Graphical abstract
Keywords: SARS-COV-2, Chinese medicine, Clinical treatment
Highlights
-
•
The data derive from Chinese medicine prescription according to the fifth National recommendations during the SARS-CoV pandemics.
-
•
The alterations of inflammatory agents, immune function and leukocyte differential count in severe pneumonia of SARS-COV-2 patients after yidu-toxicity blocking lung decoction was given.
-
•
The inflammatory agents, IL-6 and TNF-α,was found to be decreased by Yidu-toxicity blocking lung decoction in vitro observations.
-
•
Yidu-toxicity blocking lung decoction could relieving inflammation of SARS-COV-2 patients and offering anther option in clinical settings.
Abstract
The present study investigates the differences in inflammatory agents alterations, immune function, and leukocyte differential count evaluation in severe pneumonia of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome after the recommended Chinese medicine prescription of Yidu-toxicity blocking lung decoction. A total of 40 patients with yidu-toxicity blocking lung syndrome, diagnosed as severe pneumonia of SARS-COV-2 following the latest Chinese national recommendations for the diagnosis and treatment of pneumonia caused by SARS-COV-2 (the 5th edition), were recruited. They were randomly divided into the pure western medicine therapy group (PWM) and integrated into Chinese and Western medicine therapy group (ICW). The general strategies were given to both groups according to the national recommendations, and the ICW group was given Yidu-toxicity blocking lung decoction extraorally. A radioimmunoassay method was adopted to detect the content of IL-6, IL-8,IL-2R,TNF-α, procalcitonin (PCT) and high-sensitivity C-reactive protein (hs-CRP) in sera. Flow cytometry was used to determine the peripheral blood lymphocyte subsets (the levels of CD3+, CD4+, CD8+, and the ratios of CD4+/CD8+). The white blood cell counts (WBC#), neutrophils count(N#), and lymphocyte counts (L#) were measured using a fully automatic blood rheological instrument. The t-test or Rank Sum Test and Spearman analysis were conducted to evaluate the differences. The results showed that IL-6 (P = 0.013) and TNF-α (P = 0.035) levels in the PWM group were significantly higher than those in the ICW group after treatment. Infection related indicators such as WBC#, N#, L#, hs-CRP showed no differences. The analysis showed that there was no statistical difference in the values of CD4 and CD8 between the two groups. By the end of Day 29, all patients were discharged and the final cure rate for both group were 100 %.
Taken together, we conclude that Yidu-toxicity blocking lung decoction could relieve inflammation of SARS-COV-2 patients with yidu-toxicity blocking lung syndrome by eliminating inflammatory agents. CM can serve as a complementary medication to western medicine, which should be highlighted in clinical settings.
1. Introduction
In late 2019, Wuhan in China became the focus of the world owing to an ongoing outbreak of pneumonia with a novel coronavirus named SARS-COV-2 [[1], [2], [3]].Up to February 24, 2020, more than 77,779 cases in China have been confirmed. SARS-COV-2 infections are mostly mild, but it can spread quickly. Currently, the infection has affected 28 countries and 5 continents. Middle-aged and elderly patients with underlying comorbidities are susceptible to respiratory failure and may have a poorer prognosis [4]. Early identification, effective isolation measures, and appropriate treatment play an important role in improving its prognosis [1,5]. Unfortunately, no evidence-based western medicine treatment has been recommended for coronavirus infection, except for meticulous supportive treatments, until now [6].
Recently, Chinese medicine has not only for many common diseases had a significant effect, but also has played an important role in prevention and control of major diseases and emerging infectious diseases. Unlike Western medicine, which has comprehensive and logical theories, CM treatments are based on functional analysis of the patient's entire body and use the body's self-healing abilities along with drugs and other medications. One classical CM symptoms of SARS-COV-2 named yidu-toxicity blocking lung syndrome are unflagging fever or alternating chills and fever, cough (little sputum or yellow sputum), abdominal distension or constipation, shortness of breath, dyspnea, red tongue, yellow greasy or burned furred tongue, rolling, and rapid pulse. From the CM dialectical point of view, the core pathogenic characteristics of this disease attributed to damp-toxin blocking lung. Hence, aromatic Chinese medicine remedies which can repel foulness and dispersing Lung-Qi are believed to be therapeutic principles. The recommendation of CM prescription of SARS-COV-2 treatment in the fifth National recommendations are as follows: Kuxingren, Shengshigao, Gualou, Shengdahuang, Shengmahuang, Zhimahuang, Tinglizi, Taoren, Caoguo, Binglang and Cangzhu. Over time there has been little emphasis on studying the combination of levels of white blood cell, immune function, inflammatory agents, and CM treatments, and therefore, the objective of this study is to evaluate the role of CM in the treatment of SARS-COV-2.
2. Methods and materials
2.1. Ethics statement
The study was carried out with the approval of the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. All participates were recruited by random selection and written informed consent was obtained prior to our study.
2.2. Patients and inclusion criteria
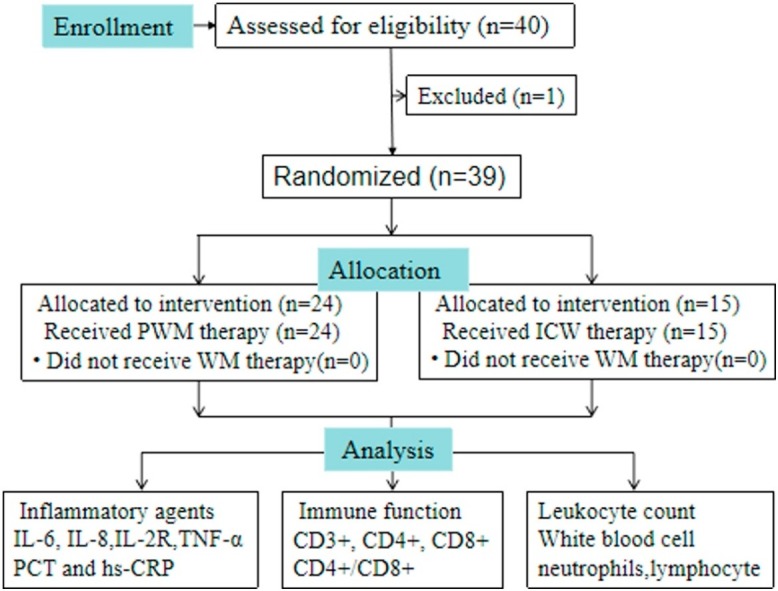
From January 20 to February 24, 2020, a total of 40 patients with severe pneumonia of SARS-COV-2 admitted to the respiratory departments and infectious disease departments of the First Affiliated Hospital of Anhui Medical University were selected. All the cases were diagnosed with SARS-COV-2 by combining clinical evidence with results from chest computed tomography (CT) and the real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR) amplification of the viral DNA from a pharyngeal examination sample twice. The inclusion procedures and criteria were as follows. Confirmed cases meeting the diagnostic criteria of severe pneumonia and yidu-toxicity blocking lung syndrome. Disease progresses to meet any of the following conditions named severe pneumonia:
-
1
Significantly increased respiration rate: RR ≥ 30/min;
-
2
Hypoxia in resting state: SpO2 ≤ 93 %;
-
3
Blood gas analysis: PaO2 / FiO2 ≤ 300 mmHg (1mmHg = 0.133 kPa).
Patients with syndromes of unflagging fever or alternating chills and fever, cough (little sputum or yellow sputum), abdominal distension or constipation, shortness of breath, dyspnea, red tongue, yellow greasy or burned furred tongue, rolling, and rapid pulse. Inclusion criteria were as follows:
-
(1)
Patients with normal liver and kidney function, no concomitant metabolic, infectious or inflammatory respiratory disease;
-
(2)
Patients were not treated with steroids and immunosuppressants;
-
(3)
Patients with no extra intestinal malignancy or history of chemotherapy or radiotherapy;
-
(4)
Patients meeting the diagnostic criteria of severe pneumonia and Yidu-toxicity blocking lung syndrome. Exclusion criteria were as follows:
-
(5)
Patients who did not meet the above inclusion criteria;
-
(6)
Patients with severe dysfunction of vital organ (heart, lung, kidney, and liver);
-
(7)
Patients with psychiatric comorbidity or poor compliance.
2.3. Therapeutic regimens
The general strategies were given to both groups according to the National recommendations for diagnosis and treatment of pneumonia caused by SARS-COV-2 (the 5th edition), including bed rest and supportive treatments; ensuring sufficient calories and water intake; maintaining water electrolyte balance and homeostasis, and strengthening psychotherapy for elder children when necessary. The ICW group received the 5th edition recommendation’s CM prescription extraorally for two weeks. All crudes of the decoction drugs were purchased from Guangdong E-fong Pharmaceutical. Drugs with License number of Yue 20160214 are as follows (Table 1 ).
Table 1.
Recommendation of CM prescription.
| Chinese name | Scientific name | Generic names | Dose(g) | Lot number |
|---|---|---|---|---|
| Kuxingren | Bitter Apricot Seed | ArmeniacaeSemenAmarum | 10 | 9052581 |
| Shengshigao | Calcium sulfate dihydrate | Gypsum Fibrosum | 30 | 8100671 |
| Gualou | Trichosanthes kirilowii Maxim | Fructus Trichosanthis | 30 | 9085801 |
| Shengdahuang | Radix et Rhizoma Rhei | Rhubarb | 6 | 9045611 |
| Shengmahuang | Ephedra saxatilis Royle ex Florio | Herba Ephedra | 6 | 9055641 |
| Zhimahuang | 6 | 9015931 | ||
| Ting li zi | Lepidium apetalum Willd | Pepperweed Seed | 10 | 8036141 |
| Taoren | Semen Persicae | Peach seed | 10 | 9082401 |
| Caoguo | Amomum tsaoko | Tsaoko Amomum Fruit | 6 | 9106161 |
| Binglang | Arecae Semen | Betelnutpalm Seed | 10 | 9060541 |
| Cangzhu | Atractylodis Rhizoma | Rhizoma Atractylodis | 10 | 9121041 |
2.4. Measurements
To characterize the effect of herbal medicine, immune function, and inflammatory agents, levels of white blood cells were detected for all patients according to the manufacturer’s instructions at the beginning and at the end of the two weeks. Flow cytometry was used to determine the peripheral blood lymphocyte subsets (the levels of CD3+, CD4+, CD8+, and the ratios of CD4+/CD8+). White blood cell counts (WBC#), neutrophils count (N#), and lymphocyte counts (L#) were measured using a fully automatic blood rheological instrument. An adopted radioimmunoassay method was used to detect the content of IL-6,IL-8,IL-2R,TNF-α (Siemens Healthcare Diagnostics Products Limited), PCT (bioMerieux,sa, France) and high-sensitivity C-reactive protein (hs-CRP) in sera. Peripheral blood samples of patients with were taken, and flow cytometry (FACSaliur, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used to determine peripheral blood lymphocyte subsets which included CD3+ (total T cells), CD4+ (helper T cells), CD8+ (cytotoxic T cells), the ratios of CD4+/CD8+, and Fluorescent labeled monoclonalantibodies (BD Pharmingen, San Diego, CA, USA) of CD3+,CD4+,CD8 + . The median time for being transferred to a designated hospital to the blood sample collection was 4 days.
2.5. Statistical analysis
The GraphPad Prism 8.01 software (San Diego, CA, USA) was used for statistical analysis. The t-test or Rank Sum Test was used to compare the difference between two individual groups, and One-Way Analysis of Variance (ANOVA) was used to compare the difference among multiple groups. All the data normality had been checked before applying the parametric test. Data with normal distribution are presented as the Mean ± SD while IQR (interquartile range) is used for non-normal distribution. A P value less than 0.05 was considered statistically significant. Since all cases were cured, a cure rate curve is drawn according to the cure time of each patient.
3. Results
3.1. Participants' characteristics
The baseline demographic characteristics including gender, age, exposure to epidemic area, signs and symptoms, onset of symptom to hospital admission and dyspnea (median (IQR), d) and Respiratory rate (median (IQR)) of both groups are shown in Table 2 . Of these patients, 10(27.0 %) cases were exposed to Wuhan area, and 27(73 %) cases were infected from other places. Fever (23 [62.16 %]) and dry cough (19 [51.35 %]) were the most common signs and symptoms. There were no statistical differences regarding these variables between the two groups (P > 0.05).
Table 2.
Characteristics for Patients with severe pneumonia of SARS-COV-2.
| Group (Mean ± Standard deviation) |
||||
|---|---|---|---|---|
| Baseline Characteristics | Total (N = 39) |
PWM (N = 24) |
ICW (N = 15) |
P Valuea |
| Gender | ||||
| Male n | 22 | 14 | 8 | 1.000 |
| Female n | 17 | 10 | 7 | |
| Age (years) | ||||
| ≤50 n | 27 | 16 | 11 | 0.734 |
| >50 n | 12 | 8 | 4 | |
| Exposure to epidemic area | ||||
| Wuhan | 10 | 9 | 1 | 0.065 |
| Others | 27 | 15 | 12 | |
| Signs and symptoms | 0.619 | |||
| Fever | 37 | 23 | 14 | 1.000 |
| Dry cough | 31 | 19 | 12 | 1.000 |
| Others | 20 | 11 | 9 | |
| Onset of symptom to, median (IQR), d | ||||
| Hospital admission | 6(3.0−7.0) | 5.5(3.0−7.0) | 6.5(4.0−7.5) | 0.732 |
| Dyspnea | 1 | 0 | 1.000 | |
| Respiratory rate, median (IQR) | 20(18−25) | 20(19−25) | 20(18−24) | 0.514 |
3.2. Yidu-toxicity blocking lung decoction could relieving inflammation of SARS-COV-2 patients with yidu-toxicity blocking lung syndrome
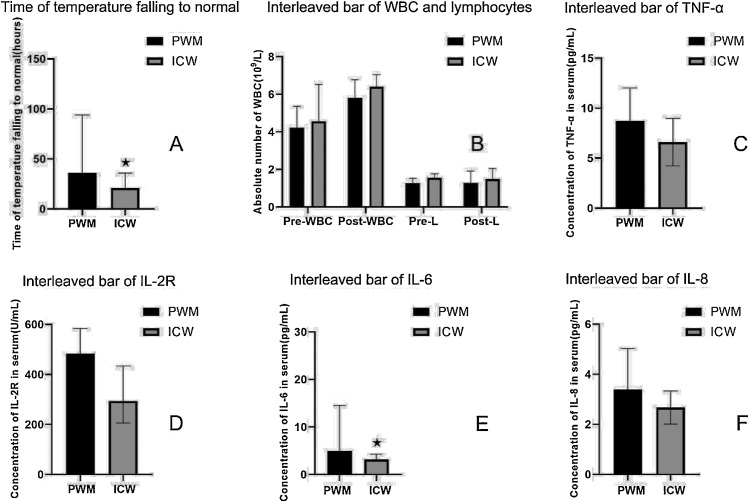
IL6, IL8, IL-2R and TNF -α were selected as inflammatory factors, and t-test or Rank Sum Test was used to compare the difference between two groups. Analysis showed that the IL-6 (P = 0.013) and TNF-α(P = 0.035) levels in the pure western medicine therapy group(PWM) were significantly higher than those in the integrated Chinese and western medicine therapy group (ICW). Infection related indicators such as white blood cell counts (including leukocytes on admission and before discharge), Lymphocyte counts (on admission and discharge), and CRP were compared between the two groups. Procalcitonin (PCT) was excluded because only one of the patients had a higher than normal procalcitonin. The analysis showed that there were no statistical differences in these indicators between the two groups of patients. The results for all the above indicators were listed in Table 3 and Table 4 and Fig. 1 .
Table 3.
Characteristics for patients underwent different therapies (t-test).
| Group (Mean ± Standard deviation) |
t | P | ||
|---|---|---|---|---|
| Pure western medicine therapy group (PWM) | Integrated Chinese and western medicine therapy group (ICW) | |||
| δ-L | 0.22 ± 0.39 | 0.22 ± 0.34 | 0.147 | 0.884 |
| TNF-α | 8.75 ± 3.28 | 6.61 ± 2.37 | 2.187 | 0.035 |
| CD4 | 604.67 ± 227.80 | 546.95 ± 280.54 | 0.521 | 0.607 |
Hour (Days): Length of hospital stay; δ-L(109/L): Difference between the absolute number of lymphocytes before discharge and at admission; TNF-α(pg/mL): Tumor necrosis factor α. CD4:CD4 cell count.
Table 4.
Characteristics for patients underwent different therapies (Rank sum test).
| Group (median, IQR (25,75)) |
Z | P | ||
|---|---|---|---|---|
| Pure western medicine therapy group (PWM) | Integrated Chinese and western medicine therapy group (ICW) | |||
| Hours | 32.00(16.00,73.00) | 21.00(4.00,36.00) | −1.970 | 0.049 |
| Pre-WBC | 4.22(3.90,5.36) | 4.57(3.63,6.52) | −0.346 | 0.729 |
| Post-WBC | 5.83(4.30,6.78) | 6.40(4.69,7.05) | −0.303 | 0.762 |
| δ-WBC | 1.17(0.30,1.65) | 1.56(0.81,1.76) | −0.115 | 0.908 |
| Per-L | 1.27(0.97,1.53) | 1.29(1.11,1.91) | 0.987 | 0.284 |
| Post-L | 1.29(1.11,1.91) | 1.49(1.24,2.05) | 0.810 | 0.528 |
| IL-6 | 4.96(3.5,14.55) | 3.27(1.00,4.43) | −2.486 | 0.013 |
| IL-8 | 2.50(2.50,4.42) | 2.50(2.50,2.50) | 0.633 | 0.818 |
| IL-2R | 494.50(281.25,610.75) | 299.00(216.00,435.00)[[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]] | 0.069 | 0.071 |
| CD8 | 394.00(206.00,428.50) | 271.00(175.00,356.00) | −1.510 | 0.323 |
| CRP | 4.70(0.325,20.40) | 2.50(-1.60,29.80) | −0.260 | 0.795 |
Hour(Days):Time of temperature falling to normal; Per-WBC(109/L): The absolute number of white blood cell before discharge; Post-WBC(109/L): The absolute number of white blood cell at admission; δ-WBC(109/L): Difference between the absolute number of white blood cell before discharge and at admission; Per L(109/L): The absolute number of lymphocytes before discharge; Post L(109/L): The absolute number of lymphocytes at admission; IL-6(pg/mL): Interleukin 6;IL-8: Interleukin 8(pg/mL);IL-2R(U/mL): Interleukin 2R.
Fig. 1.
Interleaved bar of indicators for patients in two groups.
A: PWM: Western medicine therapy group; ICW: Integrated Chinese and western medicine therapy group;B: Pre-WBC: Per-WBC(109/L): The absolute number of white blood cell before discharge; Post-WBC(109/L): The absolute number of white blood cell at admission; Per-L(109/L): The absolute number of lymphocytes before discharge; Post-L(109/L): The absolute number of lymphocytes at admission; C: TNF-α:Tumor necrosis factor α;D: IL-2R(U/mL): Interleukin 2R; E: IL-6(pg/mL): Interleukin 6;F: IL-8(pg/mL): Interleukin 8. In part A, B, D, E and F, the values of the data were shown in quartiles (25, 75), while in part C, the values of data were shown with mean ± standard deviation.★P < 0.05.
3.3. Changes of leukocyte differential count alterations and immune function in severe pneumonia of SARS-COV-2 patients with yidu-toxicity blocking lung syndrome
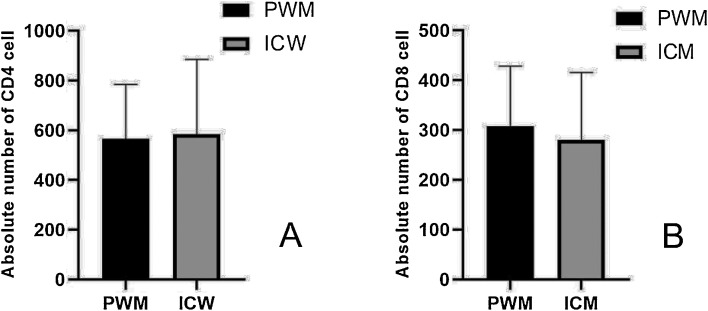
CD4 and CD8 in two different groups were also collected. The analysis showed that there was no statistical difference in the values of CD4 and CD8 between the two groups. The results were listed in Table 3, Table 4. We performed Spearman analysis for CD4 and IL6, while the results suggest that there was no significant relationship between the two indicators (P = 0.772). Because the value of IL6 was not normal distribution, we try to calculate the Lg (IL-6) and Lg (IL-6 + 1) to convert IL-6 value to normal distribution, and then did Pearson analysis between the converted value and CD4, however the results still do not indicate that there was a significant correlation (P = 0.864,P = 0.935) (Fig. 2 ).
Fig. 2.
Interleaved bar of CD4 and CD8 for patients in two groups.
In part A, the values of the data were shown with mean ± standard deviation, while in part B, the values of data were shown in quartiles (25, 75).
3.4. Cure rate and hospital stay shows no difference for the two group
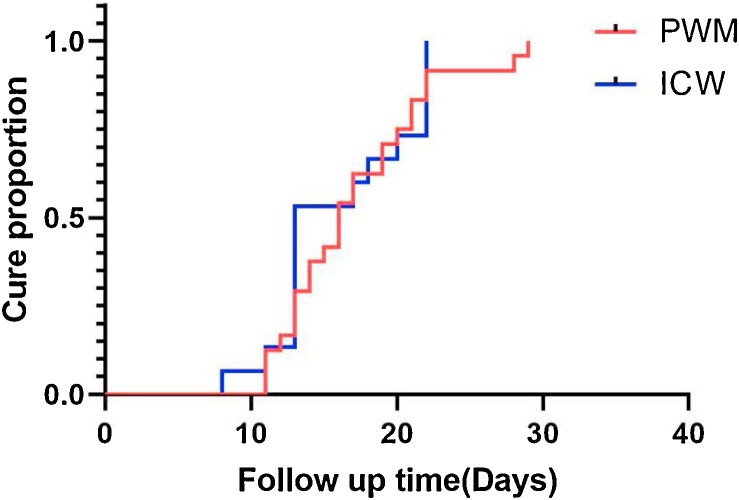
Length of hospital stays for two group were collected and the mean hospital stay for PWM group was 17.08 days(17.058 ± 4.98),while median survival time of the ICW group was 13.00days(13.00(13.00,22.00)). No significant difference was shown between them (P = 0.662). Cure curve according to the cure time of patients in each group as showed in Fig. 3 . Log-rank test was used to analyze the two cure rate of the two group and there seemed to be no significant difference between them (P = 0.878).By the end of Day 29, all patients were discharged and the final cure rate for both group were 100 %.
Fig. 3.
Cure curve of PWM and ICW groups.
4. Discussion
No specific vaccines has been recommended for preventive control of this disease [7]. Currently, the four principles “early identification”, “early isolation”, “early diagnosis”, and “early treatment” were adopted [8]. Antibacterial agents are ineffective. In addition, no clinical trial have assessed the efficacy and safety of antiviral agents used clinically such as lopinavir, arbidol, resochin and prezista [9]. Methylprednisolone is an option for improving shortness of breath and the dose varied depending on disease severity [1,10]. However, no effective outcomes were observed. For thousands of years, CM has gained plenty of experience in infectious diseases prevention and control. CM therapies were recommended throughout the whole process of SARS-COV-2 treatments. In our study, 40 patients of yidu-toxicity blocking lung syndrome, diagnosed as severe pneumonia of SARS-COV-2 following the latest National recommendations for diagnosis and treatment of pneumonia caused by SARS-COV-2 were recruited. The inflammatory agents’ alterations, immune function and leukocyte differential count evaluation after given the 5th edition recommendation’s CM prescription were compared. And we found that the IL-6 (P = 0.013) and TNF-α(P = 0.035) levels in the pure western medicine therapy group(PWM) were significantly higher than those in the integrated Chinese and western medicine therapy group(ICW).Infection related indicators such as levels of white blood cell and Lymphocyte, CRP, PCT of the patients treated with integrated Chinese and western medicine therapy showed no difference. Thus, we may cautiously conclude that the 5th edition recommendation’s CM prescription could relieving inflammation of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome.
Modern pharmacological studies have demonstrated that each single drug in Yidu-toxicity blocking lung decoction could be beneficial in respiratory system and infectious disease. For example, Mahuang and Kuxingren had been taken in this CM prescription. In traditional Chinese medicine (TCM), Herba Ephedrae (Mahuang in Chinese, the dried herbaceous stems of Ephedra sinica Stapf.) and Semen Armeniacae Amarum (Xingren in Chinese, the dried, ripe seeds of Prunus armeniaca L. var. ansu Maxim.) constitute an herb pair known as Mahuang-Xingren according to the principles of mutual reinforcement and mutual assistance, which is used wildly to treat asthma and bronchitis. As a classical CM combination, Mahuang-Xingren is a core component of many herbal prescriptions and Chinese patent drugs for asthma and cough, due to the synergistic efficacy of its components and its few associated side effects [11,12]. Shengshigao (Gypsum Fibrosum), another popular partner of Mahuang in respiratory disease, is frequently working for the purpose of removing heat from the lung and controlling asthma [13]. The CM Channel tropism theory, which identifies the area the drug acts on, and is the selective action of the drug on the different areas of the human body. Compatible use of Shengshigao is part of the theory of Chinese Materia Medica [14]. Gualou (Trichosanthis Fructus) is considered an essential medicine to treat thoracic obstruction. Thoracic obstruction is a professional description of CM syndrome including oppression in chest, ache in chest, back, shoulder and arms, as well as shortness of breath. It can clear away lung-heat, resolve phlegm, soothe chest oppression, ease the chest and disperse lumps, lubricate the intestine and relax the bowels (Committee for the pharmacopoeia of China, 2015). Modern pharmacological studies proved that Gualou exhibit an anti-inflammatory effect [15,16]. Semen Persicae’s beneficial effects on various pathological situations has been indicated and most commonly blood stasis, which can be expressed as anti-coagulate, anti-inflammatory and antioxidant activity [[17], [18], [19]]. Atractylodis Rhizoma possesses an anti-allergic and anti-inflammatory potential through suppressing various immune effector cells, thus can ameliorate symptoms of allergic diseases may potentially prevent the development of subsequent atopic disorder such as allergic asthma through the influence of the gut microbiota [20,21].
Inflammation is a natural defense mechanism of the body that involves migration of leucocytes to the damaged tissues to destroy the inflammatory trigger or challenge [22]. Infectious and allergic triggers induce an initial phase, and uncontrolled inflammatory response to the virus in combination with viral virulence can lead to severe lung devastation [[23], [24], [25]]. Acute inflammation is characterized by infiltration of neutrophilic cells followed by various cytokines [26]. Therefore, it has been broadly suggested that disease severity depends on the number of cytokines released during a cytokine storm in response to infection. In symptomatic individuals, the major cytokine which undergoes the earliest increase is IL-6 [22,[26], [27], [28]]. IL-6 is multifunctional cytokine in lungs, is produced from epithelial cells, interstitial fibroblasts, macrophages and other inflammatory cells in response to a variety of stimuli that include allergens, respiratory viruses, exercise, environmental particles, and inhaled toxic particles [23,29,30]. IL-6 is an important modulator for the transition from acute phase to chronic phase of inflammation, participates actively in inflammatory and immunomodulatory mechanisms, plays both pro-inflammatory and anti-inflammatory roles in humans [31]. Herein, it regulates the CD4 + T cell-mediated responses that include cytokine production (IL-4, IL-13, IL-17 and IL-21), sIL-6R production, and suppressive activity on Treg cells [24,32]. These mediators or responses, in turn, contribute to damage lung through their effects on mucus production, matrix deposition, and proteases release from granulocytes among others [33,34]. In this study, peripheral blood was prepared for radioimmunoassay method to detect the content of IL-6, IL-8, TNF-α, PCT and hs-CRP. Compared with PMW, patients in ICW group had lower levels of IL-6 and TNF-α after treatment. It is known to all that IL-6 is the one that not only elicits acute phase reactions but also leads to the development of specific cellular and humoral immune responses via end-stage B-cell differentiation, immunoglobulin secretion and T-cell activation [35,36]. The decreased IL-6 may play a role in alleviating inflammatory response and protecting against development of severe illness.
IL-6 is a maturing agent for B lymphocytes which stimulates the synthesis and secretion of various immunoglobulins, and induces the proliferation of thymic and peripheral T-cells [37,38]. These killer cells participate in causative mechanisms that are responsible for specific diseases associated with the inflammatory or immune system alternations. But in this article, we performed Spearman analysis for the counts of peripheral CD4 and IL6, the result suggested that there was no significant relationship between the two indicators (P = 0.772). As the value of IL6 was not normal distribution, we try to calculate the Lg (IL-6) and Lg (IL-6 + 1) to convert IL-6 value to normal distribution, and then did Pearson analysis between the converted value and CD4, however the results still do not indicate that there was a significant correlation(P = 0.864,P = 0.935).The results were different from the pathological findings of COVID-19 patient, which showed that the counts of peripheral CD4 and CD8 T cells were substantially reduced, while their status was hyperactivated, as evidenced by the high proportions of HLA-DR (CD4 3·47 %) and CD38 (CD8 39·4%) double-positive fractions. Moreover, there was an increased concentration of highly proinflammatory CCR4+CCR6+ Th17 in CD4 T cells. Additionally, CD8 T cells were found to harbor high concentrations of cytotoxicgranules [39]. Meanwhile, IL-6 also plays functional roles in a variety of other processes including macrophage differentiation, neural cell differentiation and proliferation [40]. In this study, the counts of white cells, immune function, and inflammatory agents’ alterations in treating severe pneumonia with CM were observed. The analysis showed that there were no statistical differences in these indicators between the two groups. A possible explanation is that the amount of samples and lack of repeats in this study.
In conclusion, our study suggests that the 5th edition recommendation’s CM prescription, can be safely used in the treatment of severe pneumonia of SARS-COV-2 with yidu-toxicity blocking lung syndrome. CM can serve as a complementary medication to western medicine, though not reversible at the late stage in most cases, organ failure, which may have great significance in the prognosis of the disease, can be ameliorated and the inflammatory medium could be eliminated with early and timely diagnosis and treatment, which should be highlighted in clinical settings.
Author contributions
Jie Zhao and Xiaodong Yang participated in its design and coordination and evaluated the statistical analysis and critically revised the manuscript and guaranteed of integrity of the entire study. Chenghua Wang, Shuai Song and Kun Cao carried out the experiment. Taohua Wei, Qiaoxue Ji and Wanqun Zheng performed statistical analysis and edit the manuscript. Jiali Li, Xue Zhou and Jie Liu were involved in designing the study and drafting the manuscript. All authors read and approved the final manuscript.
Funding
This study was sponsored by the National Natural Science Fund (81903994).
Declaration of Competing Interest
All authors declare no competing interests.
References
- 1.Yang W. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kui L. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl.) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S. COVID-19 control in China during mass population movements at New Year. Lancet. 2020 doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohrabi C. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020 doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Carinci F. Covid-19: preparedness, decentralisation, and the hunt for patient zero. BMJ. 2020;368 doi: 10.1136/bmj.m799. p. bmj m799. [DOI] [PubMed] [Google Scholar]
- 9.Xiang Y.T. Timely research papers about COVID-19 in China. Lancet. 2020;395(10225):684–685. doi: 10.1016/S0140-6736(20)30375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo H. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020 doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song S. Stereoselective metabolism of amygdalin-based study of detoxification of Semen Armeniacae Amarum in the Herba Ephedrae-Semen Armeniacae Amarum herb pair. J. Ethnopharmacol. 2016;179:356–366. doi: 10.1016/j.jep.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Song S. Concurrent quantification and comparative pharmacokinetic analysis of bioactive compounds in the Herba Ephedrae-Semen Armeniacae Amarum herb pair. J. Pharm. Biomed. Anal. 2015;109:67–73. doi: 10.1016/j.jpba.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Xu W. (1)H NMR-based metabonomics study on the toxicity alleviation effect of other traditional Chinese medicines in Niuhuang Jiedu tablet to realgar (As2S2) J. Ethnopharmacol. 2013;148(1):88–98. doi: 10.1016/j.jep.2013.03.073. [DOI] [PubMed] [Google Scholar]
- 14.Gao Z., Li F.S., Upur H. A study of the law of herbal administration in treating lung-distension by TCM physicians through history using cluster analysis. J. Tradit. Chin. Med. 2011;31(4):303–307. doi: 10.1016/s0254-6272(12)60008-9. [DOI] [PubMed] [Google Scholar]
- 15.Yu X. Trichosanthis Fructus: botany, traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018;224:177–194. doi: 10.1016/j.jep.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Li H. The ethanol extract of fructus trichosanthis promotes fetal hemoglobin production via p38 MAPK activation and ERK inactivation in K562 cells. Evid. Complement. Alternat. Med. 2011;2011 doi: 10.1093/ecam/neq022. p. 657056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji S. Antioxidant effect of aqueous extract of four plants with therapeutic potential on gynecological diseases; Semen persicae, Leonurus cardiaca, Hedyotis diffusa, and Curcuma zedoaria. Eur. J. Med. Res. 2017;22(1) doi: 10.1186/s40001-017-0293-6. p. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.W. Effect of an herbal mixture of Cinnamon Cortex, Persicae Semen, and Natril Sulfas on collagen-induced arthritis and lipopolysaccharides-induced nuclear factor-kappa B signaling. Chin. J. Integr. Med. 2016 doi: 10.1007/s11655-016-2517-y. [DOI] [PubMed] [Google Scholar]
- 19.Xi S. Toxicity and clinical reasonable application of Taoren (Semen Persicae) based on ancient and modern literature research. J. Tradit. Chin. Med. 2013;33(2):272–279. doi: 10.1016/s0254-6272(13)60139-9. [DOI] [PubMed] [Google Scholar]
- 20.Xu W. Molecular mechanisms associated with macrophage activation by Rhizoma Atractylodis Macrocephalae polysaccharides. Int. J. Biol. Macromol. 2020;147:616–628. doi: 10.1016/j.ijbiomac.2020.01.081. [DOI] [PubMed] [Google Scholar]
- 21.Tsang M.S. Anti-inflammatory activities of Pentaherbs formula and its influence on gut microbiota in allergic asthma. Molecules. 2018;23(11) doi: 10.3390/molecules23112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur S. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020;28(5) doi: 10.1016/j.bmc.2020.115327. p. 115327. [DOI] [PubMed] [Google Scholar]
- 23.Godel P., Shimabukuro-Vornhagen A., von Bergwelt-Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2017;44(3):371–373. doi: 10.1007/s00134-017-4943-5. [DOI] [PubMed] [Google Scholar]
- 24.Betakova T. Cytokines induced during influenza virus infection. Curr. Pharm. Des. 2017;23(18):2616–2622. doi: 10.2174/1381612823666170316123736. [DOI] [PubMed] [Google Scholar]
- 25.Frey N. Cytokine release syndrome: who is at risk and how to treat. Best Pract. Res. Clin. Haematol. 2017;30(4):336–340. doi: 10.1016/j.beha.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Liu D., Zhao J. Cytokine release syndrome: grading, modeling, and new therapy. J. Hematol. Oncol. 2018;11(1) doi: 10.1186/s13045-018-0653-x. p. 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy H. Cytokine release syndrome: current perspectives. Immunotargets Ther. 2019;8:43–52. doi: 10.2147/ITT.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimabukuro-Vornhagen A. Cytokine release syndrome. J. Immunother. Cancer. 2018;6(1) doi: 10.1186/s40425-018-0343-9. p. 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooney C., Sauer T. Modeling cytokine release syndrome. Nat. Med. 2018;24(6):705–706. doi: 10.1038/s41591-018-0068-9. [DOI] [PubMed] [Google Scholar]
- 30.Obstfeld A.E. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017;130(23):2569–2572. doi: 10.1182/blood-2017-08-802413. [DOI] [PubMed] [Google Scholar]
- 31.Lee D.W. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fathi M.A.A. 1,3,4-Oxadiazole/chalcone hybrids: design, synthesis, and inhibition of leukemia cell growth and EGFR, Src, IL-6 and STAT3 activities. Bioorg. Chem. 2018;84:150–163. doi: 10.1016/j.bioorg.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang H.J. Inhibitory effects of compounds and extracts from ampelopsis brevipedunculata on IL-6-induced STAT3 activation. Biomed Res. Int. 2020;2018 doi: 10.1155/2018/3684845. p. 3684845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang S. Inhibitory effects of suppressor of cytokine signaling 3 on inflammatory cytokine expression and migration and proliferation of IL-6/IFN-gamma-induced vascular smooth muscle cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2018;33(5):615–622. doi: 10.1007/s11596-013-1168-x. [DOI] [PubMed] [Google Scholar]
- 35.Hansen M.B. Interleukin-6 signaling requires only few IL-6 molecules: Relation to physiological concentrations of extracellular IL-6. Immun. Inflamm. Dis. 2013 doi: 10.1002/iid3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiesel B.F. Toxicity, pharmacokinetics and metabolism of a novel inhibitor of IL-6-induced STAT3 activation. Cancer Chemother. Pharmacol. 2016;78(6):1225–1235. doi: 10.1007/s00280-016-3181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. p. a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norelli M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giladi N.D. RTVP-1 promotes mesenchymal transformation of glioma via a STAT-3/IL-6-dependent positive feedback loop. Oncotarget. 2015;6(26):22680–22697. doi: 10.18632/oncotarget.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]