Abstract
Background
Children with congenital Zika syndrome (CZS) maintain severe motor impairments at the end of the first year of life. Presence of certain symptoms and comorbidities increase these children's vulnerability.
Aims
To evaluate gross motor function of a group of Brazilian children with CZS at 24 months of age and to investigate the association between the presence of CZS symptoms and comorbidities with gross motor development.
Methods and procedures
Fifty children with CZS participated in the study. Information was collected from medical charts, and gross motor development was evaluated by the Gross Motor Function Measure (GMFM)-88. GMFM-88 scores were compared among comorbid groups. Three subgroups of children were identified by cluster analysis, based on information from head circumference at birth, symptoms, comorbidities and gross motor function.
Outcomes and results
Significant associations (p < 0.001) were observed between number of comorbidities/symptoms and dimensions A (r = -0.57) and B (r = -0.58) of the GMFM-88. Children were grouped into 3 clusters, with different gross motor skills. Children with epilepsy and dysphagia composed the cluster with smaller median scores for dimensions A and B of the GMFM-88.
Conclusions and implications
The presence of CZS symptoms and comorbidities compromise the gross motor repertoire of children with CZS at 24 months.
Keywords: Neuroscience, Virology, Disability, Musculoskeletal system, Nervous system, Pediatrics, Health promotion, Zika virus, Microcephaly, Dysphagia, Epilepsy, Motor skills
Neuroscience; Virology; Disability; Musculoskeletal system; Nervous system; Pediatrics; Health promotion; Zika virus; Microcephaly; Dysphagia; Epilepsy; Motor skills
What this paper adds?
This study evaluated the gross motor development of children with congenital Zika syndrome (CZS) at 24 months of age. Although severity in gross motor development is observed in a number of these children, they cannot be considered a homogeneous group of children. Rather, their motor repertoire combined with the presence of certain symptoms and comorbidities formed 3 clusters of children with different abilities in the A (lying down and rolling) and B (sitting) dimensions of the GMFM-88. Poorer motor performance was observed in children with dysphagia and epilepsy. Previous studies argued that head circumference at birth is significantly associated with gross motor delay of children with CZS, at 12 months of age. Our results demonstrate that, at 24 months of age, the presence of CZS symptoms such as dysphagia and epilepsy, not head circumference at birth, is associated with poorer gross motor skills repertoire.
1. Introduction
Congenital Zika syndrome (CZS) is present in infants whose mothers were infected by the virus during pregnancy. In Brazil, the first cases were recorded in the Northeast region in 2015 (Brazil, Ministério da Saúde. Secretaria de Vigilância em Saúde, 2017). These cases were initially described as microcephaly. As clinical studies advanced and confirmed the causal relationship between the clinical findings, mainly neurological in nature, and children with vertical exposure to the Zika virus, the spectrum of the syndrome was established (Costello et al., 2016; Mlakar et al., 2016; World Health Organization [WHO], 2016; Pan American Health Organization [PAHO]/World Health Organization [WHO], 2017). The CZS spectrum is characterized by multiple sensory, neurological and orthopedic manifestations of varying severity. It is possible that other manifestations will be identified as these children age and with the advancement of knowledge on this new syndrome (Albuquerque et al., 2018).
Different comorbidities and symptoms compromise the prognosis of children with CZS. For example, arthrogryposis in the hip, knee, ankle and wrist joints may negatively affect child development (Alvino et al., 2016). Dysphagia, a symptom often present in children with CZS (Silva et al., 2016), impairs oral function and increases the risk of aspiration (Leal et al., 2017). Children with CZS show multifocal and focal forms of epilepsy (Silva et al., 2016; Kapogiannis et al., 2017). Frequent severe seizure episodes negatively influence the motor development of these children (Einspieler et al., 2019). A recent literature review contended that epilepsy is common in children with CZS (Pessoa et al., 2018). Some clinical signs found in these children include increased muscle tone, hyperreflexia, irritability (Schuler-Faccini et al., 2016), as well as visual and auditory impairments (Satterfield-Nash et al., 2017). Satterfield-Nash et al. (2017) also reported sleep-related and eating difficulties.
Two recent studies evaluated movement patterns and motor skills of children with CZS. Einspieler et al. (2019) documented the movement patterns of 35 infants born with microcephaly who were ZIKV-exposed in the first semester post term; they followed this group until 12 months of age. At 3–5 months, infants had absent fidgety movements. After the first year of life, all children with CZS presented severe motor impairments compatible with spastic CP (Einspieler et al., 2019). The authors found an association between head circumference at birth and the movement patterns between 3 and 5 months of life. In another study, Melo et al. (2019) evaluated the gross motor function of 59 children with CZS at mean age 14.7 months (range 5–29) and investigated the factors associated with this outcome. The results revealed severely impaired gross motor function in 81% of the sample, as assessed by the Gross Motor Function Measure (GMFM). Factors associated with children's gross motor function included malformations of cortical development, head circumference at birth and family per capita income (Melo et al., 2019). These two studies confirm the severe motor repertoire of children with CZS after 12 months of life and suggest an association between motor impairment or compromised movement patterns at the end of the first year of life with their head circumference at birth.
The severity of cases and the multiplicity of comorbidities increase the clinical vulnerability and jeopardize the developmental outcome of these children beyond their first year of life. To date, the relationship between symptoms, comorbidities and the motor skills of children with CZS has not been investigated. It is possible that the presence of comorbidities worsens motor development outcomes in this group of children. The objective of the present study was to describe the gross motor function of a group of Brazilian children with CZS at 24 months of age and to evaluate the association between presence of symptoms, comorbidities and gross motor development of these children.
2. Methods
2.1. Study design
This is a retrospective study. Data from the study are part of an institutional project that followed children diagnosed with CZS since 2016. Children with CZS who were treated at a child rehabilitation service located in a large urban center in northeastern Brazil, were evaluated at 24-month-old. The clinical information from children and their families at admission were recorded in the medical records and accessed from the institutional information system.
2.2. Participants
All those responsible for the children (N = 50) treated at the child rehabilitation center were invited and accepted to participate in the study. The inclusion criteria were children with a neurological diagnosis of microcephaly resulting from Zika virus infection who were admitted to the institution for treatment.
This study complied with all legal precepts of research involving human subjects and was approved by the Ethics Committee of the Federal University of Minas Gerais (Universidade Federal de Minas Gerais), Brazil, under number: CAAE 56768916.1.0000.5149.
2.3. Procedures and instruments
Children's and families' data were collected from the medical records and other sources of information between August and December 2018, from a complete list of all children with CZS admitted to the child rehabilitation center for treatment. Children were assessed for their gross motor development at 24 months of age.
The clinical data of the sample were obtained from medical records of different specialties, namely, pediatrics, neurology, orthopedics, and ophthalmology, as well as from laboratory tests. The information included data on each infant at birth (e.g., gestational age, birth weight, height, head circumference, 1-minute Apgar score and 5-minute Apgar score) and after admission to the center (e.g., neurological diagnosis). In addition, maternal and gestational data (e.g., maternal age, number of pregnancies, number of prenatal visits, gestational period at time of Zika virus infection and type of delivery) were also obtained from the child rehabilitation center's records. Information about children's symptoms and comorbidities were gathered throughout their first two years of life.
At 24 months of age, the gross motor function of the children was evaluated with the GMFM-88 (Russell et al., 2013). This test consists of 88 items distributed into 5 dimensions: A) lying and rolling-17 items; B) sitting-20 items; C) crawling and kneeling-14 items; D) standing-13 items; and E) walking, running and jumping-24 items. Each item is scored on an ordinal scale from 0-3, with a score of 0 given when the child is unable to initiate the motor activity (does not initiate), a score of 1 given when the child performs less than 10% of the activity (initiates), a score of 2 given when the child performs more than 10 and less than 100% of the task (partially completes), and a score of 3 is given when the child completes the task. The score for each of the 5 dimensions is expressed as a percentage considering the maximum score of that dimension. The total score is given by the sum of the percentages of all dimensions divided by 5 (total number of dimensions). Therefore, each dimension equally contributes to the total score (Russell and Rosenbaum, 2013).
Five physical therapists were trained to administer the GMFM-88. The interexaminer reliability indicated high consistency, with intraclass correlation coefficients (ICCs) ranging from 0.97 to 0.98.
The Gross Motor Function Classification System (GMFCS) determined the level of gross motor function skills of the children (Palisano et al., 2007). The GMFCS is a classification system for children and young people with CP aged 0–18 years that uses posture and mobility information to classify the gross motor function into 5 levels, which are stratified by age group. Considering the ages of children in this study, between 24 and 48 months, children classified as level I can sit with both hands free to manipulate objects; at level II, children of this age may have difficulty with sitting balance when both hands are free to manipulate objects; they pull to stand on a stable surface, and they crawl on their hands and knees and walk using assistive mobility devices as preferred methods of mobility; at level III, children of this age require adult assistance to assume a sitting position, and their primary means of self-mobility is creeping on their stomach or crawling on their hands and knees; at level IV, children of this age require adaptive equipment for sitting and standing, and their self-mobility for short distances is achieved by rolling, creeping on their stomach, or crawling; and at level V, all motor functions are limited, including their ability to maintain antigravity head and trunk postures. The GMFCS was used in other studies to classify the gross motor function of children with CZS (Melo et al., 2019; Einspieler et al., 2019).
2.4. Statistical analyses
The normality assumption was initially evaluated for the GMFM's total score and sub-scales (dependent variables). The children were grouped according to symptoms and comorbidity and the GMFM-88 scores of these groupings were compared using Student's t-test in the case of a normal distribution or the Mann-Whitney U test when the data distribution was non-normal.
The Spearman correlation coefficient tested the association between the number of symptoms/comorbidities (i.e., frequency) and the GMFM-88 scores. Lastly, cluster analysis was used to identify subgroups of children with CZS using the variables head circumference at birth, comorbidities and gross motor function at 24 months (GMFM-88). This analysis classified individuals into groups based on data from a set of variables, with clusters that maximized the similarities of the intragroup data as well as the differences between groups. For analysis of the mixed numerical and categorical data, a two-step algorithm was applied, allowing the computation of these data (Shih et al., 2010). Fisher's exact test tested the association of categorical variables with the clusters, and the Kruskal-Wallis test compared the clusters on the continuous variables.
The analyses were performed with the statistical packages R® version 3.3.1 and SPSS version 22, with the significance level set at α = 0.05.
3. Results
A total of 50 infants with CZS and their mothers were included in the study. Most children had dysphagia (90%) and visual impairment (58%), followed by epilepsy (48%), hip dislocation (20%) and arthrogryposis (14%). Tables 1 and 2 provide descriptive information from the children at birth (n = 50) as well as sociodemographic information from mothers and families.
Table 1.
Information from families and mothers of children with congenital Zika syndrome (CZS) (n = 50). Fortaleza, Ceará, Brazil.
| Information | n (%) |
|---|---|
| City of residence | |
| Fortaleza (capital) | 41 (82) |
| Interior of Ceará state | 9 (18) |
| Mother's education | |
| Up to elementary school | 20 (40) |
| High school | 25 (50) |
| College | 5 (10) |
| Marital status | |
| Married | 40 (80) |
| Single | 5 (10) |
| Divorced | 4 (8) |
| Widow | 1 (2) |
| Number of prenatal care visits | |
| <7 care visits | 14 (28) |
| ≥7 care visits | 36 (72) |
| Gestational period in which signs/symptoms of Zika virus infection were reported | |
| First trimester | 32 (64) |
| Second trimester | 7 (14) |
| Third trimester | 1 (2) |
| Not reported | 10 (20) |
| Type of delivery | |
| Vaginal birth | 23 (46) |
| Cesarean birth | 27 (54) |
| Mother's age (years) when she became pregnant∗ | 25.3 ± 6.6 |
Numbers represent mean (standard deviation).
Table 2.
Characteristics at birth of children with congenital Zika syndrome (CZS) (n = 50). Fortaleza, Ceará, Brazil.
| Characteristic | N (%) |
|---|---|
| Sex∗ | |
| Male | 31 (62) |
| Female | 19 (38) |
| Gestational age at birth (weeks)∗ | |
| Term | 44 (88) |
| Preterm | 6 (12) |
| Birth weight (g)∗∗ | 2768 ± 501 |
| Birth height (cm)∗∗ | 45.6 ± 3.2 |
| Head circumference at birth (cm)∗∗ | |
| Girls | 29.3 ± 1.9 |
| Boys | 30.2 ± 2.0 |
| 1-minute Apgar score | |
| 5 | 1 (2) |
| 7 | 3 (6) |
| 8 | 20 (40) |
| 9 | 24 (48) |
| N/A∗∗∗ | 2 (4) |
| 5-minute Apgar score | |
| 8 | 5 (10) |
| 9 | 40 (80) |
| 10 | 3 (6) |
| N/A∗∗∗ | 2 (4) |
Information refers to frequency (percentage).
Information refers to mean (standard deviation).
N/A: Two children had non-available information.
Out of the 50 children who agreed to participate in the study, 46 children were evaluated for the gross motor development at 24 months of age. Three children were absent from the scheduled evaluation due to health issues: 1 due to hospital admission for pneumonia, another because of viral infection, and the third due to surgery. One child did not attend the evaluation session due to personal/family reasons.
At 24 months of age, out of the 46 children who returned for evaluation, 71.7% were classified as level V, 23.9% as level IV and 4.3% as levels I and II based on the GMFCS; no child was classified as level III. The GMFM-88 scores according to the GMFCS level of the children are shown in Table 3.
Table 3.
Median and interquartile range (25–75) of GMFM-88 scores of children (n = 46)∗ with congenital Zika syndrome (CZS), at 24 months of age, according to GMFCS level. Fortaleza, Ceará, Brazil.
| GMFM-88 test dimensions |
GMFM-88 Total |
||||||
|---|---|---|---|---|---|---|---|
| GMFCS Level∗∗∗ |
A (Lying & Rolling) | B (Sitting) | C (Crawling & Kneeling) | D (Standing) | E (Walking, Running & Jumping) | Raw score | Percentage |
| I (n = 1) | 39∗∗ | 53∗∗ | 26∗∗ | 26∗∗ | 32∗∗ | 176 | 67.5 |
| II (n = 1) | 51∗∗ | 54∗∗ | 27∗∗ | 6∗∗ | 11∗∗ | 149 | 56.9 |
| IV (n = 11) | 18 (13–37) | 10 (8–19) | 0 (0–3) | 0 (0–0) | 0 (0–0) | 28 (21–59) | 10.3 (7.8–22.1) |
| V (n = 33) | 6 (3–8) | 4 (2–6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 9 (6–13.5) | 4 (2.6–5.1) |
| All children | 8 (5–13.75) | 6 (3–9) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 13 (7–23) | 4.9 (3.2–8.4) |
Four children did not perform the GMFM evaluation at 24 months of age.
Numbers refer to the child's score in each dimension.
There was no participant classified as GMFCS III.
As the scores of the sample in dimensions C, D and E were minimal, the results of the gross motor function evaluation of the children were reported based on the scores from dimensions A and B of the GMFM-88.
Children with epilepsy and dysphagia had lower scores on dimensions A and B of the GMFM-88 compared to children without them (p < 0.05). Table 4 shows the results from the comparative analyses of the comorbidity subgroups on these GMFM-88's dimensions. Other comorbidities were not associated with the scores of dimensions A and B of the GMFM-88 (see Table 5).
Table 4.
Frequency of symptoms and comorbidities in children with congenital Zika syndrome (CZS) (n = 50) and scores from dimensions A and B of the GMFM-88. Fortaleza, Ceará, Brazil.
| Comorbidities | N | Dimension A∗ (Lying & Rolling) |
Dimension B∗ (Sitting) |
|---|---|---|---|
| Median (p25 - p75) | Median (p25 - p75) | ||
| Arthrogryposis | |||
| Yes | 7 | 6 (3–7) | 3 (2–5) |
| No | 43 | 9 (5–15) | 6 (4–10) |
| p-value | 0.068 | 0.038 | |
| West syndrome | |||
| Yes | 1 | 3 (3–3) | 0 (0–0) |
| No | 49 | 8 (5–14) | 6 (3–9) |
| p-value | 0.199 | 0.096 | |
| Hip dislocation | |||
| Yes | 10 | 6 (4–9) | 4 (3–5) |
| No | 40 | 8 (6–14) | 6 (3–9) |
| p-value | 0.304 | 0.267 | |
| Visual impairments | |||
| Yes | 29 | 7 (4–13) | 5 (2–9) |
| No | 21 | 9 (6–25) | 7 (3–17) |
| p-value | 0.187 | 0.325 | |
| Symptoms | |||
| Epilepsy | |||
| Yes | 24 | 6 (3–8) | 5 (2–6) |
| No | 26 | 12 (7–23) | 8 (4–11) |
| p-value | 0.005 | 0.003 | |
| Dysphagia∗∗ | |||
| Yes | 45 | 7 (5–11) | 6 (2–8) |
| No | 4 | 44 (39–50) | 41 (24–54) |
| p-value | <0.001 | <0.001 | |
Dimensions A (Lying and Rolling) and B (Sitting) of the Gross Motor Function Measure (GMFM-88) test.
Four children were not administered the GMFM at 24 months of age.
One child had missing information.
Table 5.
Composition and comparison of clusters according to the variables motor development (dimensions A and B of GMFM-88), head circumference at birth, and presence of epilepsy and dysphagia (n = 46). Fortaleza, Ceará, Brazil.
| Variables | Cluster 1 (n = 21) | Cluster 2 (n = 5) | Cluster 3 (n = 20) | p-value |
|---|---|---|---|---|
| Dimension A (Lying & Rolling)∗ | 6 (3–8) | 39 (38–49) | 10 (5.5–14.5) | <0.001a,b,c |
| Dimension B (Sitting)∗ | 4 (2–6) | 51 (29–53) | 7 (4–9.5) | <0.001a,b,c |
| Head circumference at birth∗ | 29 (28–30) | 31.5 (31–32.5) | 30 (28.5–31) | <0.01a,c |
| Epilepsy∗∗ | <0.001 | |||
| Yes† | 21 (100%) | 1 (20%) | 0 (0%) | |
| No | 0 (0%) | 4 (80%) | 20 (100%) | |
| Dysphagia∗∗ | <0.001 | |||
| Yes†† | 21 (100%) | 1 (20%) | 20 (100%) | |
| No | 0 (0%) | 4 (80%) | 0 (0%) | |
a = Cluster 1×Cluster 2.
b = Cluster 1×Cluster 3.
c = Cluster 2×Cluster 3.
Numbers indicate median and interquartile range (25 and 75).
Numbers indicate frequency (percentage).
Out of the 24 children with epilepsy at admission to the child rehabilitation center, 2 children did not return for the GMFM-88 assessment, at 24 months.
Out of the 45 children with dysphagia at admission to the child rehabilitation center, 1 child had non-available information and the other 2 did not return for the GMFM-88 assessment, at 24 months.
There was a moderate negative association (p < 0.001) between the number of comorbidities and the total scores from dimensions A (rs = -0.57) and B (rs = -0.58) of the GMFM-88.
Cluster analysis identified 3 groups of children. The fitted model was evaluated with the silhouette coefficient, which is a measure of cohesion and separation of clusters, with values ranging from -1 to 1. To be considered a good model, the mean value of the silhouette coefficient must be equal to or greater than 0.5 (Rousseeuw, 1987). In the present study, the value of this coefficient was greater than 0.5, thus indicating good cluster classification quality.
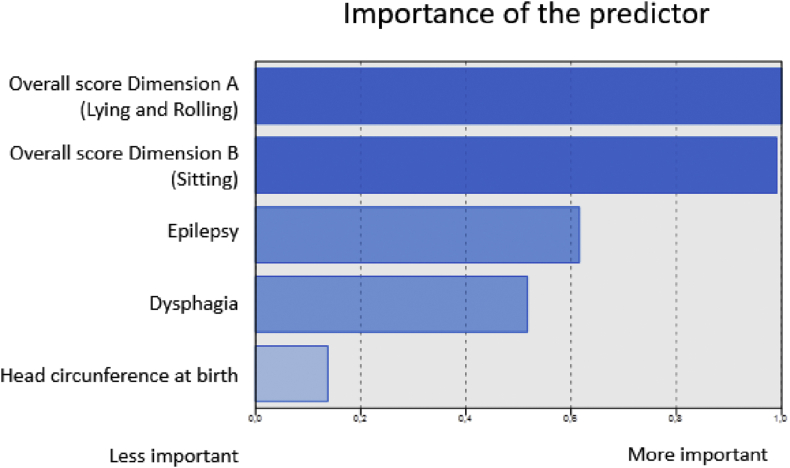
The ratings of predictors' importance for cluster formation vary from 0 (least important) to 1 (most important) (Hastie et al., 2008). Figure 1 shows the ranking of variables according to their importance for clusters formation. The scores from dimensions A and B of the GMFM-88 were the most important variables, followed by the presence of epilepsy and dysphagia. The variable head circumference at birth was not relevant for the clusters' composition.
Figure 1.
Importance of predictors for cluster classification. Fortaleza, Ceará, Brazil.
In cluster 1 (n = 21), 95.2% of the children were classified as GMFCS level V and 4.8% as level IV; these children had the lowest scores from dimensions A and B of the GMFM-88. In addition, all children in this cluster had both epilepsy and dysphagia. Cluster 2 (n = 5) consisted of 60% of children classified as GMFCS level IV and 20% of children classified as levels I and II, each. This cluster included the children with higher scores from dimensions A (Md = 39) and B (Md = 51) of the GMFM-88; most children (80%) did not have epilepsy or dysphagia. Lastly, cluster 3 (n = 20) consisted of 65% of children classified as GMFCS level V and 35% of children classified as level IV, with intermediate scores from dimensions A (Md = 10) and B (Md = 7) of the GMFM-88. None of the children in this cluster had epilepsy, but all had dysphagia.
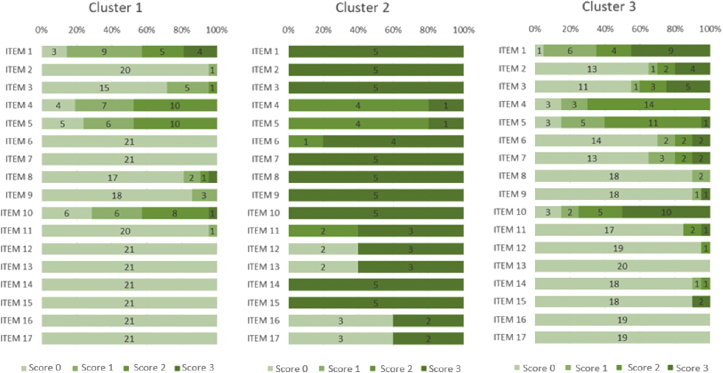
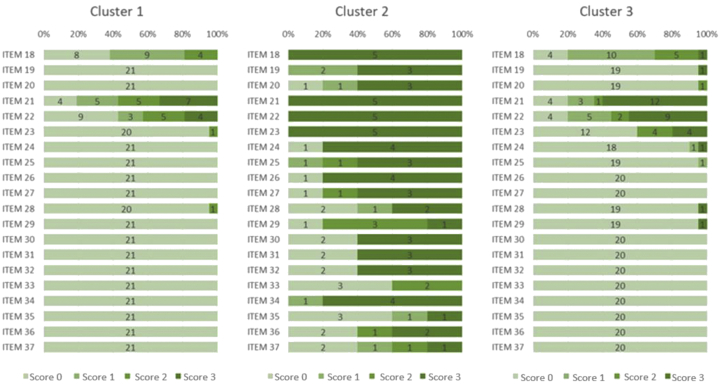
Figures 2 and 3 details the scores received in each item that composed dimensions A and B of the GMFM-88, by children from the three clusters.
Figure 2.
Scores received for items (1–17) from dimension A (Lying and Rolling) of the GMFM-88 by the children in the 3 clusters. Fortaleza, Ceará, Brazil. Score 0 = did not initiate; Score 1 = initiated; Score 2 = partially completed; Score 3 = completed. The depicted numbers refer to number of children achieving scores 0–3 on the GMFM-88 items.
Figure 3.
Scores received for items (18–37) from dimension B (Sitting) of the GMFM-88 by the children in the 3 clusters. Fortaleza, Ceará, Brazil. Score 0 = did not initiate; Score 1 = initiated; Score 2 = partially completed; Score 3 = completed. The depicted numbers refer to number of children achieving scores 0–3 on the GMFM-88 items.
4. Discussion
This study reports the gross motor skills and the presence of main comorbidities and symptoms of a group of children with CZS at 24 months of age. The results showed that 96% of the children from our sample were classified as GMFCS levels IV and V, thus confirming the severe motor impairment of children with CZS. However, these children formed three distinct subgroups, based on their gross motor skills and presence of dysphagia and epilepsy, thus, suggesting they are not a homogeneous group.
The gross motor skills of children from our sample were more important in determining the three clusters, relative to the other factors. The scores from dimensions A (lying and rolling) and B (sitting) of the GMFM-88 were the most important predictors. Given the functional characteristics of the sample, we chose to use the GMFM-88 to assess the gross motor function of the participants. This version of the GMFM has a larger number of items in dimensions A (lying down and rolling) and B (sitting) than does the GMFM-66 version. It is, therefore, the most appropriate version for the evaluation of children with more severe motor impairment, such as CZS (Russell et al., 2000). In the present study, the gross motor skills repertoire of children was scored mainly using dimensions A and B of the GMFM-88, as the median of dimensions C, D and E was zero. These values confirm the important gross motor function limitation presented by children with CZS at 24 months.
To date, few studies on the gross motor development of children with CZS have been published as this is a new condition in the Brazilian scenario. A recent publication that used the GMFM to evaluate the gross motor development (Melo et al., 2019) reported median scores of 13 and 6 in dimensions A and B, respectively, and a total score of 18. Although our participants had similar functional levels (GMFCS IV and V), we found lower scores in the supine and prone positions (dimension A: Md = 8) and in the total gross motor function score (Md = 13). Such differences may be attributed to the occurrence of comorbidities in our sample.
The combination of dysphagia and epilepsy in children with CZS seem to have very negative impact on gross motor function. In the present study, 90% of our sample had dysphagia and 48% had epilepsy. Dysphagia is one of the most frequently observed symptoms in children with CZS (Silva et al., 2016). According to Leal et al. (2017), all children with CZS in a series of cases (n = 9) showed dysphagia, with severe implications to their development. The high frequency of dysphagia in children with CP classified as GMFCS levels IV and V was identified by Venkateswaran and Shevell (2008), Cameron et al. (2013) and Yi et al. (2013). The study by Cameron et al. (2013) included 23 children with CP of cytomegalovirus etiology, 18 of whom had severe dysphagia. That study found a significant relationship between more severe gross motor function impairment (i.e., GMFCS V) and indication for gastrostomy. The association between the GMFM-88 scores and dysphagia in individuals with CP was also observed by Yi et al. (2013), with dysphagia contributing to lower gains from rehabilitation.
Another symptom that influenced gross motor development at 24 months was epilepsy. Such condition is very frequent in children with CZS, as described by Silva et al. (2016) and Kapogiannis et al. (2017). Leal et al. (2017) suggested that severe brain injury in children with CZS may have negative impact in several aspects of development, including oral motor function. Einspieler et al. (2019) also reported the presence of epilepsy in 79.4% of 34 children with CZS, whose motor impairment at the end of the first year of life was severe. In addition, vision problems have been described as frequent in children with this syndrome (Campos et al., 2016; Pessoa et al., 2018; Saad et al., 2018). Our results confirm the symptoms and comorbidities observed in children with CZS and further explore their impact on children's gross motor repertoire.
Cluster 2 included children who had relatively better gross motor function compared to children in the other two clusters. Although this cluster mostly had children classified as GMFCS IV, 20% of them were classified as GMFCS I and II, and the majority (80%) did not have dysphagia or epilepsy. Notably, few children in this cluster had a score of zero on the items related to lying, rolling and sitting (dimensions A and B) of the GMFM-88. At 24 months of age, many of the children in cluster 2 received full scores on items related to the ability to sit with support (i.e., items 21, 22 and 23 of dimension B). Our results reveal that despite the severity of the neurological condition, children with CZS have different gross motor function repertoires at 24 months of age, characterizing three subgroups of children. These results confirm the trend reported by Melo et al. (2019), who found some cases of milder motor impairment in a group of children with CZS, evaluated at 14 months of age. Thus, there is still much to learn about the developmental progression of children with CZS (Eickmann et al., 2016).
Our results showed that certain comorbidities such as arthrogryposis and hip dislocation had no impact on children with CZS gross motor function, at 24 months of age. Taken in isolation, arthrogryposis and hip dislocation are known to compromise children's gross motor development (Kroksmark et al., 2006; Donohoe et al., 2019; Zarrinkalama et al., 2011). However, in the clinical complexity of the CZS, considering the damage to the central nervous system of these children, the co-occurrence of specific conditions that compromise their joint structure and function do not seem to impose greater impairment to their gross motor development.
Head circumference at birth had no relevance in the composition of the three clusters from our study. Children from cluster 1, who showed worse motor development and had epilepsy and dysphagia, showed smaller head circumferences compared to those of cluster 2, with children with better gross motor skills. However, the results showed no difference in head circumference at birth between children from clusters 1 and 3. These results do not confirm evidence from two other recent studies that evaluated the movement and motor skills of children with CZS (Einspieler et al., 2019; Melo et al., 2019). Both Einspieler et al. (2019) and Melo et al. (2019) found an association of head circumference at birth with abnormal posture and movement patterns at 3–5 months of age (Einspieler et al., 2019) and gross motor function at 14 months. Considering that gross motor function is a multifactorial phenomenon that is influenced by the environment in which the child lives and by the parent-child relationship, it is possible that the head circumference at birth measure loses its relevance in determining the motor repertoire at 24 months of age in children with CZS. Another explanation for the discrepancy between our results and those of the two cited studies may be attributed to the nature of the relationship between head circumference at birth and movement of children with CZS in the first two years of life. It is possible that the direct relationship reported by Einspieler et al. (2019) and by Melo et al. (2019) is actually mediated by the presence of CZS symptoms such as dysphagia and epilepsy. Future studies can clarify this argument. The survival of these children requires systematic follow-up with information that can help determine the impact and repercussions of CZS on further development of these children and on the families' routine.
4.1. Study limitations
The present study has some limitations. The sample consisted of children treated at a single child rehabilitation center in one of the cities of Northeast Brazil most affected by the Zika virus infection outbreak. Asymptomatic children exposed to the infection were not included in the present study. The small number of children in Cluster 2 (n = 5) may have restricted the description of their profile of gross motor skills. Lastly, the effect and relevance of the variables associated with the three clusters of children, formed at 24 months, need to be confirmed by prospective longitudinal studies that follow the motor function of children with CZS.
5. Conclusion
This study confirms the severe impairments in gross motor skills of children with CZS at 24 months of age. Three subgroups were formed with children with different motor repertoires. Head circumference at birth did not contribute to the composition of clusters or subgroups of children. The combined presence of dysphagia and epilepsy negatively affected the severity of gross motor dysfunction in these children. Monitoring the clinical conditions and manifestations, as well as the development of these children, may help to better understand this syndrome and its impact. The characteristics of the distinct profiles of gross motor skills described in our study help guide intervention strategies specific to each subgroup.
Declarations
Author contribution statement
Lêda Maria da Costa Pinheiro Frota: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rosana Ferreira Sampaio, Marisa Cotta Mancini: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
José Lucivan Miranda, Rita Maria Cavalcante Brasil: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Ana Paula Bensemann Gontijo, Juliana Vaz de Melo Mambrini, Marina de Brito Brandão: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the following Brazilian agencies: National Council for Scientific and Technological Development (CNPq), the Research Support Foundation from the State of Minas Gerais (FAPEMIG), the Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001), and the Office of the Vice Provost for Research (Pró-Reitoria de Pesquisa) from the Universidade Federal de Minas Gerais (UFMG).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the parents of the children for their participation in this study and the Center for Treatment and Early Stimulation (Núcleo de Tratamento e Estimulação Precoce-NUTEP) for permission to access and use information from the children's medical records and for the site to conduct the study.
References
- Albuquerque M.F.P.M., Souza W.V., Araújo T.V.B., Braga M.C., Miranda D.B., Filho Ximenes R.A.A., Melo D.A, Filho., Brito C.A.A., Valongueiro S., Melo A.P.L., Brandão S.P. Filho., Martelli C.M.T. The microcephaly epidemic and Zika virus: building knowledge in epidemiology Cad. Saude Publica. 2018;34 doi: 10.1590/0102-311X00069018. [DOI] [PubMed] [Google Scholar]
- Alvino A.C.M., Mello L.R.M., Oliveira J.A.M.M. Association of arthrogryposis in neonates with microcephaly due to Zika virus - a case series. Rev. Bras. Saúde Materno Infant. 2016;16(supl):89–94. [Google Scholar]
- Cameron N.A., Mark E., Gormley M.E., Jr., Deshpandea S. Severity of disability in patients with cerebral palsy secondary to symptomatic congenital cytomegalovirus encephalopathy. J. Pediatr. Rehabil. Med.: An Interdiscip. 2013;6:239–242. doi: 10.3233/PRM-140258. [DOI] [PubMed] [Google Scholar]
- Campos A.G.M., Lira R.P.C., Arantes T.E.F. Optical coherence tomography of macular atrophy associated with microcephaly and presumed intrauterine Zika virus infection. Arq. Bras. Oftalmol. 2016;79(6):400–401. doi: 10.5935/0004-2749.20160112. [DOI] [PubMed] [Google Scholar]
- Costello A., Duran Dua, T., Gülmezoglu M., Oladapo O.T., William Perea W., Pires J., Ramon-Pardo P., Rollins N., Saxena S. Defining the syndrome associated with congenital Zika virus infection. [Editorial] Bull. World Health Organ. 2016;94:406. doi: 10.2471/BLT.16.176990. 406A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe M., Pruszcynski B., Rogers K., Bowen J.R. Predicting ambulatory function based on infantile lower extremity posture types in amyoplasia arthrogryposis. J. Pediatr. Orthop. 2019;39(7):e531–e535. doi: 10.1097/BPO.0000000000001322. [DOI] [PubMed] [Google Scholar]
- Eickmann S.H., Carvalho M.D.C.G., Ramos R.C.F., Rocha M.A.W., van der Linden V., Silva P.F.S. Zika virus congenital syndrome. Cad. Saúde Pública. 2016;32(7) doi: 10.1590/0102-311X00047716. [DOI] [PubMed] [Google Scholar]
- Einspieler C., Utsch F., Brasil P., Aizawa C.Y.P., Peyton C., Hasue R.H., Genovesi F.F., Damasceno L., Moreira M.E., Adachi K., Marschik P.B., Nielsen-Saines K. Association of infants exposed to prenatal Zika virus infection with their clinical, neurologic, and developmental status evaluated via the general movement assessment tool. JAMA Network Open. 2019;2(1) doi: 10.1001/jamanetworkopen.2018.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T., Tibshirani R., Friedman J. 2a ed. Springer; Stanford, California: 2008. The Elements of Statistical Learning: Data Mining, Inference and Prediction. [Google Scholar]
- Kapogiannis B.G., Chakhtoura N., Hazra R., Spong C.Y. Bridging knowledge gaps to understand how Zika virus exposure and infection affect child development. JAMA Pediatr. 2017;171(5):478–485. doi: 10.1001/jamapediatrics.2017.0002. [DOI] [PubMed] [Google Scholar]
- Kroksmark A.-K., Kimber E., Jerre R., Beckung E., Tulinius M. Muscle involvement and motor function in amyoplasia. Am. J. Med. Genet. 2006;140A:1757–1767. doi: 10.1002/ajmg.a.31387. [DOI] [PubMed] [Google Scholar]
- Leal M.C., Van Der Linden V., Bezerra T.P., Valois L., Borges A.C.G., Antunes M.M.C., Brandt K.G., Moura C.X., Rodrigues L.C., Ximenes C.R. Characteristics of dysphagia in infants with microcephaly caused by congenital Zika virus infection, Brazil, 2015. Emerg. Infect. Dis. 2017;23(8) doi: 10.3201/eid2308.170354. www.cdc.gov/eid [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministério da Saúde. Secretaria de Vigilância em Saúde . 2017. Vírus Zika No Brasil: a Resposta Do SUS.http://bvsms.saude.gov.br/bvs/publicacoes/virus_zika_brasil_resposta sus.pdf Brasil. Accessed at: [Google Scholar]
- Mlakar J., Korva M., Tul N., Popović M., Poljšak-Prijatelj M., Mraz J., Kolenc M., Rus K.R., Vipotnik T.V., Vodušek V.F., Vizjak A., Pižem J., Petrovec M., Županc T.A. Zika virus associated with microcephaly. N. Engl. J. Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Melo A., Gama G.L., Silva R.A., Jr., Assunção P.L., Tavares J.S., Silva M.B., Costa K.N.F.S., Vânia M.L., Evangelista M.A., Amorim M.R. Motor function in children with congenital Zika syndrome. Dev. Med. Child Neurol. 2019 doi: 10.1111/dmcn.14227. [DOI] [PubMed] [Google Scholar]
- Palisano R., Rosenbaum P., Bartlett D., Livingston M. 2007. GMFCS – E & R Gross Motor Function Classification System. Expanded and Revised.https://canchild.ca/system/tenon/assets/attachments/000/000/058/original/GMFCS-ER_English.pdf Accessed at: [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization/World Health Organization. PAHO/WHO . 2017. Zika - Epidemiological Report Brazil.https://www.paho.org/hq/dmdocuments/2017/2017-phe-zika-situation-report-bra.pdf Washington, D.C. Accessed at: [Google Scholar]
- Pessoa A., van der Linden V., Yeargin-Allsopp, Carvalho M.D.C.G., Ribeiro E.M., Braun K.V.N., Durkin M.S., Pastula D.M., Moore J.T., Moore A., A C. Motor abnormalities and epilepsy in infants and children with evidence of congenital Zika virus infection. Pediatrics. 2018;141(supl):167–179. doi: 10.1542/peds.2017-2038F. [DOI] [PubMed] [Google Scholar]
- Rousseeuw P.J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. [Google Scholar]
- Russell D.J., Avery L.M., Rosenbaum P.L., Raina P.S., Walter S.D., Palisano R.J. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys. Ther. J. 2000;80(9):873–885. [PubMed] [Google Scholar]
- Russell D.J., Rosenbaum P.L., Wright M., Avery L.M. Clinics in Developmental Medicine. second ed. Mac Keith Press; London, UK: 2013. Gross motor function measure (GMFM-66 & GMFM 88): user's manual; p. 290. 978-1-908316-88-2. [Google Scholar]
- Russell D.J., Roseanbaum P.L. 2013. Gross Motor Function Measure (GMFM) Score Sheet (Gmfm-88 and Gmfm-66 Scoring)https://canchild.ca/system/tenon/assets/attachments/000/000/218/original/gmfm-88_and_66_scoresheet.pdf Accessed at: [Google Scholar]
- Saad T., Costa A.A.P., Góes F.V., Freitas M., Almeida J.V., Ignêz L.J.S., Amancio A., Alvim R.J., Kramberger L.A.A. Neurological manifestations of congenital Zika virus infection. Childs Nerv. Syst. 2018;34:73–78. doi: 10.1007/s00381-017-3634-4. [DOI] [PubMed] [Google Scholar]
- Satterfield-Nash A., Kotzky K., Allen J., Bertolli J., Moore C.A., Pereira I.O., Pessoa A., Melo F., Santelli A.C.F.S., Boyle C.A., Peacock G. Health and development at age 19–24 Months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak — Brazil, 2017. US department of health and human services/centers for disease control and prevention. MMWR. 2017;66(49):1347–1351. doi: 10.15585/mmwr.mm6649a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler-Faccini L., Ribeiro E.M., Feitosa I.M.L., Horovitz D.D.G., Cavalcanti D.P., Pessoa A., Doriqui M.J.R., Neri J.I., Pina Neto J.M., Wanderley H.Y.C., Cernach M., El-Husny A.S., Pone M.V.S., Serao C.L.C.V., Sanseverino M.T.V. Possible association between Zika virus infection and microcephaly Brazil, 2015. US department of health and human services/centers for disease control and prevention. MMWR. 2016;65(3):59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- Shih M.Y., Jheng J.W., Lai L.F. A two-step method for clustering mixed categorical and numeric data. Tamkang J. Sci. Eng. 2010;13(1):11–19. [Google Scholar]
- Silva A.A.M., Ganz J.S.S., Sousa P.S., Doriqui M.J.R., Ribeiro M.R.C., Branco M.R.F.C., Queiroz R.C.S., Pacheco M.J.T., Costa F.R.V., Silva F.S., Simões V.M.F., Pacheco M.A.B., Lamy F., Lamy Z.C., Alves M.T.S.S.B. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg. Infect. Dis. 2016;22(11):1953–1956. doi: 10.3201/eid2211.160956. www.cdc.gov/eid [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran S., Shevell M.I. Comorbidities and clinical determinants of outcome in children with spastic quadriplegic cerebral palsy. Dev. Med. Child Neurol. 2008;50:216–222. doi: 10.1111/j.1469-8749.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2016. Director- General Summarizes the Outcome of the Emergency Committee Regarding Clusters of Microcephaly and Guillain-Barré Syndrome.http://www.who.int/mediacenter/news/statements/2016/emergency-committee-zika-microcephaly/en Accessed at: [Google Scholar]
- Yi T.I., Jin J.R., Kim S.H., Han K.H. Contributing factors analysis for the changes of the gross motor function in children with spastic cerebral palsy after physical therapy. Ann Rehabil. Med. 2013;37(5):649–657. doi: 10.5535/arm.2013.37.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinkalama R., Rice J., Brook P., Russo R.N. Hip displacement and overall function in severe cerebral palsy. J. Pediatr. Rehabil. Med.: An Interdiscip. Approach. 2011;4:197–203. doi: 10.3233/PRM-2011-0175. [DOI] [PubMed] [Google Scholar]