Abstract
Background:
There are limited data on the safe interval from diagnosis to surgery in patients with stage I esophageal adenocarcinoma. We hypothesized that increased time to surgery would be associated with worse survival and increased nodal upstaging.
Methods:
The National Cancer Database (NCDB) was used to identify patients with cT1N0M0 esophageal adenocarcinoma (2004–2015) who underwent esophagectomy without induction therapy. The primary outcome was survival and the secondary outcomes were the rate of margin-positive resection and pathologic nodal upstaging. Time to surgery was modeled as a categorical variable, dividing patients into quartiles (Q1–4), and as a continuous variable using piecewise linear splines centered on 50 and 100 days.
Results:
A total of 2495 patients met study criteria. When examined in quartiles, there was no difference in survival between groups based on time to surgery in both unadjusted and multivariable analysis. As a continuous variable, increasing time to surgery less than 50 days was associated with improved survival (HR 0.99; 95%CI 0.98–1.00), Time to surgery greater than 100 days was associated with worse survival (HR 1.00; 95%CI 1.00–1.01) and increased margin-positive resection (OR 1.01; 95%CI 1.00–1.02). Treatment at a high volume center, government insurance, and diagnosis and treatment at different centers were associated with surgery beyond 100 days
Conclusions:
Increasing time to surgery greater than 100 days is associated with worse outcomes in patients with stage I esophageal adenocarcinoma. In this patient population, esophagectomy should be offered as soon as safely possible.
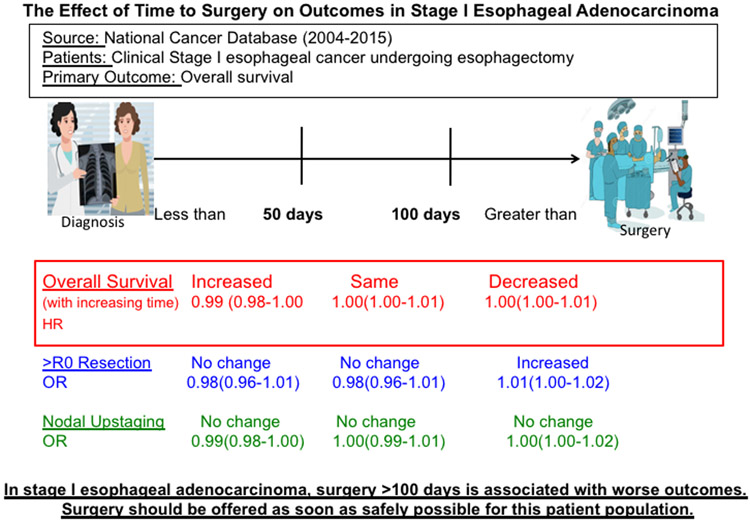
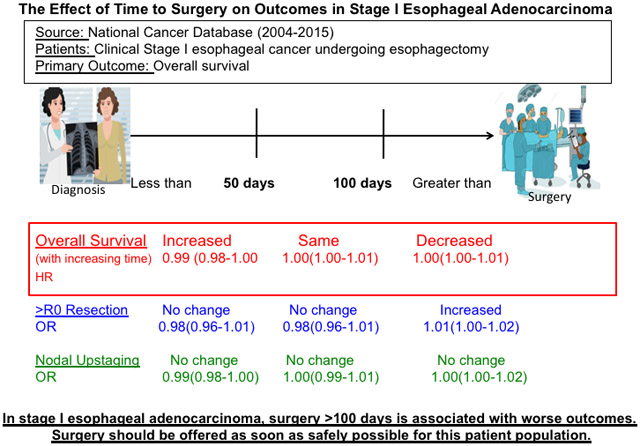
Graphical Abstract
Central Figure. In stage I esophageal adenocarcinoma, survival worsens if surgery is offered >100 days.
Introduction
The National Comprehensive Cancer Network (NCCN) guidelines1 recommend surgery without induction therapy for cT1a-bN0M0 esophageal adenocarcinoma. However, the guidelines do not specify a safe time interval to surgery in this population. While there are observational studies examining the optimum interval between induction chemoradiotherapy and surgery in esophageal cancer2-5, there are no studies, to our knowledge, that examine this question in patients with early esophageal cancer undergoing surgery upfront. There is conflicting literature in other malignancies, including lung, bladder, and colon cancer, about a safe interval for surgery6-10, with some studies demonstrating worse survival and higher incidence of pathologic upstaging with delayed timing of surgery6,7,9.
We performed a retrospective cohort study using a large national database to examine the effect of time to surgery on survival for patients with stage I esophageal adenocarcinoma. We hypothesized that increased time to surgery would be associated with worse survival and increased pathologic nodal upstaging and margin-positive resection.
Methods
Data Source
The National Cancer Database (NCDB) is a joint effort of the American Cancer Society and the American College of Surgeons. It contains information about approximately 80% of cancers diagnosed across the United States annually, collected by certified tumor registrars in 1500 hospitals11.
Patient Selection
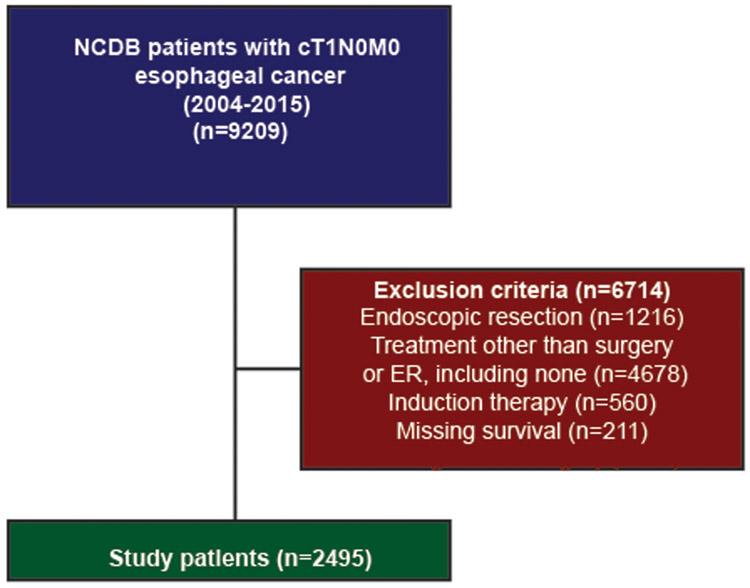
This study was deemed exempt by our Institutional Review Board. In the NCDB, patients diagnosed with cT1N0M0 esophageal adenocarcinoma who underwent esophagectomy were identified during a study period of 2004–2015. Patients receiving neoadjuvant chemotherapy or radiation, with missing survival, or missing data on time from diagnosis to surgery were excluded (Figure 1). Patients receiving endoscopic resection (ER) alone were also excluded. Because the NCDB codes only the most definitive procedure a patient underwent, patients who received ER followed by esophagectomy were catalogued as having undergone only esophagectomy, and were included in the final cohort.
Figure 1.
Scheme of patient selection for this study
Study Design
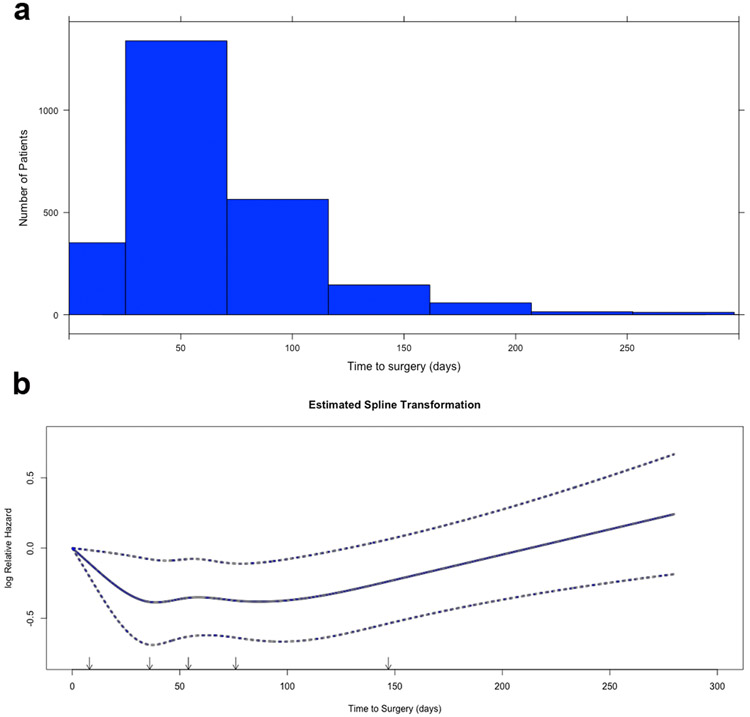
Time from diagnosis to surgery had an approximately normal distribution (Figure 2a). The median time to surgery was 54 days (interquartile range 34–80). In the first part of the study, time to surgery was modeled as a categorical variable, and patients were stratified into four groups based on quartiles: Q1 (<=35 days), Q2 (35–55 days), Q3 (56–80 days), and Q4 (>80 days). In the second part of the study, time to surgery was considered as a continuous variable. A restricted cubic spline (RCS) transformation of time to surgery from diagnosis with five pre-specified knots12 revealed a linear increase in mortality after 100 days (Figure 2b). The transformation also suggested a decrease in mortality within the first 50 days. Restricted cubic splines are cubic polynomial transformations of functions that may be nonlinear or have nonlinear components, and have been shown to better approximate the relationship between the independent and dependent variable than linear models12. Based on RCS, time to surgery was modeled as piecewise linear splines with knots at 50 and 100 days. Since there were no statistically significant or sharply non-linear components to the RCS transformation, the model was simplified into three piecewise linear splines12. The fit of the model was evaluated using the Akaike Information Criterion (AIC), likelihood ratio, and shrinkage factor.
Figure 2.
(a) Histogram of time to surgery in the overall cohort. (b) Unadjusted restricted cubic spline transformation of time to surgery. Arrows denote pre-specified knots selected in unadjusted analysis. Y-axis demonstrates the unadjusted log hazard of mortality while X-axis the time to surgery in days. Dotted lines reflect bounds of the 95% confidence interval
The primary outcome was overall survival. Continuous and categorical variables were compared using the Wilcoxon rank sum and Pearson’s chi-squared tests, respectively. Survival was estimated using the Kaplan-Meier and Cox Proportional Hazards methods. The secondary outcomes were rates of margin-positive resection and pathologic nodal upstaging. These were studied using multivariable logistic regression. Variables included in the Cox and logistic regression models were selected a priori: age, sex, race, diagnosis year, Charlson-Deyo comorbidity index (CDCC) score, insurance status, rural or urban location of treatment facility, academic or non-academic type of treatment center, distance travelled to treatment center, facility geographic location, treatment center volume for esophagectomy during the study period, tumor size, and tumor grade. In the logistic regression for factors associated with surgery beyond 100 days, whether a patient was diagnosed and treated at the same center was also included as a variable13. Subgroup analyses were performed in patients with cT1a or cT1b esophageal cancer, which was coded in the NCDB from years 2011 onwards after the AJCC introduced the distinction in 2010. Because of the small number of events in each of these groups, the following variables were included in the multivariable model: time to surgery modeled as splines (Supplemental Figures 2 and 3), CDCC score, and tumor size. Center volume was also included in the model for patients with cT1b disease. The variables with the greatest effect size in univariable estimates were chosen for the final model. Proportional hazards assumptions for each variable and the overall model were checked using visual and quantitative representations of Schoenfeld residuals.
Missing data were handled using complete case analysis given the high degree of completeness in the NCDB. R version 3.5.1 for Mac (Vienna, Austria) was used for all statistical analysis. A p value less than or equal to 0.05 was considered statistically significant.
Results
A total of 2495 patients met study criteria. The demographic characteristics of study patients, stratified by quartile, are summarized in Table 1. The median survival for patients in Q1, Q2, Q3, and Q4 was 125 months (95% confidence interval [CI] 98-N/A), 135 months (95%CI 107-N/A), 109 months (95%CI 99-N/A), and 102 months (95%CI 94-N/A), respectively. There was no significant difference in overall survival between the groups (log-rank p=0.69). In multivariable Cox Proportional Hazards regression, there was no difference in survival between the groups (Table 2).
Table 1.
Demographic characteristics of study patients
| Quartile 1 (n=675)(%) |
Quartile 2 (n=623)(%) |
Quartile 3 (n=582)(%) |
Quartile 4 (n=615)(%) |
p value |
|
|---|---|---|---|---|---|
| Age (years, median) | 65 | 64 | 66 | 66 | 0.04 |
| Sex (female) | 78(12) | 96(15) | 98(17) | 98(16) | 0.04 |
| Race | 0.22 | ||||
| White | 644(97) | 605(98) | 557(97) | 596(98) | |
| Black | 9(1.5) | 6(1) | 10(1.5) | 8(1.5) | |
| Other | 9(1.5) | 6(1) | 9(1.5) | 1(0.2) | |
| Year of diagnosis, median (inter-quartile range) | 2009(2007-2011) | 2009(2007-2012) | 2010(2008-2012) | 2010(2008-2012) | <0.001 |
| CDCC Score | 0.28 | ||||
| 0 | 465(69) | 428(69) | 383(66) | 420(68) | |
| 1 | 176(26) | 162(26) | 157(27) | 147(24) | |
| 2+ | 34(5) | 33(5) | 42(7) | 48(8) | |
| Insurance status | <0.001 | ||||
| Private | 315(48) | 295(49) | 234(41) | 200(34) | |
| Government | 333(51) | 305(50) | 323(57) | 381(65) | |
| None | 5(0.8) | 6(1) | 8(2) | 10(2) | |
| Facility location | 0.48 | ||||
| Metro | 515(80) | 502(84) | 459(82) | 481(82) | |
| Urban | 120(19) | 84(14) | 87(16) | 95(16) | |
| Rural | 11(1.5) | 13(2) | 11(2) | 14(2) | |
| Facility type | <0.001 | ||||
| Academic/research program | 389(58) | 355(58) | 369(64) | 430(70) | |
| Pathologic stage | <0.001 | ||||
| 1 | 466(80) | 432(81) | 427(83) | 444(85) | |
| 2 | 78(13) | 89(17) | 64(13) | 63(12) | |
| 3 | 42(7) | 11(2) | 21(4) | 16(3) | |
| Tumor size (median mm) | 18 | 16 | 15 | 14 | <0.001 |
| Positive margins | 24(4) | 13(2) | 9(1.5) | 9(1.5) | 0.04 |
Table 2.
Cox multivariable regression of independent predictors of survival with time to surgery modeled as a categorical variable by quartile
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.04 | 1.03 | 1.05 | <0.001 |
| Sex (female) | 0.86 | 0.68 | 1.09 | 0.20 |
| Race (reference: White) | ||||
| Black | 1.21 | 0.62 | 2.37 | 0.58 |
| Other | 1.22 | 0.45 | 3.29 | 0.70 |
| Year of diagnosis (per year) | 0.98 | 0.95 | 1.01 | 0.20 |
| CDCC score (reference: 0) | ||||
| 1 | 1.06 | 0.88 | 1.28 | 0.53 |
| 2+ | 1.23 | 0.90 | 1.69 | 0.19 |
| Insurance status (reference: private) | ||||
| Government | 1.01 | 0.81 | 1.24 | 0.96 |
| None | 1.51 | 0.78 | 2.90 | 0.22 |
| Facility location (reference: metro) | ||||
| Urban | 1.29 | 1.03 | 1.62 | 0.03 |
| Rural | 1.19 | 0.63 | 2.26 | 0.60 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 0.98 | 0.79 | 1.23 | 0.87 |
| Distance to treatment center (per mile) | 0.999 | 0.997 | 1.000 | 0.02 |
| Facility geography (reference: New England) | ||||
| Middle Atlantic (nj,ny,pa) | 1.38 | 0.93 | 2.06 | 0.11 |
| South Atlantic (dc,de,fl,ga,md,nc,sc,va,wv) | 1.12 | 0.75 | 1.66 | 0.58 |
| East North Central (il,in,mi,oh,wi) | 1.37 | 0.94 | 2.02 | 0.11 |
| East South Central (al,ky,ms,tn) | 1.17 | 0.72 | 1.92 | 0.52 |
| West North Central (ia,ks,mn,mo,nd,ne,sd) | 1.10 | 0.71 | 1.70 | 0.67 |
| West South Central (ar,la,ok,tx) | 1.18 | 0.72 | 1.94 | 0.51 |
| Mountain (az,co,id,mt,nm,nv,ut,wy) | 1.62 | 1.00 | 2.63 | 0.05 |
| Pacific (ak,ca,hi,or,wa) | 1.02 | 0.67 | 1.56 | 0.92 |
| Center volume (reference: Q1 ≤1 a year) | ||||
| Q2 (>1-3) | 0.97 | 0.69 | 1.36 | 0.86 |
| Q3 (>3-8) | 0.97 | 0.69 | 1.36 | 0.87 |
| Q4 (>8) | 0.66 | 0.46 | 0.96 | 0.03 |
| Tumor size | 1.000 | 0.999 | 1.002 | 0.96 |
| Grade (reference: well differentiated) | ||||
| Moderately differentiated | 1.11 | 0.86 | 1.43 | 0.41 |
| Poorly differentiated | 2.13 | 1.66 | 2.73 | <0.001 |
| Time to surgery (reference: Q1) | ||||
| Q2 | 0.83 | 0.67 | 1.04 | 0.10 |
| Q3 | 0.91 | 0.72 | 1.14 | 0.40 |
| Q4 | 0.93 | 0.73 | 1.18 | 0.55 |
Time to surgery was then modeled as a continuous variable using piecewise linear splines and knots at 50 and 100 days. A total of 371 patients (15%) underwent surgery after 100 days from diagnosis (Table 3). In univariable analysis, increasing time to surgery less than 50 days was associated with improved survival (HR 0.99; 95%CI 0.99–1.00; p=0.02) while increasing time to surgery beyond 100 days was associated with worse survival (HR 1.00; 95%CI 1.00–1.01; p=0.003) (Supplemental Figure 1). In multivariable analysis, increasing time to surgery within 50 days from diagnosis was associated with improved survival (Table 4). Compared to receiving surgery at 50 days, the hazard of receiving surgery at 10, 30, and 40 days from diagnosis was 1.64 (95%CI 1.17–2.32), 1.28 (95%CI 1.08–1.52), and 1.13 (95%CI 1.04–1.23), respectively. Beyond 100 days, increasing time to surgery was associated with worse survival (Table 4). Compared to receiving surgery at 100 days, the hazard at 110, 120, and 180 days was 1.03 (95%CI 1.01–1.06), 1.07 (95%CI 1.01–1.12), and 1.30 (95%CI 1.05–1.60), respectively. Between 50 and 100 days, time to surgery was not associated with a change in survival. In a subgroup analysis of 292 patients with cT1a cancer, time to surgery less than 100 days was not associated with survival but time to surgery beyond 100 days was associated with worse survival (HR 1.01; 95%CI 1.00–1.02; p=0.03). In 373 patients with cT1b disease, time to surgery less than 100 days was not associated with improved survival but time to surgery greater than 100 days was associated with worse survival (HR 1.01; 95%CI 1.00–1.01; p=0.0005).
Table 3.
Demographic characteristics of patients receiving surgery within or after 100 days
| <50 days (n=1174)(%) |
50-100 days (n=950)(%) |
> 100 Days (n=371)(%) |
p value | |
|---|---|---|---|---|
| Age (years, median) | 64 | 66 | 65 | 0.05 |
| Sex (female) | 156(13) | 160(17) | 54(15) | 0.07 |
| Race | 0.52 | |||
| White | 1131(98) | 914(97) | 357(98) | |
| Black | 13(1) | 14(2) | 6(1.5) | |
| Other | 14(1) | 10(1) | 1(0.5) | |
| Year of diagnosis, median (inter-quartile range) | 2009(2007-2011) | 2010(2008-2012) | 2010(2008-2012) | <0.001 |
| CDCC Score | 0.18 | |||
| 0 | 808(69) | 635(67) | 253(68) | |
| 1 | 307(26) | 244(26) | 91(25) | |
| 2+ | 59(5) | 71(8) | 27(7) | |
| Insurance status | <0.001 | |||
| Private | 551(48) | 367(40) | 126(35) | |
| Government | 578(51) | 538(59) | 226(64) | |
| None | 11(1) | 14(2) | 4(1) | |
| Facility location | 0.88 | |||
| Metro | 918(82) | 750(82) | 289(81) | |
| Urban | 186(17) | 141(16) | 59(17) | |
| Rural | 20(2) | 21(2) | 8(2) | |
| Facility type | <0.001 | |||
| Academic/research program | 671(58) | 614(65) | 258(70) | |
| Pathologic stage | 0.39 | |||
| 1 | 818(81) | 689(83) | 262(83) | |
| 2 | 143(14) | 110(13) | 41(13) | |
| 3 | 51(5) | 28(3) | 11(4) | |
| Path N stage | 0.91 | |||
| N0 | 848(72) | 701(74) | 278(75) | |
| N1 | 115(10) | 91(10) | 32(9) | |
| N2 | 16(1) | 8(1) | 4(1) | |
| N3 | 3(0.3) | 6(1) | 2(1) | |
| Unknown | 192(16) | 144(15) | 55(15) | |
| Tumor size (median mm with IQR) | 17(10-30) | 15(7-25) | 14(7-25) | <0.001 |
| Positive margins | 0.12 | |||
| R0 | 1097(97) | 900(98) | 349(97) | |
| R1 | 19(2) | 8(1) | 3(1) | |
| R2 | 1(0.1) | 0(0) | 2(0.6) | |
| Unspecified positive | 13(1) | 7(1) | 2(0.6) | |
| Indeterminate | 6(0.5) | 3(0.3) | 3(0.8) | |
| Time to surgery (days)(median with IQR) | 34(23-42) | 69(59-81) | 128(112-162) | <0.001 |
Table 4.
Cox multivariable regression of independent predictors of survival with time to surgery modeled as a continuous variable with linear splines and knots at 50 and 100 days
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.04 | 1.03 | 1.05 | <0.001 |
| Sex (female) | 0.86 | 0.68 | 1.09 | 0.21 |
| Race (reference: White) | ||||
| Black | 1.23 | 0.63 | 2.40 | 0.55 |
| Other | 1.26 | 0.47 | 3.41 | 0.64 |
| Year of diagnosis (per year) | 0.98 | 0.95 | 1.02 | 0.29 |
| CDCC score (reference: 0) | ||||
| 1 | 1.06 | 0.88 | 1.28 | 0.54 |
| 2+ | 1.22 | 0.89 | 1.67 | 0.21 |
| Insurance status (reference: private) | ||||
| Government | 1.00 | 0.81 | 1.23 | 0.97 |
| None | 1.56 | 0.81 | 3.00 | 0.19 |
| Facility location (reference: metro) | ||||
| Urban | 1.27 | 1.02 | 1.60 | 0.04 |
| Rural | 1.20 | 0.63 | 2.28 | 0.58 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 1.00 | 0.80 | 1.24 | 0.97 |
| Distance to treatment center (per mile) | 0.9985 | 0.9973 | 0.9997 | 0.02 |
| Facility geography (reference: New England) | ||||
| Middle Atlantic (nj,ny,pa) | 1.36 | 0.91 | 2.02 | 0.13 |
| South Atlantic (dc,de,fl,ga,md,nc,sc,va,wv) | 1.08 | 0.73 | 1.61 | 0.69 |
| East North Central (il,in,mi,oh,wi) | 1.33 | 0.91 | 1.96 | 0.14 |
| East South Central (al,ky,ms,tn) | 1.16 | 0.71 | 1.89 | 0.56 |
| West North Central (ia,ks,mn,mo,nd,ne,sd) | 1.06 | 0.69 | 1.64 | 0.78 |
| West South Central (ar,la,ok,tx) | 1.08 | 0.66 | 1.78 | 0.76 |
| Mountain (az,co,id,mt,nm,nv,ut,wy) | 1.55 | 0.95 | 2.52 | 0.08 |
| Pacific (ak,ca,hi,or,wa) | 0.99 | 0.65 | 1.52 | 0.97 |
| Center volume (reference: Q1 ≤1 a year) | ||||
| Q2 (>1-3) | 0.97 | 0.70 | 1.36 | 0.86 |
| Q3 (>3-8) | 0.96 | 0.69 | 1.34 | 0.82 |
| Q4 (>8) | 0.66 | 0.46 | 0.94 | 0.02 |
| Tumor size | 1.0000 | 0.9985 | 1.0015 | 0.98 |
| Grade (reference: well differentiated) | ||||
| Moderately differentiated | 1.11 | 0.87 | 1.43 | 0.41 |
| Poorly differentiated | 2.15 | 1.68 | 2.76 | <0.001 |
| Time to surgery (per day) | ||||
| <50 days | 0.9905 | 0.9839 | 0.9971 | 0.005 |
| 50-100 days | 1.0022 | 0.9965 | 1.0080 | 0.45 |
| >100 days | 1.0031 | 1.0005 | 1.0058 | 0.02 |
In a multivariable logistic regression, time to surgery less than 50 days (odds ratio [OR] for <50 days 0.98; 95%CI 0.96–1.01; p=0.16) or between 50 and 100 days (OR 0.98; 95%CI 0.96–1.01; p=0.15) was not associated with margin-positive resection, while time greater than 100 days was associated with increased risk of margin-positive resection (OR 1.01; 95%CI 1.00–1.02; p=0.004). In a second multivariable regression, time to surgery less than 50 days (OR 0.99; 95%CI 0.98–1.00; p=0.30), between 50 and 100 days (OR 1.00; 95%CI 0.99–1.01; p=0.15), or beyond 100 days (OR 1.00; 95%CI 1.00–1.02; p=0.53) was not associated with pathologic nodal upstaging. A third multivariable logistic regression was performed to examine factors associated with a delay in surgery beyond 100 days. Treatment at a high volume center, government insurance, and diagnosis and treatment at different centers were associated with surgery after 100 days, while increasing age and tumor size were associated with surgery within 100 days (Table 5).
Table 5.
Multivariable logistic regression of independent predictors of delay to surgery beyond 100 days
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Odds Ratio | Lower | Upper | p-value |
| Age (per year) | 0.97 | 0.95 | 0.99 | 0.005 |
| Sex (female) | 1.07 | 0.70 | 1.62 | 0.77 |
| Race (reference: White) | ||||
| Black | 1.27 | 0.33 | 4.84 | 0.72 |
| Year of diagnosis (per year) | 0.99 | 0.93 | 1.05 | 0.72 |
| CDCC score (reference: 0) | ||||
| 1 | 0.93 | 0.65 | 1.32 | 0.68 |
| 2+ | 1.71 | 0.96 | 3.06 | 0.07 |
| Insurance status (reference: private) | ||||
| Government | 2.27 | 1.55 | 3.32 | <0.001 |
| None | 1.33 | 0.27 | 6.55 | 0.72 |
| Facility location (reference: metro) | ||||
| Urban | 1.08 | 0.68 | 1.71 | 0.75 |
| Rural | 1.49 | 0.59 | 3.78 | 0.40 |
| Median income (reference: <$38,000) | ||||
| $38000-$47999 | 0.85 | 0.51 | 1.43 | 9.55 |
| $48000-$62999 | 0.72 | 0.41 | 1.28 | 0.27 |
| >$63000 | 0.71 | 0.37 | 1.37 | 0.31 |
| Education (reference: >=21% not high school grads) | ||||
| 13%-20.9% | 1.39 | 0.79 | 2.47 | 0.25 |
| 7%-12.9% | 1.46 | 0.80 | 2.66 | 0.22 |
| <7% | 1.12 | 0.56 | 2.27 | 0.74 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 1.02 | 0.65 | 1.62 | 0.92 |
| Distance to treatment center (per mile) | 1.00 | 1.00 | 1.00 | 0.15 |
| Facility geography (reference: New England) | ||||
| Middle Atlantic (nj,ny,pa) | 0.77 | 0.38 | 1.55 | 0.47 |
| South Atlantic (dc,de,fl,ga,md,nc,sc,va,wv) | 0.72 | 0.36 | 1.45 | 0.36 |
| East North Central (il,in,mi,oh,wi) | 0.99 | 0.51 | 1.92 | 0.97 |
| East South Central (al,ky,ms,tn) | 0.52 | 0.20 | 1.34 | 0.18 |
| West North Central (ia,ks,mn,mo,nd,ne,sd) | 0.41 | 0.18 | 0.96 | 0.04 |
| West South Central (ar,la,ok,tx) | 0.77 | 0.30 | 1.99 | 0.59 |
| Mountain (az,co,id,mt,nm,nv,ut,wy) | 0.39 | 0.19 | 1.30 | 0.13 |
| Pacific (ak,ca,hi,or,wa) | 1.05 | 0.50 | 2.21 | 0.89 |
| Center volume (reference: Q1 ≤1 a year) | ||||
| Q2 (>1-3) | 1.39 | 0.51 | 3.77 | 0.51 |
| Q3 (>3-8) | 2.04 | 0.77 | 5.37 | 0.15 |
| Q4 (>8) | 2.64 | 0.98 | 7.08 | 0.05 |
| Center of diagnosis vs. treatment (reference: same) | ||||
| Diagnosis and treatment at different centers | 1.63 | 1.14 | 2.31 | 0.007 |
| Tumor size (per mm) | 0.98 | 0.97 | 1.00 | 0.009 |
Discussion
We used the NCDB to examine the impact of time to surgery on outcomes in patients with stage I esophageal adenocarcinoma. We found that increasing time to surgery greater than 100 days was associated with worse survival and increased risk of margin-positive resection although these risks were small. Our study suggests that the safest interval from diagnosis to surgery in stage I esophageal adenocarcinoma is about 100 days.
This is the only study, to our knowledge, that examines the question of a safe interval to surgery in patients undergoing upfront surgery for esophageal cancer. The bulk of literature in esophageal cancer has, instead, focused on the optimum timing of surgery following induction therapy. There are observational studies in other cancers, however, that consider this question with varying results. For instance, Samson and colleagues examined outcomes in patients undergoing surgery for non-small cell lung cancer, and found that a delay in surgery beyond eight weeks was associated with decreased median survival and higher pathologic upstaging9. Similarly, Yang and colleagues used the NCDB to study the optimum timing of lobectomy in stage IA squamous cell lung cancer, and found that overall survival worsened after 38 days6. Mahmud and colleagues reported that an interval to radical cystectomy greater than 12 weeks was associated with worse survival in patients with bladder cancer7. Bagaria and colleagues, on the other hand, found that time to surgery even beyond 84 days was not associated with a change in survival for patients with colon cancer8. None of these studies reported an increase in margin-positive resection with increased time to surgery. In our analysis, time to surgery greater than 100 days was associated with a statistically significant decrease in survival even though the effect size was small: at 110 days, the adjusted risk of mortality increased by 3% compared to 100 days, and at 120 days the risk increased by 7%. However, the most judicious conclusion of this study is that esophagectomy should be offered as early as possible since outcomes worsen after 100 days. In a cancer that is managed by a multidisciplinary team of medical and radiation oncologists, gastroenterologists, surgeons, and nutritionists, offering surgery soon after diagnosis may prove challenging. The need to adequately stage the patient and both evaluate and optimize the patient for surgery should be balanced by the risks presented in this manuscript, which although small are detectable.
Our study also found that increasing time to surgery in the first 50 days after diagnosis is associated with improved survival. Patients who received surgery 20 days following diagnosis, for instance, had an estimated 28% increased mortality compared to those receiving surgery at 50 days. The reason for this finding is unclear, and most suggestive of bias that was not accounted for in patients undergoing very early surgery. These patients, for instance, may have had complications of esophageal cancer warranting early surgery. They may also have received surgery without adequate staging, even though the risk of pathologic nodal upstaging was not increased in this group and the pathologic stage distribution was similar compared to the other groups of patients.
The study’s finding that time to surgery beyond 100 days was associated with a margin-positive resection is unexpected. A clinical T1 tumor would not ordinarily have a doubling time that would result in a margin-positive resection with a wait of just over three months. Because the NCDB does not provide further information on the positive margins (e.g., location, whether frozen section was obtained), we cannot speculate on the reasons for margin-positive resection in this study. It is possible that a margin-positive resection occurred due to factors other than delay of surgery, but in our logistic regression, time to surgery was the only variable associated with a margin-positive resection, while other candidate factors like center volume, treatment at an academic center, tumor size, and grade were not significantly associated with it. However, our choice and design of variables included in the regression are limited. For instance, while we divided center volume into quartiles based on distribution, there are likely substantial differences between centers that perform 8 esophagectomies a year and those that perform more than 13 a year by Leapfrog Group standards14.
Our study has several other important limitations. It is a retrospective cohort study and carries an inherent risk of bias due to confounding factors that we may not fully understand. For instance, we do not know why patients were offered surgery at a certain interval from diagnosis, although we found that patients with government insurance and at high volume centers are more likely to have delayed surgery. The relationship between treatment at a high volume center and time to surgery did not have an appreciable effect on survival, though, because an interaction term of these two variables was found to be non-significant (p=0.23) in a multivariable Cox model. This suggests that treatment at a high volume center and time to surgery independently exerted an effect on survival in our study. In addition, the NCDB does not catalogue information about staging methods used, which may have significantly altered the time to surgery and survival via increased accuracy of staging. However, the pathologic stage distribution was not significantly different amongst patients undergoing surgery within 50 days, between 50 and 100 days, and beyond 100 days, suggesting that any variation in time due to staging methods used may not be associated with a significant difference in survival. Another explanation for the worse survival observed after 100 days may have been that patients with poor functional status, nutrition, or with significant comorbidities were offered surgery after optimization, but may also have been poorer surgical candidates at baseline, accounting for the worse survival. While we attempted to adjust for this using multivariable analysis, the NCDB does not contain information about functional status and nutrition that are important components of fitness for surgery. The delayed surgery cohort may also include patients who initially underwent ER and were found to have positive margins or deeper invasion, prompting esophagectomy15. Because the NCDB only reports the most definitive operation a patient received, we do not know how many patients in the esophagectomy group also underwent an initial ER. The NCDB also only reports overall survival rather than disease-free survival, which limits the external validity of this study. The small cohort sizes of the patients with reported T1a or T1b disease limits the value of those subgroup analyses as well. For instance, the shape of the RCS curve for patients with cT1b disease suggests that survival following a delay in surgery may be worse compared to that of cT1a patients, but this hypothesis should be tested in a larger cohort where a more meaningful multivariable analysis is possible. The results of the study are also affected by missing data because only a minority of patients with T1N0M0 esophageal adenocarcinoma met criteria for the study. However, of the patients receiving esophagectomy, only a fraction of patients had missing data on time to surgery (1.6%).
In this NCDB analysis, increasing time to surgery beyond 100 days was associated with worse overall survival and increased margin-positive resection, though these risks were small. In patients with stage I esophageal cancer, surgery should be offered as soon as safely possible because outcomes worsen after 100 days (Figure 3).
Figure 3.
In this National Cancer Database analyses, restricted cubic splines identified that survival worsens for patients with stage I esophageal adenocarcinoma if surgery is offered later than 100 days from diagnosis. The risk of margin-positive resection also increases beyond 100 days while the risk of pathologic nodal upstaging remains similar.
Supplementary Material
Supplemental Figure 1. Kaplan-Meier survival curves for patients stratified by time to surgery, in days. Y-axis reflects survival as a percentage while X-axis time, in years, from diagnosis. Shaded areas represent bounds of the 95% confidence interval
Supplemental Figure 2. Unadjusted restricted cubic spline transformation of time to surgery in patients with clinical T1a esophageal adenocarcinoma. Arrows denote pre-specified knots selected in unadjusted analysis. Y-axis demonstrates the unadjusted log hazard of mortality while X-axis the time to surgery in days. Dotted lines reflect bounds of the 95% confidence interval
Supplemental Figure 3. Unadjusted restricted cubic spline transformation of time to surgery in patients with clinical T1b esophageal adenocarcinoma. Arrows denote pre-specified knots selected in unadjusted analysis. Y-axis demonstrates the unadjusted log hazard of mortality while X-axis the time to surgery in days. Dotted lines reflect bounds of the 95% confidence interval
Central Message.
Surgery for stage I esophageal adenocarcinoma is associated with worse survival and increased margin-positive resection after 100 days from diagnosis.
Perspective Statement.
There is no literature examining the safest interval for surgery for stage I esophageal cancer. We used a large national database to demonstrate that surgery later than 100 days from diagnosis is associated with worse survival and increased margin-positive resection. Surgery should be offered as soon as safely possible for these patients.
Acknowledgements
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Drs. Raman and Voigt were supported by a National Institutes of Health T-32 grant 5T32CA093245 in surgical oncology. Dr. Jawitz was supported by a National Institutes of Health T-32 grant 5T32HL069749 in clinical research.
This work was presented at the American Association of Thoracic Surgeons Annual Meeting in Toronto in May 2019.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers. Journal of the National Comprehensive Cancer Network. 2011;9(8):830–887. doi: 10.6004/jnccn.2011.0072 [DOI] [PubMed] [Google Scholar]
- 2.Shaikh T, Ruth K, Scott WJ, et al. Increased Time From Neoadjuvant Chemoradiation to Surgery Is Associated With Higher Pathologic Complete Response Rates in Esophageal Cancer. The Annals of Thoracic Surgery. 2015;99(1):270–276. doi: 10.1016/j.athoracsur.2014.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haisley KR, Laird AE, Nabavizadeh N, et al. Association of Intervals Between Neoadjuvant Chemoradiation and Surgical Resection With Pathologic Complete Response and Survival in Patients With Esophageal Cancer. JAMA Surg. 2016;151(11):e162743–e162743. doi: 10.1001/jamasurg.2016.2743 [DOI] [PubMed] [Google Scholar]
- 4.Franko J, Voynov G, Goldman CD. Esophagectomy Timing After Neoadjuvant Therapy for Distal Esophageal Adenocarcinoma. The Annals of Thoracic Surgery. 2016;101(3):1123–1130. doi: 10.1016/j.athoracsur.2015.09.044 [DOI] [PubMed] [Google Scholar]
- 5.Chiu C-H, Chao Y-K, Chang H-K, et al. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol. 2013;20(13):4245–4251. doi: 10.1245/s10434-013-3139-7 [DOI] [PubMed] [Google Scholar]
- 6.Yang C-FJ, Wang H, Kumar A, et al. Impact of Timing of Lobectomy on Survival for Clinical Stage IA Lung Squamous Cell Carcinoma. Chest. 2017;152(6):1239–1250. doi: 10.1016/j.chest.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 7.Mahmud SM, Fong B, Fahmy N, Tanguay S, Aprikian AG. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol. 2006;175(1):78–83; discussion 83. doi: 10.1016/S0022-5347(05)00070-4 [DOI] [PubMed] [Google Scholar]
- 8.Bagaria SP, Heckman MG, Diehl NN, Parker A, Wasif N. Delay to Colectomy and Survival for Patients Diagnosed with Colon Cancer. Journal of Investigative Surgery. 2018;0(0):1–8. doi: 10.1080/08941939.2017.1421732 [DOI] [PubMed] [Google Scholar]
- 9.Samson P, Patel A, Garrett T, et al. Effects of Delayed Surgical Resection on Short-Term and Long-Term Outcomes in Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg. 2015;99(6):1906–1912; discussion 1913. doi: 10.1016/j.athoracsur.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108–113; discussion 113–114. doi: 10.1067/mtc.2003.93 [DOI] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrell FE. Multivariable Modeling Strategies. In: Harrell Jr Frank E, ed. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer Series in Statistics. Cham: Springer International Publishing; 2015:63–102. doi: 10.1007/978-3-319-19425-7_4 [DOI] [Google Scholar]
- 13.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–785. doi: 10.1097/SLA.0b013e318211cc0f [DOI] [PubMed] [Google Scholar]
- 14.Varghese TK, Wood DE, Farjah F, et al. Variation in Esophagectomy Outcomes in Hospitals Meeting Leapfrog Volume Outcome Standards. The Annals of Thoracic Surgery. 2011;91(4):1003–1010. doi: 10.1016/j.athoracsur.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Plum PS, Hölscher AH, Pacheco Godoy K, et al. Prognosis of patients with superficial T1 esophageal cancer who underwent endoscopic resection before esophagectomy-A propensity score-matched comparison. Surg Endosc. 2018;32(9):3972–3980. doi: 10.1007/s00464-018-6139-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan-Meier survival curves for patients stratified by time to surgery, in days. Y-axis reflects survival as a percentage while X-axis time, in years, from diagnosis. Shaded areas represent bounds of the 95% confidence interval
Supplemental Figure 2. Unadjusted restricted cubic spline transformation of time to surgery in patients with clinical T1a esophageal adenocarcinoma. Arrows denote pre-specified knots selected in unadjusted analysis. Y-axis demonstrates the unadjusted log hazard of mortality while X-axis the time to surgery in days. Dotted lines reflect bounds of the 95% confidence interval
Supplemental Figure 3. Unadjusted restricted cubic spline transformation of time to surgery in patients with clinical T1b esophageal adenocarcinoma. Arrows denote pre-specified knots selected in unadjusted analysis. Y-axis demonstrates the unadjusted log hazard of mortality while X-axis the time to surgery in days. Dotted lines reflect bounds of the 95% confidence interval