Abstract
Ultraviolet (UV) irradiation induces physiological and morphological skin damage, resulting in skin dryness, wrinkle formation, and loss of elasticity. The basement membrane (BM) has been shown to play crucial roles in binding epidermis to dermis tightly, regulating cell differentiation and proliferation, and signaling protein production. Dietary flavonoids have been revealed to improve the damage caused by UV exposure. Immature Citrus unshiu is known to contain high concentrations of flavonoids such as hesperidin and narirutin. In this study, the effects of immature Citrus unshiu powder (ICP) on photoaged skin were demonstrated using UVB irradiated hairless mice. Oral administration of ICP improved loss of skin hydration and increase of transepidermal water loss. The histological analyses of hairless mice dorsal skin revealed that oral administration of ICP improved UVB-induced overgrowth of epidermal cell, suppressed epidermal cell mortality and BM destruction. Therefore, the administration of ICP could improve photoaging by protecting the tissues around BM.
Keywords: Biochemistry, Cell biology, Antioxidant, Phenolic compound, Food component analysis, Extracellular matrix, Aging, Citrus unshiu, Flavonoids, Hesperidin, Narirutin, Antioxidants, Photoaging, Ultraviolet light, Hairless mice, Basement membrane, Laminin
Biochemistry, Cell biology; Antioxidant; Phenolic compound; Food component analysis; Extracellular matrix; Aging; Citrus unshiu; Flavonoids; Hesperidin; Narirutin; Antioxidants; Photoaging; Ultraviolet light; Hairless mice; Basement membrane; Laminin.
1. Introduction
Skin aging is divided into two types: age-related intrinsic aging and ultraviolet (UV) irradiation-induced photoaging. Photoaging is characterized by several clinical features, including wrinkles, dryness, roughness, and pigmentary change [1]. The physiological and biochemical features of photoaged skin include skin dehydration and increase in epidermal thickness [2]. Basement membrane (BM) is known as an important multi-molecular structure which firmly attaches the epidermis and dermis, regulating epidermal differentiation, proliferation, as well as epidermal-dermal interaction. BM is damaged and multilayered by UV irradiation, promoting aging process in dermis and epidermis, hence improving BM repair is important to maintain skin health [3].
One of the major factors of photoaging is thought to be an increase in reactive oxygen species (ROS) production. ROS is generated in both epidermis and dermis by UV irradiation, followed by degradation of BM and extracellular matrix and apoptosis [4]. One way to reduce ROS and oxidative stress is dietary antioxidants, which can enhance endogenous antioxidant activity. Consequently, there is increasing interest in dietary antioxidants that can induce skin anti-aging effects [5].
Citrus unshiu (Satsuma mandarin) is one of the most popular fruits in Japan, China and Korea. Citrus unshiu contains various bioactive substances such as flavonoids, amino acids, and carotenoids, so that its fruits and peel have been widely used as a traditional medicine to treat common colds, bronchial discomfort, and indigestion for centuries [6]. Interestingly, the constituents and the biochemical composition of Citrus unshiu change during its maturation [7]. In immature Citrus unshiu (ICP), the flavonoids content is higher than mature one [8]. Among the flavonoids, hesperidin and narirutin are well known as dominant flavonoids in Citrus unshiu. These flavonoids have been shown to possess antioxidant and anti-inflammatory activities [9] and protective effect against apoptosis [10]. Chiang et al. showed that hydrolysates of Citrus plants stimulate melanogenesis protecting against UV-induced dermal damage [11]. And Citrus has reported to be used as a skin moisturizing and protective agent on UV-induced damage [11, 12]. Choi et al showed that anti-photoaging effects of immature Unripe Citrus extracts including inhibiting the expression of matrix metalloproteinases (MMPs) and enhancing the typeⅠcollagen [13]. However, the effects of immature Citrus unshiu on BM remains unknown. In this study, we examined the effect of oral administered immature Citrus unshiu on photoaging in the skin of hairless mice and confirmed that orally supplementation of ICP might be a useful strategy to protect from photoaging via repairing BM damage.
2. Materials and methods
2.1. Reagents
Histological and immunohistochemical analyses were conducted with Mayer's hematoxylin (Muto pure chemicals, Tokyo, Japan), anti-laminin polyclonal antibody DyLight488 (Thermo Fischer Scientific, MA, USA), 10 % normal goat serum and MAX-PO (rabbit) (Nichirei bioscience, Tokyo, Japan), and ImmPACT DAB SK-4105 (Vector laboratories, CA, USA). All other reagents were obtained from Wako (Wako Pure Chemicals, Osaka, Japan).
2.2. Fruits and sample preparation
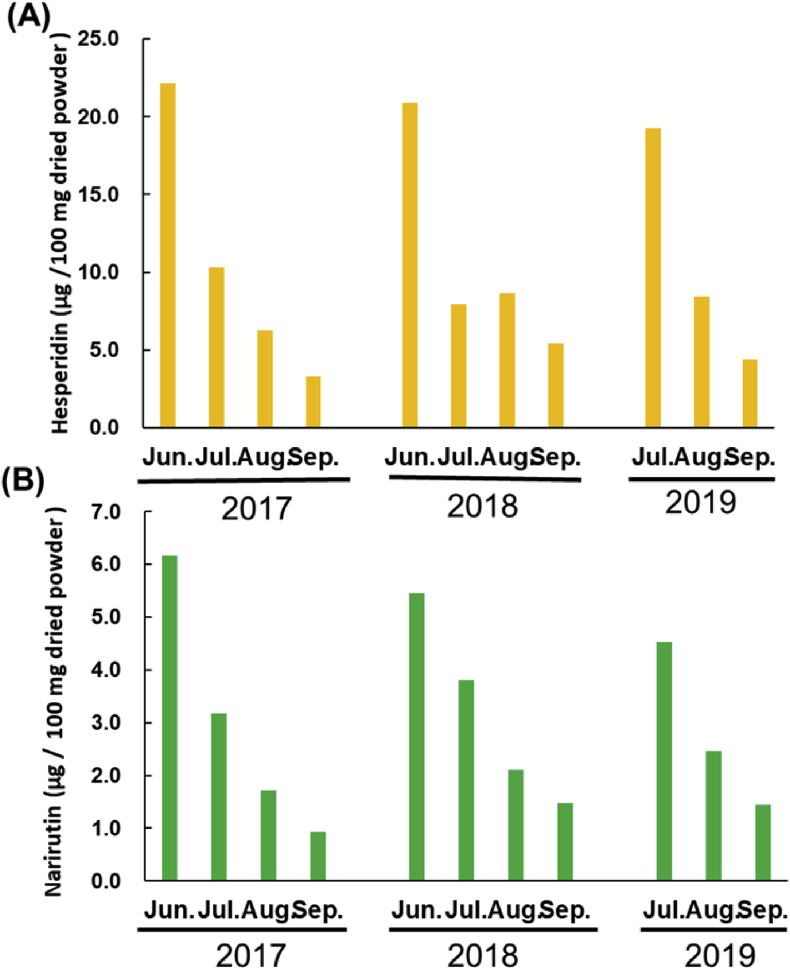
ICP was obtained from Mikkabi – cho, Hamamatsu – city, Shizuoka, Japan. ICP was stored at -30 °C before used for experiment, and was crushed and freeze-dried. Whole fruits including peel and pulp were used in the experiment. The content of hesperidin and narirutin in ICP for three consecutive years were analyzed (Figure 1). In 2019, fruits in June were not available due to bad harvest of ICP. There were no change in flavonoid content since September. They were determined with HPLC according to the method for Citrus unshiu peel analysis on The Japanese Pharmacopoeia 17th edition [14]. The HPLC analysis was performed on DP8020, AS 8021, UV8020, and CO8020 (Tosoh, Tokyo, Japan). The chromatographic separation was performed on an ODS – 80 TsQA column (4.6 × 150 mm) (Tosoh, Tokyo, Japan). ICP containing 22.9 μg hesperidin, 7.2 μg narirutin/100 g, which was processed from Citrus unshiu harvested in June 2017, was used for animal experiments.
Figure 1.
Hesperidin and narirutin contents in Citrus. unshiu. from the different developmental stages. The whole fruits were cut and homogenized. Following to lyophilized and grind into fine powder, hesperidin and narirutin was extracted with methanol for HPLC analysis. (A) hesperidin; (B) narirutin.
2.3. Animals and administration of ICP
The experimental protocol was approved by the Ethics Committee of Tokyo University of Agriculture and Technology (Tokyo, Japan; approval No. 29–71). Six-week old male Hos:HR-1 hairless mice (Japan SLC, Shizuoka, Japan) were purchased from Sankyolabo service (Tokyo, Japan). Mice were housed in collective cages at 24 ± 2 °C and at 40 ± 10% humidity on a twelve hour light and dark cycle, with free access to water and Lab MR stock diet (Nihon nosan kogyo, Tokyo, Japan). Following one week of acclimation, mice were divided into three groups: non-irradiated control mice [UV(−) control, n = 6], UV irradiated control mice [UV(+) control, n = 7 ], and UV irradiated and ICP administered mice [UV(+) ICP, n = 7 ]. The ICP was dissolved in 0.5% tragacanth gum (Wako Pure Chemicals, Osaka, Japan) and orally administered at 200 mg/kg body weight/day for seven weeks. Only 0.5 % tragacanth gum was orally administered to the control groups. In accordance with the UV irradiation method of Tanaka et al. [15], the mice were housed in cages and subjected to UVB irradiation. Mice were irradiated with UVB three times a week for seven weeks. UVB irradiation time was gradually extended. Mice were exposed to UVB irradiation for three times 60 s for the first week. Exposure time was then increased to 90, 90, 120 s for the second week, 120, 120, 150 s for the third week, 150, 180, 180 s for the fourth week, three times 210s for the fifth week, 225 s each for the sixth and seventh weeks.
The total energy of UVB that each mouse received was 2.42 J/cm2 over seven weeks. Skin moisture content and transepidermal water loss (TEWL) in the dorsal skin were measured with Corneometer CM 825 and Tewameter TM 300 (Courage + Khazaka Electronic, Koln, Germany).
2.4. Histological and immunohistochemical analyses
After seven weeks of UVB exposure, mice were sacrificed under anesthesia (SEVOFRANE; Maruishi Pharmaceutical, Osaka, Japan). Dorsal skin biopsy samples were obtained using a biopsy punch BP-80F (Kai medical, Tokyo, Japan) with a diameter of eight mm. Dorsal skin samples were fixed in tissue tek Ufix (Sakura-finetek Japan, Tokyo, Japan) and embedded in paraffin. Paraffin sections (4 μm) were deparaffinized and stained with hematoxylin and eosin (H&E). Immunostaining was conducted according to the method of Amano et al. [16]. Three sites were randomly selected in each section and photographed using microscope and a camera (C – 3040 ZOOM, OLYMPUS, Tokyo, Japan). The epidermal thickness of three groups were calculated for a total of 10 places in three visual fields and analyzed with software Image J. The mean of these 30 measurements in each group were used to calculate the mean for each experimental group.
2.5. Statistical analysis
Data are presented as the means ± standard deviation (SD). After a one-way ANOVA was performed, statistical analysis was performed using Student's t-tests followed by Statcel version 4.0. P - values <0.05 were considered significant.
3. Results
3.1. Skin moisture content and TEWL of dorsal skin from UV irradiated hairless mice
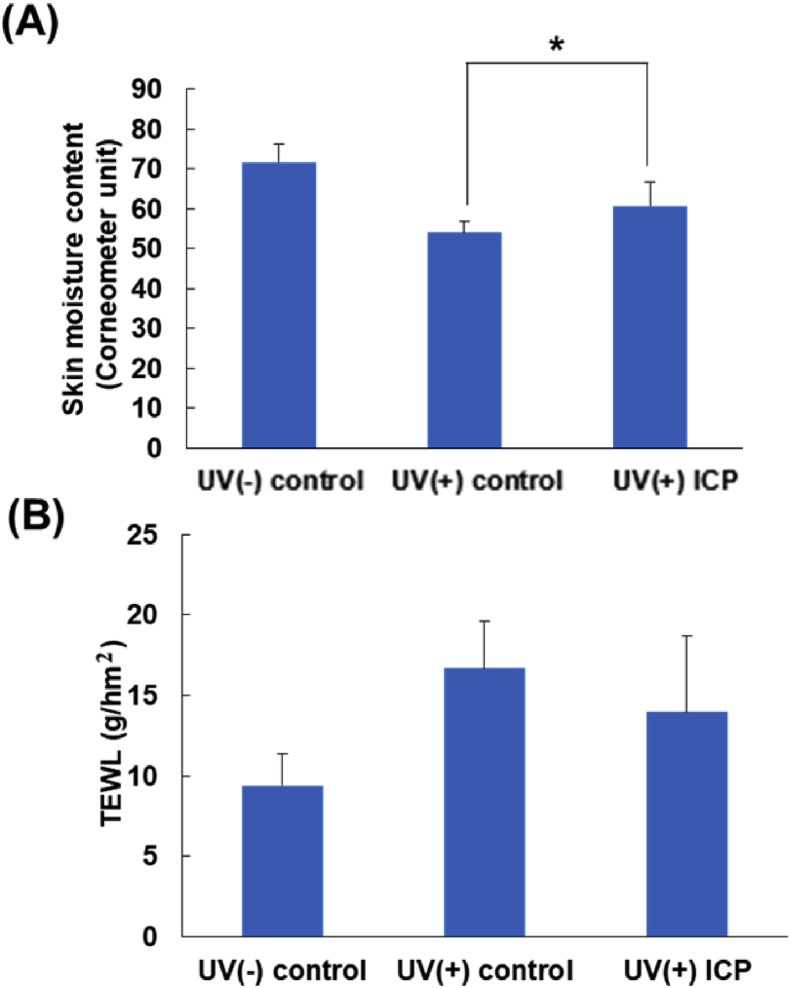
As a result of ICP administration for seven weeks, restoration of skin moisture content and TEWL were observed (Figure 2). Comparing the skin moisture content and TEWL of hairless mice back skin on the UV(-) and UV(+) control, the influence of UVB irradiation was confirmed. A photoaging model was created without causing erythema formation on the back skin. At the start of the experiment, skin moisture content in UV(-)control, UV(+) control and UV(+) ICP were 70.2 ± 3.4 and 70.5 ± 3.1, and 70.1 ± 3.4, respectively. Skin moisture content decreased with UVB irradiation. seven weeks later, skin moisture content of UV(-) control, UV(+) control and UV(+)ICP were 71.7 ± 4.7, 54.1 ± 2.6 and 60.7 ± 5.9, respectively. Skin moisture content in UV(+) ICP was significantly higher than that in UV(+) control (p < 0.05).
Figure 2.
Skin moisture content and TEWL of dorsal skin in UVB irradiated hairless mice skin after the oral administration of ICP for 7 weeks. (A) The skin moisture content and (B) TEWL of the dorsal skin was measured twice a week during the experimental period. Data are shown as means ± SD. ∗significant (p < 0.05) difference between UV(+) control and UV(+) ICP using the Student's t-test.
In this study the experiment, TEWL in UV(-)control, UV(+) control and UV(+) ICP were 7.5 ± 0.7, 7.2 ± 0.5, and 7.6 ± 0.6 g/h/m2. With seven weeks UVB irradiation, TEWL increased, and skin barrier was reduced. Finally, TEWL in UV(-) control, UV(+) control and UV(+) ICP were 9.4 ± 2.0,16.7 ± 2.9 and 14.0 ± 4.7 g/h/m2. TEWL in UV(+) control was significantly lower than that in UV(+) ICP.
3.2. Histological and immunohistochemical analyses of UV irradiated hairless mice
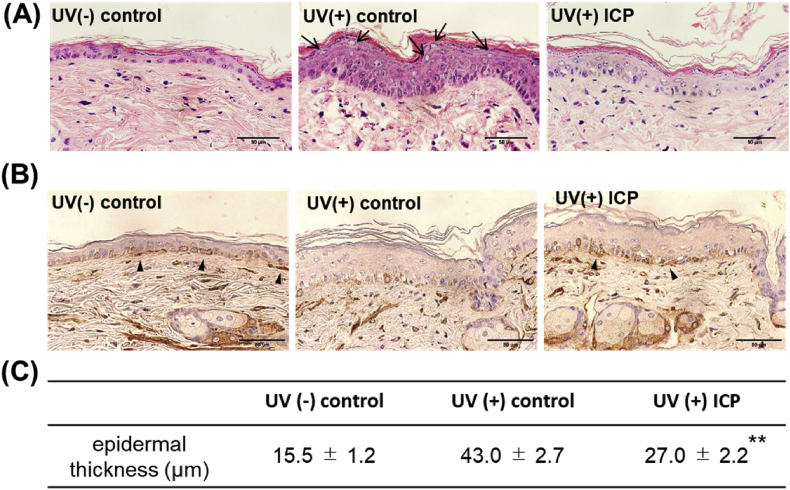
Histochemical analyses of dorsal skin were shown in Figure 3. Compared to UV(-) control, the epidermis thickened significantly by UVB irradiation with 2.8 fold in UV(+) control and 1.7 fold increase in UV(+) ICP, respectively (p < 0.01). The epidermis obviously became hyperplastic and the skin thickened in UVB irradiated groups. Especially in UV(+) control, dead cells were observed in the stratum spinosum. However, in UV(+) ICP as compared with in UV(+) control, the skin showed less thickening (p < 0.01). Moreover, abnormal growth of the epidermal cells was suppressed and the number of denucleated cells were also decreased. Immunohistochemical analysis using an antibody against laminin was conducted to identify the BM, which stain brown (Figure 3B). In UV(+) ICP, the BM was consecutively stained in a darker brown, whereas staining of BM was weakened in UV(+) control. These results suggested that the oral administration of ICP suppressed BM degradation and inhibited epidermal hyperplasia.
Figure 3.
Histological appearance of dorsal skin in UVB irradiated hairless mice skin after the oral administration of ICP. After UV irradiation and the oral administration of ICP for 7 weeks, dorsal skin tissue was removed and (A) hematoxylin and eosin (H & E) staining small arrows indicate de-nucleated cells in the upper stratum spinosum (bar = 50 μm), (B) immunostaining with anti - laminin was performed. Arrows indicate basement membrane (bar = 50 μm), and (C) epidermal thickness. Data are shown as means ± SD. ∗∗significant (p < 0.01) difference between UV(+) control and UV(+) ICP.
4. Discussion
In this study, ICP improved photoaging skin by protecting BM in UVB irradiated hairless mice. UV irradiation triggers skin disfunction through generating intracellular and extracellular ROS, which has negative effects on the skin, including lipid peroxide and protein peroxide production and DNA damage. These oxidative stresses induce chronic skin damage and change the skin construction such as destruction of cell-cell adhesion, fragmentation of intercellular collagen fiber, and disruption of BM. As a result, decreasing of skin moisture content and skin barrier disfunction are caused [17].
Hesperidin and narirutin, the major flavonoids in ICP, are known to have high antioxidant capacity and decrease ROS generation [10]. Hesperidin has been reported to have an effect to attenuate UVB-induced apoptosis mediated by mitigation of oxidative stress in human keratinocytes [18]. Narirutin also has a wide range of therapeutic properties such as an inhibitory effect against allergic skin reactions in a murine model of atopic dermatitis [19]. The orally administered flavonoids are thought to be absorbed into the body after being hydrolyzed and transformed into aglycones. They showed enhancing effect on the production of typeⅠ collagen in UV damaged human dermal fibroblasts [20]. Also, they promoted the production of hyaluronan (HA), important skin moisturizing factor, in HaCaT keratinocytes [21]. In the present study, ICP might be absorbed as bioactive compounds in other forms and attribute to suppress ROS damage in skin and protect from UVB-induced skin structure change. However, the detailed mechanism was not enough by this experiment, so additional experiments are necessary.
We confirmed the reduction of skin moisture content and the increase of TEWL caused by UVB irradiation were suppressed by orally administration of ICP, which indicated ICP protected from UVB-induced skin barrier disfunction. From H&E staining, we observed the characteristic histological changes in UVB-damaged epidermis such as epidermal hyperplasia and increase of the number of the dead cells in stratum spinosum. Epidermal thickening is known as one of the characteristics of photoaged skin. Previous reports revealed that dietary supplementation with antioxidants such as green tea polyphenols had anti-photoaging activities including epidermal hyperplasia [22]. Although dietary antioxidants do not directly neutralize free radicals, they attribute to maintain the cell's redox state, resulting in activating the endogenous antioxidant enzymes, which work as ROS scavengers [5]. We confirmed that ICP had high antioxidant activity with ORAC score 2260 μmol Trolox equivalent/L (data not shown). Our findings suggested that orally administered ICP suppressed UV-induced cell death in epidermis and prevent increase in the epidermal thickness via alleviating ROS generation.
BM is known as an important structure which strongly attaches the epidermis to the dermis. At the dermal-epidermal junction (DEJ) of sun-exposed skin, severe disruption and reduplication of BM were reported [23]. Laminin, a glycoprotein which provides links between keratinocyte and dermal extracellular matrix, is confirmed to promote the formation of BM at the DEJ in skin equivalent model [24]. Therefore, degradation of laminin may induce BM deficiency, and consequently result in cell proliferation abnormality and skin barrier disfunction. Hence enhancing BM repair could be an effective way to suppress photoaging [3]. In this study, we could not determine the function of polyphenols in Immature Citrus unshiu on the improvement of basement membrane with UV irradiation damage. It is considered that this finding suggested that ICP maintained epidermal homeostasis, normalizing dermal-epidermal adhesion and signal transduction by protecting from BM destruction. BM is conceived to be disrupted by UV radiation through activating MMP-1, 2, 3, 9 [3]. In previous reports, immature C. unshiu extract suppressed MMP-1 production in human dermal fibroblasts, MMP-2 expression in UVB-irradiated hairless mice [12], and hesperidin inhibited UVB-induced MMP-9 activation in hairless mice [25]. From these reports, it is conceivable that ICP suppressed UVB-induced BM degradation via MMPs suppression. Moreover, we confirmed that methanol extract from ICP increased HA production in HaCaT keratinocytes (data not shown). Extracellular HA in epidermis plays a crucial role to hold water and maintain cell cycle and function normally [26]. A previous study showed that decreasing of the HA content was observed in UV-irradiated HaCaT keratinocytes [27]. Together, these results indicated that ICP might act as a potent inhibitor against UV-induced HA degradation, maintaining cell homeostasis. Further investigation to determine ICP effect on promoting UVB-damaged BM repair is needed.
Finally, this study suggested that ICP might be a good candidate to improve UVB- induced photoaging skin. Flavonoids in ICP like hesperidin and narirutin are considered as the important substances in ICP.
Declarations
Author contribution statement
M. Watanabe and Y. Nomura: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
E. Tamaru: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Mr. Osamu Shimizu for Citrus unshiu supply. We are also grateful to the members of Scleroprotein and Leather Research Institute (Tokyo University of Agriculture and Technology).
References
- 1.Chung J.H. Photoaging in Asians. Photodermatol. Photoimmunol. Photomed. 2003;19:109–121. doi: 10.1034/j.1600-0781.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 2.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int. J. Mol. Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano S. Possible involvement of basement membrane damage in skin photoaging. J. Invest. Dermatol. Symp. Proc. 2009;14:2–7. doi: 10.1038/jidsymp.2009.5. [DOI] [PubMed] [Google Scholar]
- 4.Liu-Smith F., Jia J., Zheng Y. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Adv. Exp. Med. Biol. 2017;996:27–40. doi: 10.1007/978-3-319-56017-5_3. [DOI] [PubMed] [Google Scholar]
- 5.Kaliora A.C., Dedoussis G.V., Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Yoshigai E., Machida T., Okuyama T., Mori M., Murase H., Yamanishi R., Okumura T., Ikeya Y., Nishino H., Nishizawa M. Citrus nobiletin suppresses inducible nitric oxide synthase gene expression in interleukin-1beta-treated hepatocytes. Biochem. Biophys. Res. Commun. 2013;439:54–59. doi: 10.1016/j.bbrc.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Kang Y.-J., Yang M.-H., Ko W.-J., Park S.-R., Lee B.-G. Studies on the major components and antioxidative properties of whole fruit powder and juice prepared from premature Mandarin orange. Korean J. Food Sci. Technol. 2005;37:783–788. [Google Scholar]
- 8.Tanizawa H., Ohkawa Y., Takino Y., Miyase T., Ueno A., Kageyama T., Hara S. Studies on natural antioxidants in citrus species. I. Determination of antioxidative activities of citrus fruits. Chem. Pharm. Bull. (Tokyo) 1992;40:1940–1942. doi: 10.1248/cpb.40.1940. [DOI] [PubMed] [Google Scholar]
- 9.Tripoli E., Guardia M.L., Giammanco S., Majo D.D., Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104:466–479. [Google Scholar]
- 10.Jannat S., Ali M.Y., Kim H.R., Jung H.A., Choi J.S. Protective effects of sweet orange, Unshiu Mikan, and mini tomato juice powders on t-BHP-induced oxidative stress in HepG2 cells. Prev. Nutr. Food Sci. 2016;21:208–220. doi: 10.3746/pnf.2016.21.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang H.M., Lin J.W., Hsiao P.L., Tsai S.Y., Wen K.C. Hydrolysates of citrus plants stimulate melanogenesis protecting against UV-induced dermal damage. Phytother Res. 2011;25:569–576. doi: 10.1002/ptr.3302. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Sanchez A., Barrajon-Catalan E., Caturla N., Castillo J., Benavente-Garcia O., Alcaraz M., Micol V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol., B. 2014;136:12–18. doi: 10.1016/j.jphotobiol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Choi S.H., Choi S.I., Jung T.D., Cho B.Y., Lee J.H., Kim S.H., Yoon S.A., Ham Y.M., Yoon W.J., Cho J.H., Lee O.H. Anti-photoaging effect of Jeju Putgyul (Unripe citrus) extracts on human dermal fibroblasts and ultraviolet B-induced hairless mouse skin. Int. J. Mol. Sci. 2017;18:2052. doi: 10.3390/ijms18102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Ministry of Health, Labour and Welfare . seventeenth ed. 2016. The Japanese Pharmacopoeia. [Google Scholar]
- 15.Tanaka M., Koyama Y., Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009;73:930–932. doi: 10.1271/bbb.80649. [DOI] [PubMed] [Google Scholar]
- 16.Amano S., Akutsu N., Matsunaga Y., Nishiyama T., Champliaud M.F., Burgeson R.E., Adachi E. Importance of balance between extracellular matrix synthesis and degradation in basement membrane formation. Exp. Cell Res. 2001;271:249–262. doi: 10.1006/excr.2001.5387. [DOI] [PubMed] [Google Scholar]
- 17.Shindo Y., Witt E., Han D., Packer L. Dose-response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J. Invest. Dermatol. 1994;102:470–475. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- 18.Madduma Hewage S.R., Piao M.J., Kang K.A., Ryu Y.S., Han X., Oh M.C., Jung U., Kim I.G., Hyun J.W. Hesperidin attenuates ultraviolet B-induced apoptosis by mitigating oxidative stress in human keratinocytes. Biomol. Ther. (Seoul) 2016;24:312–319. doi: 10.4062/biomolther.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong W.S., Park S.W., Chung S.K. The antioxidative activity of Korean Citrus unshiu peels. Food Biotechnol. 1997;6:292–296. [Google Scholar]
- 20.Bae J.T., Ko H.J., Kim G.B., Pyo H.B., Lee G.S. Protective effects of fermented Citrus unshiu peel extract against ultraviolet-A-induced photoageing in human dermal fibrobolasts. Phytother Res. 2012;26:1851–1856. doi: 10.1002/ptr.4670. [DOI] [PubMed] [Google Scholar]
- 21.Kim C., Ji J., Ho Baek S., Lee J.H., Ha I.J., Lim S.S., Yoon H.J., Je Nam Y., Ahn K.S. Fermented dried Citrus unshiu peel extracts exert anti-inflammatory activities in LPS-induced RAW264.7 macrophages and improve skin moisturizing efficacy in immortalized human HaCaT keratinocytes. Pharm. Biol. 2019;57:392–402. doi: 10.1080/13880209.2019.1621353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vayalil P.K., Mittal A., Hara Y., Elmets C.A., Katiyar S.K. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J. Invest. Dermatol. 2004;122:1480–1487. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 23.Lavker R.M. Structural alterations in exposed and unexposed aged skin. J. Invest. Dermatol. 1979;73:59–66. doi: 10.1111/1523-1747.ep12532763. [DOI] [PubMed] [Google Scholar]
- 24.Tsunenaga M., Adachi E., Amano S., Burgeson R.E., Nishiyama T. Laminin 5 can promote assembly of the lamina densa in the skin equivalent model. Matrix Biol. 1998;17:603–613. doi: 10.1016/s0945-053x(98)90111-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.J., Im A.R., Kim S.M., Kang H.S., Lee J.D., Chae S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Compl. Alternative Med. 2018;18:39. doi: 10.1186/s12906-017-2058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern R., Maibach H.I. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008;26:106–122. doi: 10.1016/j.clindermatol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Tzellos T.G., Klagas I., Vahtsevanos K., Triaridis S., Printza A., Kyrgidis A., Karakiulakis G., Zouboulis C.C., Papakonstantinou E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp. Dermatol. 2009;18:1028–1035. doi: 10.1111/j.1600-0625.2009.00889.x. [DOI] [PubMed] [Google Scholar]