Abstract
Background and Aims
Patients with inflammatory bowel diseases may have higher incidences of non-melanoma skin cancers and non-Hodgkin lymphoma, potentially linked to underlying disease and treatments. This analysis assessed incidence rates of these malignancies in Japanese patients with ulcerative colitis or Crohn’s disease, and their association with thiopurine and/or anti-tumor necrosis factor-α treatment, using data from a nationwide administrative database in Japan.
Methods
Patients diagnosed with inflammatory bowel disease without malignancy were identified from the Medical Data Vision database. Incident cases of non-melanoma skin cancers and non-Hodgkin lymphoma diagnosed after prescription of thiopurine and/or anti-tumor necrosis factor-α were identified between April 2008 and January 2018. Age- and sex-adjusted incidence rate ratios were calculated relative to the total treated patient population.
Results
A total of 75 673 eligible patients were identified at the index date. Thiopurine prescription with or without anti-tumor necrosis factor-α agents increased incidence rate ratios for non-melanoma skin cancers relative to the overall population (3.39 and 4.03, respectively). There were no notable differences in non-Hodgkin lymphoma incidence relative to the total population in any treatment subgroup, regardless of prescription of thiopurine and/or anti-tumor necrosis factor-α (all incidence rate ratios, ~1).
Conclusions
There is no evidence for an increased incidence of non-Hodgkin lymphoma attributable to thiopurine or anti-tumor necrosis factor-α treatment in Japanese patients with inflammatory bowel disease. The impact of racial differences on non-Hodgkin lymphoma incidences should be considered. Thiopurine therapy may be a risk factor for non-melanoma skin cancers in Japanese patients.
Keywords: Anti-TNFα, immunomodulators, lymphoma, skin cancers
1. Introduction
Inflammatory bowel disease [IBD] encompasses a group of inflammatory conditions of the digestive tract, of which ulcerative colitis [UC] and Crohn’s disease [CD] are the two main forms.1 UC is characterized by erosions and/or ulcerations of the mucosal surface of the large intestine, while CD is a condition that can cause inflammation of any part of the gut, although the most common areas affected are the distal end of the small intestine and the colon.1–3
Based on the most recently available data, 170 781 patients with UC and 40 885 patients with CD were registered as receiving treatment in Japan in the 2014 fiscal year.4,5 Although the genetic susceptibilty of Asian [including Japanese] persons to IBD differs from that of other populations,6 and familial risk in Asia has traditionally been considered to be ‘low’,7 the incidence of IBD is increasing in Japan.8
Relapse after treatment is frequently seen in UC or CD patients, and the current medical approach consists of induction of remission followed by long-term maintenance therapy.3,9,10 For UC, in moderate-to-severe disease or in cases refractory to corticosteroids, immunomodulators, such as thiopurines, and biologic drugs, such as anti-tumor necrosis factor-alpha [TNFα] agents, can be used as second- and third-line treatments, respectively.3 Similarly for CD, thiopurines are effective for maintenance of remission, while anti-TNFα agents can be used to both induce and maintain remission.1 Combination treatment with thiopurines and anti-TNFα is also an option and has been shown to achieve better efficacy compared with either treatment used alone.11–13
Previous reports have suggested that IBD, as well as common therapies used to treat these conditions, may be associated with an increased incidence of malignancies.14 Increased risks of non-melanoma skin cancers [NMSCs] and non-Hodgkin lymphoma [NHL] due to thiopurines have been reported in IBD patients, although mostly in Caucasian patient populations [who have a higher baseline susceptibility to these malignancies than Asian patients].15–21 Analyses in predominantly Caucasian patient populations have also suggested that the use of anti-TNFα monotherapy, as well as thiopurine monotherapy, may be associated with a small increase in the risk of lymphoma in patients with IBD, which is increased further if both treatments are used in combination.22 In contrast, a single questionnaire-based study reported no increase in the incidence of haematologic malignancies in Japanese IBD patients treated with thiopurines.23 This study was the only published investigation of the incidence of haematologic malignancies in IBD patients in Japan, and to our knowledge no reports have investigated the incidence of NMSCs in Japanese IBD patients treated with common IBD therapies.
The evidence relating the incidence of NMSCs and NHL to IBD treatment is unclear in Japan and other Asian countries, such as China and Korea. We therefore utilized data from a large, nationwide administrative database to assess the incidence rates of NMSCs and NHL, and the association between these incidence rates and the administration of thiopurine and/or anti-TNFα agents in IBD patients in Japan.
2. Materials and Methods
2.1. Data sources
This study of incident cases of NMSCs and NHL in IBD patients was conducted using cross-sectional data from the Medical Data Vision [MDV] database. At the time of the study, the MDV database included data on approximately 17.8 million patients who had received treatment in any of the Diagnosis Procedure Combination [DPC] hospitals in Japan during the period from April 2008 to January 2018. The database includes not only hospitalization data, but also outpatient and prescription data. The MDV dataset, which is the largest private claims database accessible to pharma companies, includes information on: diagnoses coded using the World Health Organization’s International Statistical Classification of Diseases and Related Health Problems 10th Revision [ICD-10] coding scheme; disease names coded using Japanese Disease Name Codes; medical procedures coded using Japanese Procedure Codes; and prescription information, including generic drug names. It should be noted that when a patient is transferred to another hospital or clinic, his/her claims data can no longer be collected continuously.
2.2. Ethical statement
Given that data contained within the MDV database are anonymous, no informed consent was required, as per the Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labor, and Welfare. All authors had full access to all the data, and take responsibility for its integrity and the data analysis.
2.3. Study population
The study population was identified from the MDV database [study period, April 2008 to January 2018] as patients with ≥1 recorded diagnosis of IBD, using the ICD-10 codes K51 for UC and K50 for CD, and no diagnosis of malignancy at the index date [defined as the first day of the month in which IBD was first diagnosed or, for those patients with a prescription for a thiopurine and/or an anti-TNFα agent, the date of first prescription thereof]. Patients with UC and CD were evaluated as a single group in the analyses reported herein. Occurrences of malignancy [NMSCs or NHL] during the study period were identified using the ICD-10 codes shown in Supplementary Table 1, available as Supplementary data at ECCO-JCC online.
2.4. Objectives and assessments
The primary objective was to determine incidence rate ratios [IRRs] for NMSCs and NHL in treatment subgroups relative to the overall IBD patient population. As the MDV database only includes patients receiving treatment, direct comparisons with the general population were not possible; therefore, comparisons were made with the total treated IBD population in the database. Incident cases of NMSCs were identified as ≥1 recorded diagnosis of NMSCs [by ICD-10 code; laboratory confirmation of diagnosis was not required in accordance with usual practice]. The following criteria were used to identify incident cases of NHL: ≥3 recorded diagnoses of NHL [by ICD-10 code]; ≥1 recorded diagnosis of NHL and a diagnostic/staging imaging procedure [gallium scintigraphy or positron emission tomography–computed tomography scan]; or ≥1 recorded diagnosis of NHL and ≥1 treatment prescription for rituximab, cisplatin, cyclophosphamide, doxorubicin, vincristine, cytarabine, etoposide, carboplatin, brentuximab vedotin, or ifosfamide.
For all analyses, the IBD population was divided into treatment subgroups based on patient prescription records as: thiopurine [azathioprine or 6-mercaptopurine] without TNFα [infliximab or adalimumab]; anti-TNFα without thiopurine; thiopurine and anti-TNFα; and other treatment. As a sensitivity analysis, IRRs for IBD patients with a ≥3-month observation period for thiopurine or ≥3 prescriptions for an anti-TNFα after their first recorded prescription were also calculated.
The secondary objective was to determine whether there was an association between the incidences of NMSCs or NHL and the prescribing of thiopurine and/or anti-TNFα agents in the IBD population using Cox proportional hazards modelling.
2.5. Statistical analysis
2.5.1. Incidence rate ratios
Incidence of malignancy [i.e. the number of patients with a recorded diagnosis of the target malignancy [NMSCs or NHL] during the evaluation period, defined as the period from the index date until the end of the observation period or the date of malignancy diagnosis, whichever was earlier] was identified from claims data for patients with IBD and no diagnosis of malignancy at the index date. Daily and annual incidences of NMSCs and NHL were calculated. Incidence rates were then expressed as the number of malignancies per 100 000 person-years.
For the reference population, estimated age- and sex-adjusted incidences of NMSCs and NHL were calculated for all treated IBD patients in the MDV database using the following formula:
Incidence = observation period [in years] × age- and sex-adjusted annual incidence of malignancy in the overall treated IBD population.
The IRRs were calculated as the ratio of incident cases in the IBD treatment subgroups of interest to incident cases in the overall IBD population.
2.5.2. Cox proportional hazards analyses
The effect of age, sex, and treatment type on NMSCs and NHL risk in treated IBD patients from the MDV database was analysed using adjusted Cox proportional hazards models.
SAS version 9.4 [SAS Institute, Cary, NC, USA] was used for the statistical analyses. All analyses were performed by Crecon Medical Assessment, Tokyo, Japan, with financial support from Takeda Pharmaceutical Company Limited.
3. Results
During the study period [as defined above], 75 673 patients with IBD and no diagnosis of malignancy at the index date [representing more than a quarter of all patients with IBD in Japan] were identified from the MDV database. Baseline patient characteristics are summarized in Table 1. The mean age of patients was 45.7 years and 43% of patients were female.
Table 1.
Baseline characteristics at the time of cohort entry for patients with IBD and no diagnosis of malignancy at the index date.
| MDV database | |
|---|---|
| Patients, N | 75 673 |
| Mean age, years [SD] | 45.7 [18.3] |
| Female, % | 42.5 |
| Mean observation time, months [SD] | 30.8 [26.8] |
| Patients with a prescription for a thiopurine or anti-TNFα, n | 18 858 |
| Thiopurine only | 7042 |
| Anti-TNFα only | 6062 |
| Thiopurine and anti-TNFα | 5754 |
IBD, inflammatory bowel disease [ulcerative colitis/Crohn’s disease]; MDV, Medical Data Vision; SD, standard deviation; TNFα, tumor necrosis factor-alpha.
The index date was defined as the first day of the month in which IBD was first diagnosed or, for those patients with a prescription for a thiopurine and/or an anti-TNFα agent, the date of first prescription thereof.
3.1. Incidence rate ratios
3.1.1. Non-melanoma skin cancers
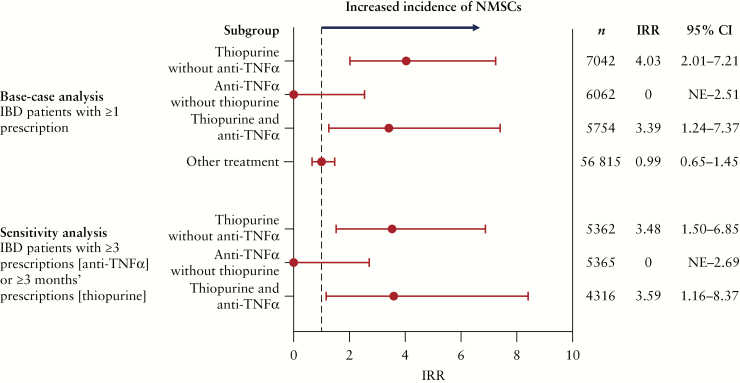
Development of NMSCs in IBD patients during the evaluation period is shown in Figure 1 and Supplementary Table 2, available as Supplementary data at ECCO-JCC online. A total of 43 cases of NMSCs were observed in 75 673 patients. IRRs for NMSCs relative to the overall IBD population [in patients with ≥1 prescription] were 4.03 in patients who were prescribed thiopurine alone and 3.39 in those who were prescribed thiopurine and anti-TNFα combined. In patients who were prescribed anti-TNFα alone, the IRR was 0. Incidence rates per 100 000 person-years for NMSCs were 4.94 for thiopurine alone, 2.51 for thiopurine and anti-TNFα combined, and 0 for anti-TNFα alone. The results of the sensitivity analysis were similar to those of the main analysis.
Figure 1.
Age- and sex-adjusted IRRs for NMSCs in IBD patient subgroups relative to the overall IBD population by treatment prescribed [MDV database]. CI, confidence interval; IBD, inflammatory bowel disease [ulcerative colitis/Crohn’s disease]; IRR, incidence rate ratio; MDV, Medical Data Vision; NE, not estimable; NMSC, non-melanoma skin cancer; TNFα, tumor necrosis factor-alpha.
3.1.2. Non-hodgkin lymphoma
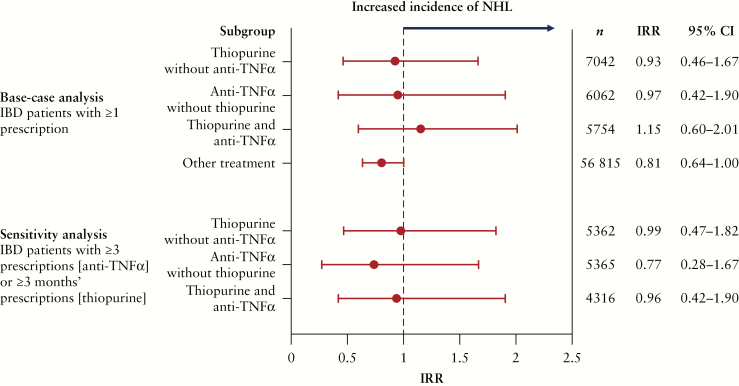
Figure 2 and Supplementary Table 3, available as Supplementary data at ECCO-JCC online, show IRRs for NHL in patients with IBD. Overall, 103 cases of NHL were seen in 75 673 patients. There were no notable differences in NHL incidence among any of the IBD treatment subgroups, regardless of prescriptions for thiopurine and/or anti-TNFα. IRRs relative to the overall IBD population (in patients with ≥1 prescription) were 0.96 for thiopurine, 1.18 for thiopurine and anti-TNFα combined, and 0.99 for anti-TNFα. The NHL incidence rates per 100 000 person-years were 4.95, 5.03, and 4.08, respectively. The results of the sensitivity analysis echoed those of the main analysis.
Figure 2.
Age- and sex-adjusted IRRs for NHL in IBD patient subgroups relative to the overall IBD population by treatment prescribed [MDV database]. CI, confidence interval; IBD, inflammatory bowel disease [ulcerative colitis/Crohn’s disease]; IRR, incidence rate ratio; MDV, Medical Data Vision; NHL, non-Hodgkin lymphoma; TNFα, tumor necrosis factor-alpha.
3.2. Cox proportional hazards analysis
In the Cox proportional hazards analysis, patient age (hazard ratio [HR], 1.09; p < 0.0001), thiopurine prescription [HR, 4.92; p < 0.0001], and combined thiopurine/anti-TNFα prescription [HR, 5.08; p = 0.001] were all significantly associated with an increased risk of NMSCs, whereas sex was not [Table 2]; the effect of anti-TNFα treatment alone on the risk of NMSCs could not be estimated accurately due to an inadequate sample size. In the lymphoma analysis, only age was significantly associated with an increased risk of NHL [HR, 1.04; p < 0.0001]; sex and treatment [thiopurine and/or anti-TNFα prescription] were not associated with NHL.
Table 2.
Cox proportional hazards analysis of the effect of age, sex, and treatment on NMSCs and NHL risk in IBD patients [MDV database].
| Malignancy and risk factor | Parameter estimate | SEM | p-value | HR |
|---|---|---|---|---|
| NMSCs | ||||
| Sex | 0.24 | 0.31 | 0.434 | 1.28 |
| Age | 0.09 | 0.01 | <0.0001 | 1.09 |
| Thiopurine only | 1.59 | 0.37 | <0.0001 | 4.92 |
| Anti-TNFα only | –13.19 | 724.68 | 0.986 | 0.00 |
| Thiopurine and anti-TNFα | 1.63 | 0.48 | 0.001 | 5.08 |
| NHL | ||||
| Sex | 0.15 | 0.20 | 0.457 | 1.16 |
| Age | 0.04 | 0.01 | <0.0001 | 1.04 |
| Thiopurine only | 0.21 | 0.32 | 0.518 | 1.23 |
| Anti-TNFα only | 0.28 | 0.38 | 0.455 | 1.33 |
| Thiopurine and anti-TNFα | 0.54 | 0.32 | 0.094 | 1.71 |
HR, hazard ratio; IBD, inflammatory bowel disease [ulcerative colitis/Crohn’s disease]; MDV, Medical Data Vision; NHL, non-Hodgkin lymphoma; NMSC, non-melanoma skin cancer; SEM, standard error of the mean; TNFα, tumor necrosis factor-alpha.
4. Discussion
To our knowledge, this is the first real-world study based on a large-scale administrative database to assess the morbidity of NMSCs and NHL in Japanese patients with IBD. Incidences of these malignancies in the Japanese population, and the Asian population as a whole, different [generally much lower] compared with Caucasian populations.24–26 Moreover, Western IBD studies have demonstrated an association between thiopurine use and NMSCs and NHL, and there is some evidence that TNFα use may be linked with a small increase in the risk of lymphoma in predominantly Caucasian patients.15–19,22,27,28 As there are almost no current data looking at the incidences of NMSCs and NHL, and their associations with the prescribing of thiopurine/anti-TNFα therapies in other populations, such as Asian patients, this study aimed to provide real-world evidence on this clinical issue.
For NMSCs, IRRs [relative to the overall IBD population] were higher in IBD patients who were prescribed treatment with a thiopurine with or without an anti-TNFα compared with patients not receiving these treatments. Furthermore, Cox regression analysis showed that thiopurine prescription without an anti-TNFα [HR, 4.92; p < 0.0001] and thiopurine prescription with anti-TNFα therapy [HR, 5.08; p = 0.001] were both significantly associated with an increased risk of NMSCs; a HR for the anti-TNFα prescription only subgroup could not be estimated due to an inadequate sample size (because no cases of NMSCs were observed during the evaluation period). Consistently, Western studies have also shown an association between NMSCs and thiopurine and/or anti-TNFα treatment in predominantly Caucasian patients with IBD, and some have reported an association between NMSCs and IBD itself.11,19,27,29–31 For example, in a large retrospective cohort study using an administrative database of American IBD patients, recent thiopurine use [≤90 days prior to NMSC diagnosis] was associated with increased odds of developing NMSCs (adjusted odds ratio [OR], 3.56; 95% confidence interval [CI], 2.81–4.50).30 In the same study, recent biologic use [including anti-TNFα therapy] in patients with CD was also associated with NMSCs [adjusted OR, 2.07; 95% CI, 1.28–3.33].30 While we also found an association between NMSCs and thiopurine with/without anti-TNFα prescription, the increase in the relative risk of NMSCs observed with IBD treatment exposure in the present study must be balanced against the low absolute risk of NMSCs in the Japanese population.32,33 Even in patients who were prescribed a thiopurine with or without anti-TNFα therapy, the incidence rates of NMSCs [2.94–4.94 per 100 000 person-years] were still in line with published figures for the general Japanese population.32,33 Consequently, unlike in Western countries [where baseline rates of NMSCs in the general population are much higher [>1000 per 100 000 person-years in Australia and 450 per 100 000 person-years in the USA],26,34 the relative increase in NMSC incidence rates associated with the prescribing of thiopurine with/without anti-TNFα treatment is likely to have minimal adverse impact on Japanese patients with IBD, and the real-world benefits of treatment on IBD signs and symptoms are likely to far outweigh the risk of malignancy. As stated above, in our analysis, no cases of NMSCs were reported among IBD patients who were prescribed anti-TNFα therapy alone.
IRRs for NHL relative to the overall IBD population were generally comparable in patients who were prescribed a thiopurine or an anti-TNFα [with IRRs of ~1]. Reassuringly, our results were in accordance with a previous questionnaire-based study in Japan, which demonstrated no significant increase in haematologic malignancies in UC or CD patients receiving thiopurines.23 In contrast, studies in Caucasian IBD patient populations have reported an increased risk of NHL attributable to thiopurine or anti-TNFα use.16–18,22,27–29,35 For instance, in a study utilizing French National Health Insurance data, use of a thiopurine or anti-TNFα as monotherapy in adults with IBD was associated with a small but significant increase in the risk of lymphoma, and the risk was further increased with the use of both agents in combination,22 as was also seen in the present study. A meta-analysis of six cohort studies also revealed an approximately 4-fold increase in the risk of lymphoma in IBD patients treated with azathioprine/6-mercaptopurine; the authors of this analysis suggested that the increase could be attributed to the medication, the severity of the underlying disease, or both.17 With regard to potential disease effects, a Danish population-based cohort study reported an increased risk of NHL in patients with UC versus the general population that was not associated with thiopurine exposure.36 It is not clear, however, whether IBD on its own increases the risk of lymphoma due to conflicting data.27,28 Overall, our results show a similar or lower incidence of NHL in the treated IBD population [4.08–5.03 per 100 000 person-years] compared with reported figures for the baseline incidence of NHL in the general population in Japan [2.0–11.3 per 100 000 person-years; 2015 data].1,33
We believe that genetic background may underlie any inconsistencies between Asian [in this case, Japanese] and Caucasian data, resulting in different baseline incidences of malignancy [note, rates of NMSCs and NHL are much lower in Japan than in Western countries],25,26,32–34,37,38 as well as different susceptibilities for the development of malignancy due to treatment exposure. In addition, standard doses of thiopurines prescribed to Japanese patients with IBD differ from those prescribed in Western countries, and this may also have affected the results.1,2 Given these data, it will be important to further evaluate the development of malignancy in IBD patients from different ethnic backgrounds. It will also be important, in future studies, to assess whether the risk of malignancy in Japanese IBD patients is affected by the duration of thiopurine prescription, given that studies in mainly Caucasian patients have reported a link between the duration of exposure to these medications and the risk of developing cancer.18,31 Interestingly, it has also been shown that the risk of lymphoma is no longer increased following discontinuation of thiopurine treatment.39
There were some limitations to our study. For example, information on some confounding factors for malignancies [e.g. disease severity and duration] was not available in the MDV database and could not be adjusted for in this analysis. Moreover, the study period was limited to that available in the database. Analyses that adjust for additional, potentially confounding factors over longer observation periods are therefore needed. Within the MDV database, it was also not possible to continuously track patients who transferred to or from another hospital or clinic, which may have resulted in some cases of malignancy being identified inaccurately. Further, due to nature of the MDV database, it was only possible to make comparisons with the total treated IBD population, as opposed to the general population. Another potential limitation relates to the analysis of IBD patients as a single group, for it is possible that the results might have been different if we had analysed UC and CD patients separately. However, due to the low number of incident cases of malignancy for NMSCs and NHL, individual analysis by IBD type was considered unlikely to generate meaningful results. The low incidence of malignancy also precluded any meaningful subgroup analyses [e.g. analysis by patients undergoing surgery]. It should be noted that while it may be possible to extrapolate the results of our study to other Asian populations with similar genetic backgrounds [e.g. Chinese and Korean patients], this may not be possible for populations of other ethnicities.
In conclusion, this large-scale, administrative database study provides an indication of the real-world risk of NMSCs and NHL in Japanese patients with IBD. This is the first study to demonstrate that thiopurine with or without anti-TNFα prescription may be associated with an increased risk of NMSCs in this population. There was, however, no evidence that NHL incidence rates are affected by thiopurine or anti-TNFα prescription. Incidence rates of both malignancies in the Japanese population are low compared with in Caucasian populations. Therefore, racial differences regarding the risk of malignancy may need to be considered in future individualized risk–benefit assessments for UC and CD management in each ethnicity, which should not focus solely on incidence data from Caucasian patient populations. Analyses that adjust for additional confounding factors over extended observation periods are warranted.
Supplementary Material
Acknowledgments
Support for the data analysis was provided, under the direction of the authors, by Sachie Inoue, PhD, an employee of Crecon Medical Assessment. The authors would also like to thank Kosuke Iwasaki, PhD, and Wentao Tang, PhD, employees of Milliman, for their support during the development of the concept [WT] and analysis of the data [KI]. They also acknowledge Catherine Crookes of FireKite, an Ashfield company, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Takeda Pharmaceutical Co. Ltd. [Tokyo, Japan], and complied with Good Publication Practice 3 ethical guidelines [Battisti et al., Ann Intern Med. 2015;163:461-464].
Glossary
Abbreviations
- CD
Crohn’s disease
- CI
confidence interval
- DPC
Diagnosis Procedure Combination
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICD-10
International Statistical Classification of Diseases and Related Health Problems 10th Revision
- IRR
incidence rate ratio
- MDV
Medical Data Vision
- NHL
non-Hodgkin lymphoma
- NE
not estimable
- NMSC
non-melanoma skin cancer
- OR
odds ratio
- SD
standard deviation
- SEM
standard error of the mean
- TNFα
tumor necrosis factor-α
- UC
ulcerative colitis
Funding
This work was supported by Takeda Pharmaceutical Company Limited.
Conflict of Interest
TK reports personal fees from Astellas, Celltrion, Covidien, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, Mitsubishi Tanabe Pharma, Pfizer, Takeda Pharmaceutical Company Limited, grants from Otsuka Holdings, grants and personal fees from Abbvie GK, Alfresa Pharma, EA Pharma Co. Ltd, JIMRO Co., Kyorin Pharmaceutical Co. Ltd, Mochida Pharmaceutical Ltd, Nippon Kayaku, Thermo Fisher Scientific, ZERIA Pharmaceutical, outside the submitted work.
AU and EU were employees of Takeda Pharmaceutical Co. Ltd. during the conduct of the study.
TH reports personal fees from Abbvie GK, Aspen Japan K.K, Bristol-Myaers Squibb, Celltrion, Eli Lilly, Ferring, Gilead Sciences, Janssen, Kissei Pharmaceutical, Mitsubishi-Tanabe Pharma, Nichi-Iko Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical, grants from Abbvie, EA Pharma, Otuska Holdings, and grants and personal fees from JIMRO, Kyorin, Mochida Pharmaceutical, Zeria Pharmaceuticals, outside the submitted work.
Author Contributions
TK devised the conceptual ideas of the study. TK, AU, EU, and TH all made substantial contributions to all of the following: design of the study, acquisition of data, analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be submitted. TK, AU, and EU also contributed to drafting the article. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018;53:305–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hibi T, Ueno F. Guidelines for the management of ulcerative colitis in Japan – developed through integration of evidence and consensus among experts. IBD Res 2010;4:189–239. [Google Scholar]
- 3. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 4. Ueno F, Nakayama Y, Hagiwara E, Kurimoto S, Hibi T. Impact of inflammatory bowel disease on Japanese patients’ quality of life: results of a patient questionnaire survey. J Gastroenterol 2017;52:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Japanese Ministry of Health, Labour and Welfare. Heisei 26 nenndo eisei-gyousei-houkokurei no gaikyou.2014. https://www.mhlw.go.jp/toukei/saikin/hw/eisei_houkoku/14/dl/kekka7.pdf Accessed July 10, 2019.
- 6. Arimura Y, Isshiki H, Onodera K, et al. Characteristics of Japanese inflammatory bowel disease susceptibility loci. J Gastroenterol 2014;49:1217–30. [DOI] [PubMed] [Google Scholar]
- 7. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol 2012;27:1266–80. [DOI] [PubMed] [Google Scholar]
- 8. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- 9. Freeman HJ. Natural history and long-term clinical course of Crohn’s disease. World J Gastroenterol 2014;20:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masaki T, Kishiki T, Kojima K, Asou N, Beniya A, Matsuoka H. Recent trends (2016–2017) in the treatment of inflammatory bowel disease. Ann Gastroenterol Surg 2018;2:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bots S, Gecse K, Barclay M, D’Haens G. Combination immunosuppression in IBD. Inflamm Bowel Dis 2018;24:539–45. [DOI] [PubMed] [Google Scholar]
- 12. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 13. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 14. Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22:4794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garg SK, Loftus EV Jr. Risk of cancer in inflammatory bowel disease: going up, going down, or still the same? Curr Opin Gastroenterol 2016;32:274–81. [DOI] [PubMed] [Google Scholar]
- 16. Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol 2013;177:1296–305. [DOI] [PubMed] [Google Scholar]
- 17. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005;54:1121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beaugerie L, Brousse N, Bouvier AM, et al. ; CESAME Study Group Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617–25. [DOI] [PubMed] [Google Scholar]
- 19. Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012;143:390–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization International Agency for Research on Cancer. Global cancer observatory: cancer today. Non-melanoma skin cancer fact sheet 2018. https://gco.iarc.fr/today/data/factsheets/cancers/17-Non-melanoma-skin-cancer-fact-sheet.pdf Accessed September 9, 2019.
- 21. World Health Organization International Agency for Research on Cancer. Global Cancer Observatory: cancer today. Non-Hodgkin lymphoma fact sheet 2018. https://gco.iarc.fr/today/data/factsheets/cancers/34-Non-hodgkin-lymphoma-fact-sheet.pdf Accessed September 9, 2019.
- 22. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA 2017;318:1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukata N, Okazaki K, Omiya M, Matsushita M, Watanabe M; Members of the Ministry of Health and Welfare of Japan’s Inflammatory Bowel Diseases Study Group Hematologic malignancies in the Japanese patients with inflammatory bowel disease. J Gastroenterol 2014;49:1299–306. [DOI] [PubMed] [Google Scholar]
- 24. Gupta AK, Bharadwaj M, Mehrotra R. Skin cancer concerns in people of color: risk factors and prevention. Asian Pac J Cancer Prev 2016;17:5257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept 2017;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am 2014;43:525–41. [DOI] [PubMed] [Google Scholar]
- 28. Siegel CA. Risk of lymphoma in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2009;5:784–90. [PMC free article] [PubMed] [Google Scholar]
- 29. Magro F, Peyrin-Biroulet L, Sokol H, et al. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohns Colitis 2014;8:31–44. [DOI] [PubMed] [Google Scholar]
- 30. Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2010;8:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. ; Cesame Study Group Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011;141:1621–28.e1-5. [DOI] [PubMed] [Google Scholar]
- 32. Fujisawa Y, Funakoshi T, Nakamura Y, et al. Nation-wide survey of advanced non-melanoma skin cancers treated at dermatology departments in Japan. J Dermatol Sci 2018;92:230–6. [DOI] [PubMed] [Google Scholar]
- 33. National Cancer Registry. Statistical data about cancer [Japanese].2019. https://ganjoho.jp/reg_stat/statistics/dl/index.html#incidence4pref Accessed July 10, 2019.
- 34. Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151:1081–6. [DOI] [PubMed] [Google Scholar]
- 35. Farrell RJ, Ang Y, Kileen P, et al. Increased incidence of non-Hodgkin’s lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut 2000;47:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol 2013;108:1869–76. [DOI] [PubMed] [Google Scholar]
- 37. Loh TY, Ortiz A, Goldenberg A, Brian Jiang SI. Prevalence and clinical characteristics of nonmelanoma skin cancers among Hispanic and Asian patients compared with white patients in the United States: a 5-year, single-institution retrospective review. Dermatol Surg 2016;42:639–45. [DOI] [PubMed] [Google Scholar]
- 38. National Cancer Institute. Surveillance, epidemiology, and end results program. Cancer stat facts: non-Hodgkin lymphoma.2019. https://seer.cancer.gov/statfacts/html/nhl.html Accessed July 10, 2019.
- 39. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 2015;13:847–58.e4; quiz e48–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.