55.Zushi S, Akagi M, Kishimoto H, et al. Induction of bovine articular chondrocyte senescence with oxidized low-density lipoprotein through lectin-like oxidized low-density lipoprotein receptor 1. Arthritis Rheum 2009; 60: 3007–3016. [DOI] [PubMed] [Google Scholar]
- 56.Kishimoto H, Akagi M, Zushi S, et al. Induction of hypertrophic chondrocyte-like phenotypes by oxidized LDL in cultured bovine articular chondrocytes through increase in oxidative stress. Osteoarthr Cartil 2010; 18: 1284–1290. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa M, Ito H, Akiyoshi M, et al. Lectin-like oxidized low-density lipoprotein receptor 1 signal is a potent biomarker and therapeutic target for human rheumatoid arthritis. Arthritis Rheum 2012; 64: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto K, Mori S, Oda Y, et al. Lectin-like oxidized low density lipoprotein receptor 1-deficient mice show resistance to instability-induced osteoarthritis. Scand J Rheumatol 2016; 45: 412–422. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto K, Oda Y, Nakamura F, et al. Lectin-like, oxidized low-density lipoprotein receptor-1-deficient mice show resistance to age-related knee osteoarthritis. Eur J Histochem 2017; 61: 2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Wang X, Hu Z, et al. Possible involvement of the oxLDL/LOX-1 system in the pathogenesis and progression of human intervertebral disc degeneration or herniation. Sci Rep 2017; 7: 7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto K, Oda Y, Nakagawa K, et al. LOX-1 deficient mice show resistance to zymosan-induced arthritis. Eur J Histochem 2018; 62: 2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gkretsi V, Simopoulou T, Tsezou A. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Prog Lipid Res 2011; 50: 133–140. [DOI] [PubMed] [Google Scholar]
- 63.Vukelic L, Sosa I, Cvijanovic O, et al. Correlation of endothelin-1 mRNA expression and bone structure in advanced osteoarthritis. Med Hypotheses 2011; 77: 927–929. [DOI] [PubMed] [Google Scholar]
- 64.Tsukamoto I, Akagi M, Inoue S, et al. Expressions of local renin-angiotensin system components in chondrocytes. Eur J Histochem 2014; 58: 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsukamoto I, Inoue S, Teramura T, et al. Activating types 1 and 2 angiotensin II receptors modulate the hypertrophic differentiation of chondrocytes. FEBS Open Bio 2013; 3: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamagishi K, Tsukamoto I, Nakamura F, et al. Activation of the renin-angiotensin system in mice aggravates mechanical loading-induced knee osteoarthritis. Eur J Histochem 2018; 62: 2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taye A, El-Sheikh AA. Lectin-like oxidized low-density lipoprotein receptor 1 pathways. Eur J Clin Invest 2013; 43: 740–745. [DOI] [PubMed] [Google Scholar]
- 68.Catar RA, Muller G, Heidler J, et al. Low-density lipoproteins induce the renin-angiotensin system and their receptors in human endothelial cells. Horm Metab Res 2007; 39: 801–805. [DOI] [PubMed] [Google Scholar]
- 69.Morawietz H, Rueckschloss U, Niemann B, et al. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation 1999; 100: 899–902. [DOI] [PubMed] [Google Scholar]
- 70.Li D, Saldeen T, Romeo F, et al. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: the potential role of transcription factor NF-KappaB. Circulation 2000; 102: 1970–1976. [DOI] [PubMed] [Google Scholar]
- 71.Chait A, Den Hartigh LJ. Adipose tissue distribution, inflammation, and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 2020; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev 2018; 44: 38–50. [DOI] [PubMed] [Google Scholar]
- 73.Matsuzawa Y. White adipose tissue and cardiovascular disease. Best Pract Res Clin Endocrinol Metab 2005; 19: 637–647. [DOI] [PubMed] [Google Scholar]
- 74.Kanter JE, Hsu CC, Bornfeldt KE. Monocytes and macrophages as protagonists in vascular complications of diabetes. Front Cardiovasc Med 2020; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roy S, Metya SK, Sannigrahi S, et al. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine 2013; 44: 369–379. [DOI] [PubMed] [Google Scholar]
- 77.Headley CA, Di Silvestro D, Bryant KE, et al. Nitrones reverse hyperglycemia-induced endothelial dysfunction in bovine aortic endothelial cells. Biochem Pharmacol 2016; 104: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen YJ, Chan DC, Lan KC, et al. PPARγ is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J Orthop Res 2015; 33: 373–381. [DOI] [PubMed] [Google Scholar]
- 79.Sahajpal NS, Goel RK, Chaubey A, et al. Pathological perturbations in diabetic retinopathy: hyperglycemia, AGEs, oxidative stress and inflammatory pathways. Curr Protein Pept Sci 2019; 20: 92–110. [DOI] [PubMed] [Google Scholar]
- 80.Collins KH, Herzog W, MacDonald GZ, et al. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol 2018; 9: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kattoor AJ, Pothineni NVK, Palagiri D, et al. Oxidative stress in atherosclerosis. Curr Atheroscler Rep 2017; 19: 42. [DOI] [PubMed] [Google Scholar]
- 82.Trovato FM, Martines GF, Brischetto D, et al. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int 2016; 36: 427–433. [DOI] [PubMed] [Google Scholar]
- 83.Aiello FC, Trovato FM, Szychlinska MA, et al. Molecular links between diabetes and osteoarthritis: the role of physical activity. Curr Diabetes Rev 2017; 13: 50–58. [DOI] [PubMed] [Google Scholar]
- 84.Castrogiovanni P, Di Rosa M, Ravalli S, et al. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci 2019; 20: E511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szychlinska MA, Castrogiovanni P, Trovato FM, et al. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur J Nutr 2019; 58: 565–581. [DOI] [PubMed] [Google Scholar]
- 86.Szychlinska MA, Imbesi R, Castrogiovanni P, et al. Assessment of vitamin D supplementation on articular cartilage morphology in a young healthy sedentary rat model. Nutrients 2019; 11: E1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81: 353–373. [DOI] [PubMed] [Google Scholar]
- 88.Li S, Niu G, Dong XN, et al. Vitamin D inhibits activities of metalloproteinase-9/-13 in articular cartilage in vivo and in vitro. J Nutr Sci Vitaminol (Tokyo) 2019; 65: 107–112. [DOI] [PubMed] [Google Scholar]
- 89.Wintermeyer E, Ihle C, Ehnert S, et al. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016; 8: E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pascual-Garrido C, Angeline ME, Ma R, et al. Low levels of vitamin D have a deleterious effect on the articular cartilage in a rat model. HSS J 2016; 12: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsai KL, Chen LH, Chiou SH, et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol Nutr Food Res 2011; 55: S227–S240. [DOI] [PubMed] [Google Scholar]
- 92.Kume N, Mitsuoka H, Hayashida K, et al. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome–comparison with other biomarkers. J Cardiol 2010; 56: 159–165. [DOI] [PubMed] [Google Scholar]
- 93.Sawamura T, Wakabayashi I. and Okamura T. LOX-1 in atherosclerotic disease. Clin Chim Acta 2015; 440: 157–163. [DOI] [PubMed] [Google Scholar]
- 94.Johansson M, Ricci F, Aung N, et al. Proteomic profiling for cardiovascular biomarker discovery in orthostatic hypotension. Hypertension 2018; 71: 465–472. [DOI] [PubMed] [Google Scholar]