Abstract
Background
Rheumatoid arthritis (RA) is an autoimmune disease with systemic inflammatory arthritis. This meta-analysis was conducted to examine the association between occupational exposure to silica and the risk of developing RA among different workers.
Methods
In this meta-analysis, we searched relevant published studies using major electronic databases including Scopus, PubMed, ISI Web of Science, and Google Scholar search engine up to October 2019, and the references of retrieved articles were also checked for further possible sources. A random-effects model was used to account for heterogeneity among the results of the studies using the pooled odds ratios (ORs) and their 95% confidence intervals (CIs). The Q-statistic and I2 tests were calculated to assess heterogeneity between the studies.
Results
The pooled calculation of OR indicated a significant association between occupational exposure to silica and risk of developing RA among different workers (OR = 2.59, 95% CI = 1.73 to 3.45). In addition, the pooled estimates of OR in smokers were statistically significant (OR = 2.49, 95% CI = 1.13 to 3.86).
Conclusions
The findings of the present study reveal that occupational exposure to silica may be associated with increased risk of developing RA.
Keywords: Meta-analysis, Occupation, Rheumatoid arthritis, Silica exposure
1. Introduction
Autoimmune diseases as a general term refer to a group of immune disorders, including scleroderma, renal disease, lupus, and rheumatoid arthritis (RA), which has been recognized in the past decade. RA is a chronic inflammatory disease characterized by symmetric polyarthritis [1]. In this chronic disease, painful inflammation of the synovial tissues of multiple joints can ultimately lead to joint destruction and disabilities. This autoimmune diseases affect approximately 0.5–1% of the population around the world. According to the findings of many previous studies, some parameters such as genetics, environmental pollutants, certain underlying diseases and their complications, types of food diet, including consumption of vitamin D supplements and antioxidants, and consumption of alcohol are important factors in the etiology of RA [2]. There is an increase in the number of studies about the genetic role, but data regarding environmental factors that may cause RA are rare [3]. In recent years, many researchers are interested to investigate the relation between occupational exposures (e.g., silica) and risk of RA [4].
Crystalline silica is a ubiquitous mineral and a basic component of soil, sand, granite, and many other minerals. There are two kinds of silica, organic and inorganic silica. Occupational exposure to inorganic silica occurs in a large number of industries and settings, such as mines, stone quarries and granite production, ceramic and pottery industries, steel production, tunnel building, and many others [5]. Experimental studies have shown that mineral oils can induce arthritis in animals, and it has been suggested that they may act as the so-called adjuvants enhancing immune reactions. A similar mode of action has been suggested for silica [6].
The occupational exposure to crystalline silica may generally occur all over the world via various industries such as those of construction, glass, mining, agriculture, ceramics, and electronics [7]. Studies conducted by Blanc et al. [8] on construction workers, by Vihlborg et al. [9] on iron foundry workers, and by Turner and Cherry [10] on pottery industry workers, who were exposed to silica, showed that there was a significant relationship between exposure to silica and increased risk of autoimmune disorders. In addition to silica, cigarette smoking has been documented to be an environmental risk factor, and its interaction with silica has been shown to be associated with a more severe form of RA [[11], [12], [13]]. Stolt et al. [14]reported that combined exposure to silica and cigarette smoking in the Swedish population was a significant risk factor in the development of RA. The association between occupational exposure to silica and the risk of developing RA has been investigated previously. Several studies showed a significant association between exposure to silica and risk of developing RA [6,15,16], whereas other studies demonstrated no association between them [10]. Therefore, this meta-analysis was conducted to pool all case–control, cross-sectional, and cohort studies in broader databases to obtain the association between occupational exposure to inorganic dusts (crystalline silica) and the risk of developing RA among different workers.
2. Materials and methods
2.1. Data sources
We performed a systematic review and meta-analysis study to investigate the relationship between occupational exposure to silica and the risk of developing RA. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were used to improve the reporting quality of the systematic review and provide transparency in the selection process of articles [17]. To select the relevant studies, a systematic search was carried out on leading databases, including ISI Web of Science, Scopus, Google Scholar search engine, and PubMed up to October 2019.
2.2. Search strategy
The electronic search was carried out using the following keywords: (“Arthritis rheumatoid” OR “rheumatoid arthritis”) AND (“silicon-dioxide” OR “silica” OR “silicon” OR “silic” OR “tridynite”) Filters: Humans; English.
2.3. Inclusion and exclusion criteria
Only manuscripts in English were included. The relevant documents in association with occupational exposure to silica and the risk of developing RA were identified, and their titles, abstracts, and also full texts were completely reviewed. In addition, references of the searched studies were evaluated to seek additional relevant studies and prevent missing information. In this study, cross-sectional, case–control, and cohort studies were included. Case series, case reports, review articles, letters to the editors, and also duplicate articles were excluded from this study.
2.4. Identification of RA and exposure assessment
In the present study, cases of RA were defined as those subjects for whom the examining physician had responded yes to the question on RA on the last available medical examination form. In addition, assessment of occupational exposure was performed based on job title, postal questionnaire about occupational histories, telephone interview about occupational histories or using the medical record [developing anticitrullinated protein antibody (ACPA), ACPA-positive and ACPA-negative RA], and diagnoses of silicosis as a proxy for silica exposure, according to American College of Rheumatology criteria for RA.
2.5. Data extraction and quality assessment
The quality of each retrieved study was independently reviewed by two authors (F.M. and S.K.), and any disagreement between the two authors was discussed to reach a consensus. In this meta-analysis, to reduce data collection errors, data extraction was performed for all eligible studies using the extraction form. In this form, information such as first author, publication's year, age (mean or range), sample size, adjustment, estimated effect size (overall and in sub-groups), and occupation was collected.
2.6. Quality assessment
The quality assessment of the studies was performed by the improved Newcastle–Ottawa scale [18]. In this scale, there are three sections, including election (four items), comparability (two items), and exposure or outcomes (three items), and it ranges from 0 to 9. Two investigators conducted the quality assessment independently, and scores of the studies were considered low quality (<7 points) and high quality (≥7 points).
2.7. Statistical analysis
The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated from eligible studies using the random-effects model. The subgroup analyses were conducted based on the adjustment or crude reporting of association. The heterogeneity of results between the studies was checked using the Q value and I2 statistic [19]. The low, moderate, and high heterogeneity was considered by I2 statistic values of 25%, 50%, and 75%, respectively. The publication bias was assessed using the funnel plot as well as Begg's and Egger's tests [20]. All statistical analyses were performed at a significance level of 0.05 using Stata software version 13 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Description of the studies
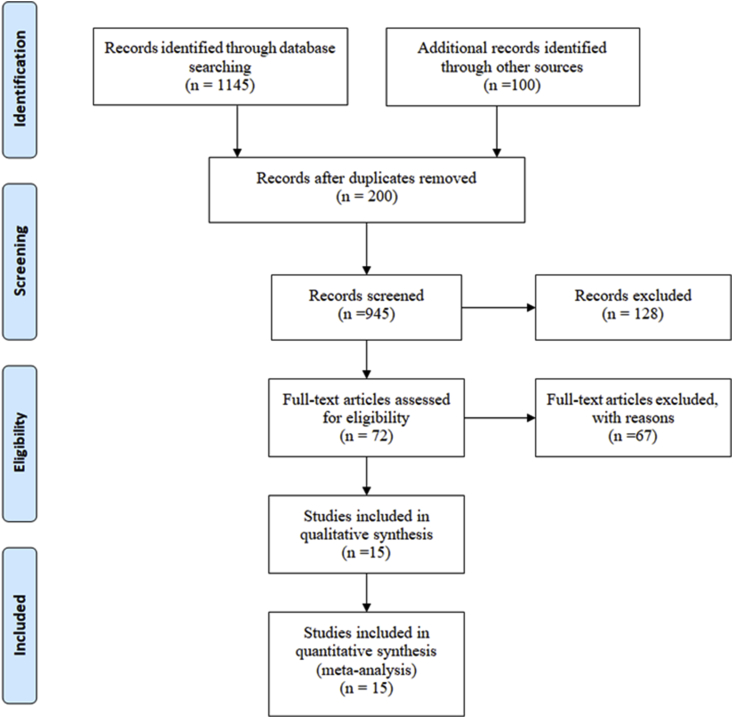
A total number of 1245 studies were retrieved at the beginning of our systematic search on the mentioned databases until October 2018. We excluded 200 duplicates and 817 irrelevant topics through screening titles and abstracts. From the remaining 72 articles, 67 studies were excluded because they were letters to editors, commentaries, and reviews or they did not fulfill our inclusion criteria. Finally, 15 articles remained for the meta-analysis. Fig. 1 shows the search process. The included studies in this meta-analysis were as follows: five cohort studies [8], [9], [10], [16], [39], eight case control studies [14], [21], [22], [23], [24], [25], [27], [28], and two cross-sectional studies [4,26]. Estimation was based on OR in 11 studies, based on risk ratio and rate ratio in three studies, and based on hazard ratio in one study. All included articles were published in English language.
Fig. 1.
Flow diagram of study selection process.
3.2. Main analysis
The association between occupational exposure to silica and the risk of developing RA, which was evaluated using the Forest plot, are indicated in Fig. 2 and Fig. 3. The pooled estimates of OR were according to the crude and adjusted ORs (OR = 1.95, 95% CI = 0.89 to 3.01 and OR = 2.59, 95% CI = 1.73 to 3.45, respectively) between occupational exposure to silica and the risk of developing RA among different workers. Four studies evaluated the association between smoking occupational exposure to silica and the risk of developing RA separately based on rate ratio (RR) and hazard ratio [9,16,27,28], and all of them showed a significant association between exposure to silica and the risk of developing RA. There was moderate heterogeneity among the studies that investigated the risk of developing RA among different workers according to adjusted OR (I2 = 52.8%, p = 0.024). In addition, as shown in Fig. 5, asymmetric shape of the funnel plot indicates an evidence of the publication bias in the included studies. The p values for Begg's and Egger's regression were 0.049 and 0.057, respectively.
Fig. 2.
A Forrest plot for estimated crude OR of RA for silica exposure. CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis; ES, effect size.
Fig. 3.
A Forrest plot for estimated adjusted OR of RA for silica exposure. CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis; ES, effect size.
Fig. 5.
Funnel plot of included studies in the meta-analysis. OR, odds ratio.
3.3. Subgroup analysis
We also separately pooled the ORs with regard to the association between exposure to silica and the risk of developing RA in studies that reported this association in smokers. As shown in Fig. 4, the pooled estimates of OR in smokers were statistically significant (OR = 2.49, 95% CI = 1.13 to 3.86).
Fig. 4.
A Forrest plot for estimated adjusted OR of RA for silica exposure in smokers. CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis; ES, effect size.
3.4. Quality of the studies
Three of the included studies in the present meta-analysis were of low quality, and others were of high quality according to the Newcastle–Ottawa scale (Table 1).
Table 1.
Characteristics of the studies used for meta-analysis.
| 1st author, year | Reference number | Country | Design | Sample | Age (years) | Estimate | Quality | HR/OR/RR (95% CI) in subgroups | Exposure assessment | Duration of exposure | Occupation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paul Blanc et al. (2016) | [8] | Sweden | Cohort | 2551 (30 RA cases) | — | HR | High | Overall: 1.57 (1.2–2.06) | Occupational histories or using the medical record | 9 years | Foundry workers with exposure for >7.5 years |
| Paul D. Blanc et al. (2015) | [16] | Sweden | Cohort | 240,983 (713 RA cases) | 30–84 | AOR | High | Overall: 1.39 (1.17–1.64) Smokers: 1.36 (1.11–1.68) |
Occupational histories or using the medical record | 13 years | Construction workers |
| Jiang et al. (2017) | [22] | Sweden | CC | 599 | — | AOR | High | Overall: 7.5 (4.2–13.2) | Medical record [anticitrullinated protein antibody (ACPA)–positive RA] | 12–19 years | Swedish Epidemiological Investigation of RA project |
| Klockars et al. (1987) | [27] | Finland | Cohort | 1026 | 15–72 | RR | High | Overall: 5.08 (3.31–7.79) | Medical record [sarcoidosis and seropositive rheumatoid arthritis (RA)] | 12 years | Granite workers |
| Olsson et al. (2004) | [4] | Sweden | CS | 2204 control, 715 cases | 16–75 | AOR | Low | Overall: 2.2 (1.3–3.5) | Postal questionnaire about occupational histories | 6 months | Farmers and farm workers |
| Stolt et al. (2005) | [24] | Sweden | CC | 276 control, 276 cases | 18–70 | AOR | High | 18–70 years: 2.2 (1.2–3.9) >70 years: 2.7 (1.2–5.8) |
Criteria of the American College of Rheumatology (ACR) | 5 years | Worked with rock drilling or stone crushing |
| Yahya et al. (2013) | [28] | Malaysian | CC | 213 control, 149 cases | 18–70 | COR | High | Overall: 2.4 (1.0–5.6) Smokers: 7.5 (2.3–24.2) |
Medical record (ACPA-positive RA) | 4 years | Occupational exposure to rock drilling, stone crushing |
| Stolt et al. (2010) | [14] | Sweden | CC | 659 control, 577 cases | 18–70 | AOR | High | Stone dust: 1.67 (1.13–2.48) Rock drilling: 2.34 (1.17–4.68) Smokers: 7.36 (3.31–16.38) |
Criteria of the ACR for RA | 10 years | Exposure to stone dust, rock drilling |
| Too et al. (2015) | [21] | Malaysian | CC | 910 control, 910 cases | 18–70 | AOR | High | Overall: 2.8 (1.6–5.2) | Medical record (developing ACPA- positive and ACPA-negative RA) | 1–4 years | Textile dust workers |
| Trupin et al. (2018) | [26] | US | CS | 973 | 66 ± 10 | AOR | High | Overall: 4.4 (2.7, 7.2) Smokers: 2.1 (1.1, 3.9) |
Telephone interview about occupational histories | Coal mining | |
| Turner et al. (2000) | [10] | United Kingdom | Cohort | 8325 (58 RA cases) | — | COR | Low | Overall: 5.36 (1.92–15.03) Smokers: 1.2 (0.57–2.49) |
Occupational histories or using the medical record | 4.4 and 5.6 years | Coal mining |
| Vihlborg et al. (2017) | [9] | Swedish | Cohort | 2187 | 20–45 | RR | High | Overall: 1.7 (1.01–2.69) | Medical record [sarcoidosis and seropositive rheumatoid arthritis (RA)] | NM | Workers in Swedish iron foundries |
| Noonan et al. (2006) | [23] | Montana | CC | 387 control, 129 cases | ≥65 | AOR | Low | Overall: 3.23 (1.31–7.96) | Occupational histories or using the medical record | At least 6 months | Occupational and environmental exposure to asbestos-contaminated vermiculite |
| Olsson et al. (2000) | [25] | Sweden | CC | 859 control, 422 cases | 25–75 | COR | High | Farmers: 1.8 (1.0–3.51) Textile workers: 2.0 (0.3–16.2) Asphalts: 14.0 (1.2–799.0) Service stations: 2.2 (0.5–9.5) |
Postal questionnaire about occupational histories | 27 months | Workers of textile asphalts and service stations and farmers |
| Steenland et al. (1995) | [39] | US | Cohort | 3328 | — | RR | High | Overall: 2.19 (1.27–3.50) | NM | NM | Gold miners |
AOR, adjusted OR; CC, case–control, CI, confidence interval; CS, cross-sectional, COR: crude OR; HR, hazard ratio; OR, odds ratio; RA, rheumatoid arthritis; NM, not mentioned; RR, rate ratio.
4. Discussion
In this systematic review, we evaluated the association between occupational exposure to silica and the risk of developing RA in different workers. The findings obtained from this study are important and useful in implementing different programs for the prevention of development of RA among health workers. In this meta-analysis, the association between occupational exposure to silica and the risk of developing RA was evaluated among various workers using all case–control, cross-sectional, and cohort studies that were evaluated and conducted up to now. The results of the present study were similar to the results of ten included studies, which showed that there was a significant association between occupational exposure to silica and the risk of developing RA among various workers. The reports of the pooled estimate and heterogeneity among the included studies showed that the obtained OR was 2.59 (moderate heterogeneity). Among the previous related studies, up to now, only one meta-analysis has been performed on the association between exposure to silica and the risk of developing RA. Khuder et al. [29] investigated the association between exposure to silica and increased mortality rate due to developing RA among workers (RR = 3.43, 95% CI = 2.22 to 5.22). Ten studies were included in this meta-analysis, in which the electronic search was limited to the MEDLINE database. In addition, the previous relevant studies were those that had been conducted until 2000, whereas in the current meta-analysis, in addition to PubMed, we searched ISI Web of Science, Scopus, and Google scholar databases up to October 2019 and included 15 studies. The results of the studies by Pollard [30] and Vihlborg et al. [9] showed that environmental factors have an important role in the development of RA. These factors included food, fluids, air pollution, chemical compounds, kind of infections, by-products obtained from different processes, radiation, and occupational and environmental exposure to silica [31,32]. Although epidemiological studies showed that exposure to silica can increase risk of developing RA, the exact mechanism of silica that may lead to development of RA is not clear. Possible mechanism that was suggested by various studies is stimulation of the immune system. Silica is a selective toxin for macrophages that binds to their cell membrane and causes disturbance in T lymphocyte–macrophage immune regulation and can lead to secretion of inflammatory factors that are responsible for stimulating DNA synthesis in synovial cells [33]. In addition to exposure to silica by various industries and mines, lifestyle, genetic factors, alcohol consumption, and cigarette smoking have been investigated in recent studies [15,34,35]. In our meta-analysis, association between exposure to silica and risk of developing RA in smoking workers was significant because cigarette smoke contains more than 4,000 chemicals that are very toxic or carcinogenic. The metabolism of this component in the body is performed by (Phase I and Phase II) enzymes in the liver that play a critical role in decreasing oxidative stress and toxicity of smoking in the body [36]. Despite multiple reports indicating relationship between smoking and exposure to silica and risk of developing RA, it depends on various factors such as second-hand or passive exposure to smoking and also degree of heavy and low exposures to silica in different jobs, such as rock drilling, stone crushing, and stone dust [22]. Few studies have investigated the association between amount and dose of silica or duration of exposure to silica and development of RA. Several studies showed that there was a significant association between risk of development of RA and cigarette smoking [16,37]. The results of the study conducted by Zeng [37] showed a significant relationship between exposure to silica and smoking and development of RA among the Swedish population; the results of the study also indicated that the effects of cumulative dose and duration of smoking in development of RA can remain after 10 years of smoking cessation. In other study performed by Karlson et al. [38], they showed that there was a dose–risk relationship between cigarette smoking and the risk of developing RA and this risk was higher in workers with heavy smoking and longer duration. Therefore, according to the results of several previous studies and the present study, there was a relationship between smoking and exposure to silica and risk of developing RA [37].
The present meta-analysis has two limitations: first, there was no specific categorization for occupations, and we could not have subgroup analysis according to different occupations. Second, baseline characteristics in the included studies such as duration of exposure, age-group, and so on were substantially different and imposed residual confounding to the results.
5. Conclusions
Investigations regarding exposure to silica or risk of developing RA are sufficient, but few studies have paid much attention to their relationship. The results of pooled effects analysis on fifteen studies in the present study indicate a significant association between exposure to silica and risk of developing RA. These findings can provide a new and useful approach for the prevention of developing RA in workers exposed to silica in different occupational environments.
Ethical approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgments
The authors would appreciate the Deputy of Research and Technology, Hamadan University of Medical Sciences, for financial support of the study.
References
- 1.Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Klareskog L., Malmström V., Lundberg K., Padyukov L., Alfredsson L., editors. Semin Immunol. 2011 doi: 10.1016/j.smim.2011.01.014. Elsevier. [DOI] [PubMed] [Google Scholar]
- 2.Cooper G.S., Gilbert K.M., Greidinger E.L., James J.A., Pfau J.C., Reinlib L., Richardson B.C., Rose N.R. Recent advances and opportunities in research on lupus: environmental influences and mechanisms of disease. Environ Health Perspect. 2008;116(6):695–702. doi: 10.1289/ehp.11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacGregor A.J., Snieder H., Rigby A.S., Koskenvuo M., Kaprio J., Aho K., Silman A.J. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43(1):30–37. doi: 10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Olsson Å.R., Skogh T., Axelson O., Wingren G. Occupations and exposures in the work environment as determinants for rheumatoid arthritis. Occup Environ Med. 2004;61(3):233–238. doi: 10.1136/oem.2003.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc P.D., Trupin L., Yelin E.H., Schmajuk G. Occupational exposure to coal and silica dust and risk of rheumatoid arthritis in Appalachia. Am J Respir Crit Care Med. 2018;197 [Google Scholar]
- 6.Parks C.G., Conrad K., Cooper G.S. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107(Suppl. 5):793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yassin A., Yebesi F., Tingle R. Occupational exposure to crystalline silica dust in the United States, 1988–2003. Environ Health Perspect. 2004;113(3):255–260. doi: 10.1289/ehp.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc P., Andersson L., Bryngelsson L. European Respiratory Society; 2016. Risk of rheumatoid arthritis in a cohort of silica-exposed Swedish foundry workers. [Google Scholar]
- 9.Vihlborg P., Bryngelsson L., Andersson L., Graff P. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: a retrospective cohort study. BMJ Open. 2017;7(7) doi: 10.1136/bmjopen-2017-016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner S., Cherry N. Rheumatoid arthritis in workers exposed to silica in the pottery industry. Occup Environ Med. 2000;57(7):443–447. doi: 10.1136/oem.57.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costenbader K.H., Feskanich D., Mandl L.A., Karlson E.W. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503. doi: 10.1016/j.amjmed.2005.09.053. e1-. e9. [DOI] [PubMed] [Google Scholar]
- 12.Stolt P., Källberg H., Lundberg I., Sjögren B., Klareskog L., Alfredsson L. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(4):582–586. doi: 10.1136/ard.2004.022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Källberg H., Ding B., Padyukov L., Bengtsson C., Rönnelid J., Klareskog L., Alfredsson L., EIRA Study Group Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–511. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolt P., Yahya A., Bengtsson C., Källberg H., Rönnelid J., Lundberg I., Klareskog L., Alfredsson L., EIRA Study Group Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann Rheum Dis. 2010;69(6):1072–1076. doi: 10.1136/ard.2009.114694. [DOI] [PubMed] [Google Scholar]
- 15.Hoovestol R.A., Mikuls T.R. Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep. 2011;13(5):431. doi: 10.1007/s11926-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 16.Blanc P.D., Järvholm B., Torén K. Prospective risk of rheumatologic disease associated with occupational exposure in a cohort of male construction workers. Am J Med. 2015;128(10):1094–1101. doi: 10.1016/j.amjmed.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M. 2017. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2009 September 15.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from: [Google Scholar]
- 19.Higgins J.P., Green S. John Wiley & Sons; 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 20.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ: Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Too C.L., Muhamad N.A., Ilar A., Padyukov L., Alfredsson L., Klareskog L., Murad S., Bengtsson C., My EIRA Study Group Occupational exposure to textile dust increases the risk of rheumatoid arthritis: results from a Malaysian population-based case-control study. Ann Rheum Dis. 2016;75(6):997–1002. doi: 10.1136/annrheumdis-2015-208278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X., Bengtsson C., Kallberg H., Klareskog L., Alfredsson L. Amount of smoking, duration of smoking cessation, and their interaction with silica exposure in the risk of rheumatoid arthritis: results from the Swedish epidemiological investigation of rheumatoid arthritis study. Arthritis Rheumatol. 2014;66 doi: 10.1136/annrheumdis-2017-212145. S1262-S. [DOI] [PubMed] [Google Scholar]
- 23.Noonan C.W., Pfau J.C., Larson T.C., Spence M.R. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ Health Perspect. 2006;114(8):1243–1247. doi: 10.1289/ehp.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolt P., Padyukov L., Kallberg H., Lundberg I., Klareskog L., Alfredsson L. Silica exposure is associated with increased risk of a restricted sub-group of rheumatoid arthritis linked to antibodies to cyclic citrullinated peptides. Arthritis Rheum. 2005;52(9) S404-S. [Google Scholar]
- 25.Olsson A.R., Skogh T., Wingren G. Occupational determinants for rheumatoid arthritis. Scand J Work Environ Health. 2000;63:243–249. doi: 10.5271/sjweh.538. [DOI] [PubMed] [Google Scholar]
- 26.Trupin L., Yelin E.H., Schmajuk G., Blanc P. Occupational exposure to coal and silica dust is associated with elevated risk of rheumatoid arthritis in coal mining areas of US. Arthritis Rheumatol. 2018;70 [Google Scholar]
- 27.Klockars M., Koskela R., Järvinen E., Kolari P., Rossi A. Silica exposure and rheumatoid arthritis: a follow up study of granite workers 1940-81. Br Med J (Clin Res Ed). 1987;294(6578):997–1000. doi: 10.1136/bmj.294.6578.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahya A., Bengtsson C., Larsson P., Too C.L., Mustafa A.N., Abdullah N.A., Muhamad N.A., Klareskog L., Murad S., Alfredsson L. Silica exposure is associated with an increased risk of developing ACPA-positive rheumatoid arthritis in an Asian population: evidence from the Malaysian MyEIRA case-control study. Mod Rheumatol. 2014;24(2):271–274. doi: 10.3109/14397595.2013.854076. [DOI] [PubMed] [Google Scholar]
- 29.Khuder S.A., Peshimam A.Z., Agraharam S. Environmental risk factors for rheumatoid arthritis. Rev Environ Health. 2002;17(4):307–315. doi: 10.1515/reveh.2002.17.4.307. [DOI] [PubMed] [Google Scholar]
- 30.Pollard K.M. Environment, autoantibodies, and autoimmunity. Front Immunol. 2015;6:60. doi: 10.3389/fimmu.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayapal M., Bhattacharjee R.N., Melendez A.J., Hande M.P. Environmental toxicogenomics: a post-genomic approach to analysing biological responses to environmental toxins. Int J Biochem Cell Biol. 2010;42(2):230–240. doi: 10.1016/j.biocel.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Pollard K.M. Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun. 2012;38(2–3):J177–J186. doi: 10.1016/j.jaut.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown J.M., Pfau J.C., Pershouse M.A., Holian A. Silica, apoptosis, and autoimmunity. J Immunotoxicol. 2005;1(3–4):177–187. doi: 10.1080/15476910490911922. [DOI] [PubMed] [Google Scholar]
- 34.Silman A.J., Pearson J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res Ther. 2002;4(S3):S265. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ollier W., Thomson W. Population genetics of rheumatoid arthritis. Rheum Dis Clin N Am. 1992;18(4):741–759. [PubMed] [Google Scholar]
- 36.Kiyohara C., Otsu A., Shirakawa T., Fukuda S., Hopkin J.M. Genetic polymorphisms and lung cancer susceptibility: a review. Lung Canc. 2002;37(3):241–256. doi: 10.1016/s0169-5002(02)00107-1. [DOI] [PubMed] [Google Scholar]
- 37.Zeng P. Institutet för miljömedicin/Institute of Environmental Medicine; 2017. Occupational factors and risk of rheumatoid arthritis: studies on silica, physical workload and cold work environment. [Google Scholar]
- 38.Karlson E.W., Chang S.-C., Cui J., Chibnik L.B., Fraser P.A., De Vivo I., Costenbader K.H. Gene–environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steenland K., Brown D. Mortality study of gold miners exposed to silica and nonasbestiform amphibole minerals: An update with 14 more years of follow-up. Am J Ind Med. 1995;27(2):217–229. doi: 10.1002/ajim.4700270207. [DOI] [PubMed] [Google Scholar]