Abstract
Influenza continues to be a significant public health challenge. Two glycoproteins on the surface of influenza virus, hemagglutinin and neuraminidase, play a prominent role in the process of influenza virus infection and release. Monoclonal antibodies targeting glycoproteins can effectively prevent the spread of the virus. In this review, we summarized currently reported human monoclonal antibodies targeting glycoproteins of influenza A and B viruses.
Keywords: Monoclonal antibodies, Influenza A virus, Influenza B virus, Glycoproteins, Hemagglutinin, Neuraminidase
Currently, the main influenza viruses are classified into four types: A, B, C and D. Influenza viruses are easily transmitted by droplets, but only influenza A and B viruses (IAV and IBV) are currently capable of causing a pandemic [1]. Over the past century, IAVs have caused several pandemics, including H1N1 in 1918, in which about 500 million people worldwide were infected, accounting for one-third of the world’s population at the time, with at least 50 million deaths; H2N2 in 1957, about 1.1 million global deaths; and H3N2 in 1968, resulting in a total of about 1 million deaths, mostly those aged 65 and older [2]. The 2009 H1N1 pandemic caused 151,700-575,400 deaths worldwide [2]. In addition to human influenza viruses, highly pathogenic avian influenza also poses a potential threat to human health. In 1997, human infection with H5N1 was first reported in Hong Kong, and in 2003, another one was also reported in Hong Kong. So far, 861 confirmed H5N1 infections worldwide were reported to World Health Organization (WHO), including 455 deaths, with a fatality rate of 53% [[3], [4], [5]]. In 2013, H7N9 was detected in China, with 1568 confirmed infections globally as of February 2020, with a fatality rate of nearly 40% [4], [6].
In addition to the above influenza pandemics, the spread of seasonal influenza can also pose a major threat to human health. According to the WHO [1], worldwide, about 3 million to 5 million cases of severe illness and 290,000 to 650,000 respiratory deaths are associated with seasonal influenza each year [1]. People with seasonal influenza can suffer from fever, cough, runny nose and other symptoms, and serious outcomes of influenza infection can result in death [1]. Therefore, control and prevention of the disease are vitally important, and vaccination is currently the most effective way to combat influenza virus [1].
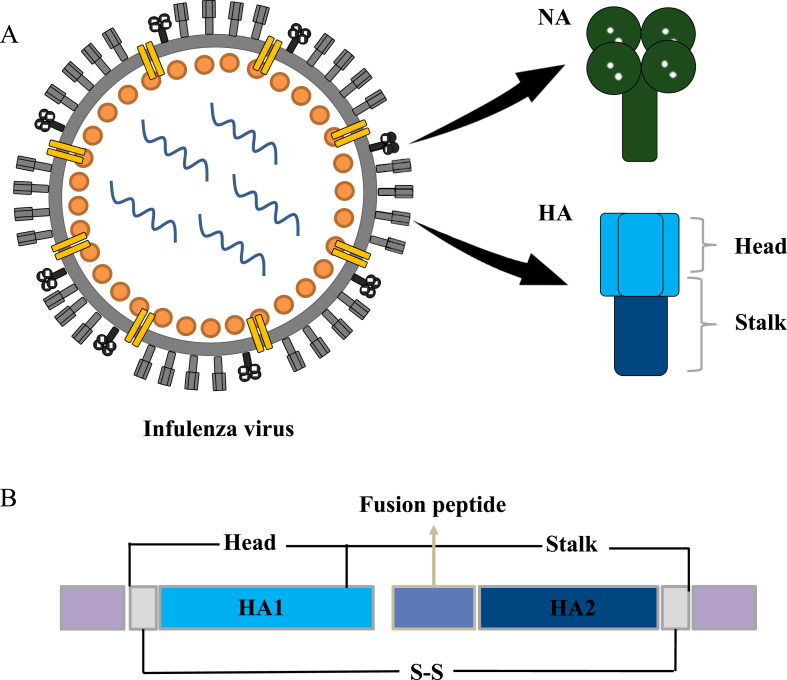
Hemagglutinin (HA) of the trimer and neuraminidase (NA) of the tetramer are two glycoproteins on the IAV and IBV surface. They play an important role in the process of virus infection and escape, each with high variability owing to adaption to environmental changes and host immune escape response (Fig. 1 A) [7,8]. According to genetic and antigenic variation, the current 18 subtypes of IAV HA (H1–H18) are divided into group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18) and group 2 (H3, H4, H7, H10, H14, and H15), while IAV of NA has 11 subtypes (N1–N11). IBV consists mainly of two lineages, Victoria and Yamagata [[9], [10], [11]]. HA consists of three single HA0 protein precursors, which form stable HA1 and HA2 linked by disulfide bonds during maturation [12,13]. HA-Head is composed of HA1 residues, and HA-Stalk is composed of partial residues of HA1 and complete HA2 (Fig. 1B). HA-Head binds to sialic acid of the host cell receptor when the influenza virus makes contact with the host surface. The cell membrane then envelops the virus to form vesicles. At low pH, HA-Stalk-mediated virus fuses with host endosomal membrane and releases genetic material into the host to be replicated [7,14,13,12,15]. The relatively less abundant NA, the main function of which is to hydrolyze glycoside bonds between sialic acid and host glycoproteins, helps mature influenza viruses escape from host cells (Fig. 2 ) [7,16,10].
Fig. 1.
The structure of glycoproteins.A. The structure of the virus, HA and NA. B. Comparison of Head and Stalk with HA1 and HA2.
Fig. 2.
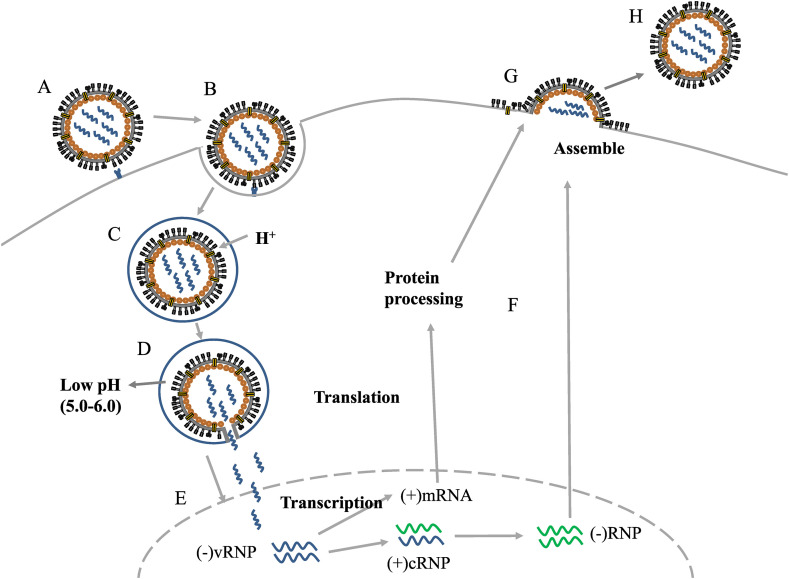
An overview of the process of virus infection, replication and release. A, B The virus binds to host sialic acid by HA and enters the cell by endocytosis; NA enhances the HA-mediated membrane fusion process, facilitating virus entry. C, D The low pH of the endosome causes the viral envelope to fuse with the endosomal membrane, and the viral genetic material, vRNPs, is released into the cytoplasm. E vRNPs enter the nucleus and initiate the transcription process, replicating various viral proteins and RNAs. F, G The synthesized viral components and vRNPs are processed and transported by Golgi bodies and endoplasmic reticulum to complete the assembly of virions on the host cell membrane. H The progeny virus is released.
As a biotargeted drug, monoclonal antibodies have been widely studied in the field of anti-infection in recent years, including anti-HIV antibodies 3BNC117, 10-1074 and VRC01, which are currently in clinical trials [17,18]; anti-Coronavirus antibodies [19,20]; anti-Ebola antibodies [21,22] and anti-Hendra and Nipah virus antibodies [23]. Getting vaccinated each year is currently the best way to combat the influenza virus. Antibodies produced by vaccination neutralize the corresponding virus, thereby effectively preventing the occurrence of the corresponding subtype influenza pandemic. Nevertheless, this solution can be thwarted by the long production cycle of the vaccine and the high degree of mutation of the virus, reducing long-term protection [24,25]. As candidate vaccines, mAbs have the advantages of high sensitivity, strong specificity, as well as short research and development cycle. They have also played an important role in scientific research, medical assistance, and industrial production, thus establishing their immeasurable economic value [26]. HA is the main target of mAbs produced by host immunity or natural infection, and the development of anti-HA mAbs has generated considerable scientific interest. Compared with HA, few studies have reported on anti-NA mAbs, but the relative stability of its structure has also attracted attention.
Mouse-derived mAbs are simple to prepare and relatively inexpensive, but they can induce immunity in the body, and they are difficult to apply clinically. On the contrary, human mAbs can substantially reduce immune side effects caused by heterogeneous antibodies to the human body, and this is a future research direction of influenza antibody drugs [27]. Therefore, we herein review human mAbs targeting HA and NA, focusing on their broad antiviral spectrum and mechanisms of action (see in the Table 1, Table 2, Table 3).
Table 1.
MAbs targeting HA-Head.
| Name | Binding To | VH Genes | HCDR of Interaction with Antigen | In Vivo | Clinical Trials |
|---|---|---|---|---|---|
| CH65 | H1 | 1–2 | 3 | – | N |
| 5J8 | H1 | 4-b | 3 | – | N |
| F045-092 | H1,H2,H3,H5 | 1–69 | – | – | N |
| F026-427 | H1,H2,H3,H5 | 1–69 | – | – | N |
| 8F8 | H2 | 3–33 | 3 | – | N |
| 8M2 | H2 | 1–69 | 2,3 | – | N |
| 2G1 | H2 | 1–69 | 2,3 | – | N |
| CR8033 | Influenza B | 3–9 | 1,2,3 | mice | N |
| 3A2 | Yamagata | – | – | – | N |
| 10C4 | Influenza B | – | – | – | N |
| F005-126 | H3 | 1–18 | 3 | – | N |
| D1-8 | H3 | – | – | mice | N |
| HNIgGA6 | H7 | 4–31 | – | mice | N |
| HNIgGB5 | H7 | 4–31 | – | mice | N |
| FLD194 | H5 | – | 1,2,3 | mice | N |
| mammalian-produced KPF1 | H1 | – | – | mice | N |
| tobacco-produced KPF1 | H1 | – | – | Guinea pigs | N |
| M826 | H7 | 1–69 | 1,2,3 | – | N |
| FluA-20 | All HA | 4–61 | 1,3 | mice | N |
| H7.5 | H7 | – | 2 | – | N |
| CR8071 | Influenza B | 1–18 | 1,2,3 | mice | N |
| 5A7 | Yamagata | – | – | mice | N |
| 46B8 | Influenza B | – | – | mice | Y |
| C12G6 | Influenza B | – | – | mice, ferrets | N |
Table 2.
MAbs targeting HA-Stalk.
| Name | Binding To | VH Genes | HCDR of Interaction with Antigen | In Vivo | Clinical Trials |
|---|---|---|---|---|---|
| CR6261 | H1, H3, H5, H6, H8, H9 | 1–69 | 2 | mice | Y |
| CR8020 | H1, H3, H4, H5, H7, H9, H10 | 1–18 | 1,3 | mice | Y |
| CR8043 | H3, H10 | 1–3 | 1,3 | mice | N |
| F10 | ALL Group 1 | 1–69 | 1,2,3 | mice | N |
| CR9114 | H1, H3, H7, H9, flu B | 1–69 | 1,2,3 | mice | N |
| 27F3 | H1,H3,H5,H6,H7,H10 | 1–69 | 2,3 | – | N |
| FI6 | H1-16 | 3–30 | 1,3 | mice, ferrets | N |
| 05-2G02 | H1, H3, H5 | 1–18 | – | – | N |
| 09-2A06 | H1 | 1–69 | – | – | N |
| 09-3A01 | H1 | 4–39 | – | – | N |
| 39.29 | H1, H2, H3 | 3–30 | 2,3 | mice, ferrets | Y |
| 81.39 | H1, H2, H3 | 3–30 | – | – | N |
| 81.39a | Group 1, Group 2 | 3–30 | – | mice, ferrets | N |
| 3.1 | H1, H2, H5, H6 | 3–30 | 1,3 | mice | N |
| CT149 | H1, H2, H3, H7, H9 | 1–18 | 2,3 | mice | N |
| VIS410 | H1, H2, H5 | – | – | mice | Y |
| MEDI8852 | All HA | 6–1 | 1,2,3 | mice | Y |
Table 3.
MAbs targeting NA.
| Name | Binding To | VH Genes | HCDR of Interaction with Antigen | In Vivo | Clinical Trials |
|---|---|---|---|---|---|
| 1000-3B06 | N1 | – | – | mice | N |
| 1000-1D05 | N1 | – | – | mice | N |
| 294-A-1C02 | N1 | – | – | mice | N |
| 294-A-1D05 | N1 | – | – | mice | N |
| 229-1D05 | N2 | – | – | mice | N |
| 235-1C02 | N2 | – | – | mice | N |
| 235-1E06 | N2 | – | – | mice | N |
| 1086C12 | Influenza B NA | 3-30 | 3 | mice | N |
| 1086F8 | Influenza B NA | 3-30 | 3 | mice | N |
| 1092D4 | Influenza B NA | 3-23 | 3 | mice | N |
| 1122C6 | Influenza B NA | 4-59 | 3 | mice | N |
| 1092E10 | Influenza B NA | 3-15 | 3 | mice | N |
| 1122C7 | Influenza B NA | 3-23 | 3 | – | N |
1. Monoclonal antibodies targeting HA
1.1. Head
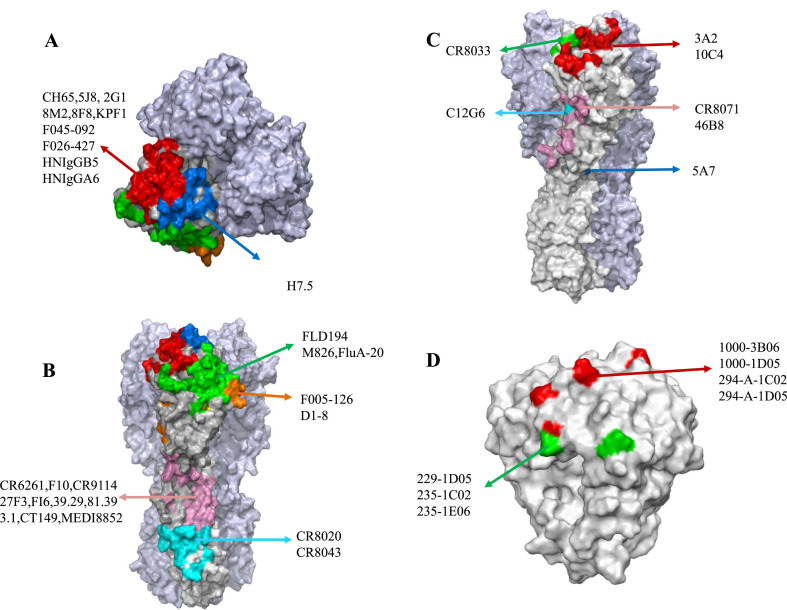
Neutralizing antibodies produced by influenza virus infection or vaccination usually target HA-Head antigens. Five antigenic sites on the H1- and H3-Head have been characterized: Sa, Sb, Ca1, Ca2, and Cb, or as sites A through E [28]. However, HA-Head has a strong ability to escape immunity. In contrast, its shallow pocket-shaped receptor binding sites (RBS) are relatively conserved and contain the 130-loop, the 150-loop, the 190-helix, and the 220-loop [[28], [29], [30],12,15]. MAbs that target the head often cross these two domains (Fig. 3A–3C) (Table 1).
Fig. 3.
Epitope overview of mAbs targeting influenza virus glycoproteins. The gray part represents the glycoproteins, the other color parts represent the mAbs recognition epitopes, similar epitopes are represented by the same color. A. Top view of influenza A HA. B. Front view of influenza A HA. C. Front view of influenza B HA. D. Top view of influenza A NA.
CH65 was obtained by separating and recombining the heavy and light chains from a single plasma cell isolated from a subject receiving a trivalent influenza vaccine [31]. CH65 mimics sialic acid and can widely neutralize and inhibit H1N1 viruses by its 19-residue HCDR3 binding with H1-Head RBS [31]. Another mAb, 5J8, is similar to CH65 in that its HCDR3 binds with H1 RBS from different directions [32]. Two mAbs, F045–092 and F026-427, with extensive cross-reactivity were screened out from the antibody library constructed by phage display technology [33]. These mAbs targeting HA-Head can neutralize various subtypes, including H1, H2, H3 and H5, by inhibiting the binding of HA to sialic acid. Furthermore, the recognition sites of F045-092 are located near antigen sites A and B [33]. Xu et al. isolated three H2N2-specific mAbs, 8F8, 8M2 and 2G1, from human peripheral blood. Tyr100 from HCDR3 of 8F8 and Phe54 from HCDR2 of 8M2 and 2G1 were responsible for binding with RBS, which inhibited the binding of the virus to sialic acid by steric hindrance [34]. The epitope of CR8033 partially overlaps with RBS such that the mAbs can neutralize both lineages, but have HA inhibition activity only against the Yamagata lineage, possibly by neutralizing the Victoria lineage in other ways [35]. In vivo, 0.6 mg/kg and 0.2 mg/kg of the mAbs could protect mice inoculated with both lineages of IBVs from lethal challenges, respectively [35]. Then Yasugi et al. obtained two types of mAbs, 3A2 and 10C4, from the peripheral lymphoid blood of volunteers. Although able to widely neutralize IBVs, these mAbs could only identify a susceptible mutation in the 190-helix region near the RBS of the Yamagata lineage of IBV [36]. F005-126 was isolated from the phage display library and could broadly neutralize the H3N2 virus [37]. The epitope of F005-126 crosses the crack of two HA monomers, and one of the peptides corresponding to the Ca1 site, which is relatively conserved in the H3 subtype with only a few amino acid differences, could neutralize the H3N2 virus by inhibiting low pH-induced, HA-mediated membrane fusion [37]. D1-8 is a unique mAb which comes from human B cells and targets a newly conserved epitope proximal to antigenic site Ca2 in the H3-Head [28]. D1-8 has broad neutralizing activity to inhibit H3 virus [28]. In addition, the mAb was shown to be more effective than oseltamivir in treating infected mice in a dose-dependent manner [28]. Two mAbs, HNIgGA6 and HNIgGB5, were obtained by screening phage antibody libraries of recovered H7N9 patients [38]. Two amino acids, 186 V and 226 L, are keys to the recognition of H7 HA by mAbs at the RBS, and they showed high neutralization activity against H7N9 in a dose-dependent manner. In an HA inhibition assay, the titration of HNIgGA6 and HNIgGB5 was as low as 0.8 μg/ml and 1.6 μg/ml, respectively. HNIgGA6 and HNIgGB5 could effectively treat H7N9 infection in mice [38]. FLD194 could neutralize the tested H5N1 subtypes with 90% inhibitory concentration (IC90) as low as 0.007 μg/ml. A dose of FLD194 before and after infection protected the mice [39]. It binds to multiple antigenic sites by five CDRs and inhibits the virus by blocking HA binding to sialic acid [39]. Nogales et al. isolated KPF1 from peripheral blood cells of vaccinated subjects, and the mAb could widely neutralize the H1 strains in vitro [40]. In vivo, 1 mg/kg or 10 mg/kg KPF1 protected mice from lethal dose challenges of the tested H1 viruses [40]. It recognizes a highly conserved amino acid between antigen sites Ca and Cb [40]. The team then used tobacco to produce KPF1, which had the same activity as mammalian-produced KPF1 in neutralizing the H1N1 strains, and the titration value was as low as 0.271 μg/ml [41]. Twenty mg/kg of the tobacco-produced KPF1 both prevented infection and treated guinea pigs infected with the H1N1 viruses, preventing their spread [41]. This is the first time that a plant-based human mAb had been reported to be active, both in vitro and in vivo, providing a new immunotherapy for the production of new mAbs [41]. C12G6 is a chimeric mAb and has shown its efficacy in neutralizing a variety of IBVs since 1940 with 50% effective concentration (EC50) of 7.68–60.39 ng/ml [42]. With doses greater than 1 mg/kg and 5 mg/kg of C12G6 in mice, respectively, it can prevent and treat IBVs infection, and is equally effective in ferrets models [42]. It targets the conserved sites overlapped with RBS and blocks entry, inhibiting membrane fusion and mediating antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity responses (CDC) [42]. M826, a human germline mAb, has a subnanometer affinity with H7N9 HA at acidic pH [43]. The crystal structure indicates that the binding site of m826 is completely exposed when the conformation of HA trimer is changed by pH induction. This is a unique epitope that exists in H7 HA [43]. Through mediating ADCC, the mAb can protect mice from lethal dose challenge of H7N9 [43]. A newly discovered human mAb, FluA-20, recognizes the head region of almost all IAV subtypes with EC50 of 5–142 ng/ml [44]. Unlike other mAbs, FluA-20 targets a new site buried at HA trimer interface that is adjacent to, but not overlapping with, RBS, and it is highly conserved in different subtypes of flu A viruses. The site was previously considered stable and inaccessible to antibodies [44]. FluA-20 inhibits the influenza virus by destroying the trimeric structure of HA through brief contact with the trimer interface [44]. Ten mg/kg of the mAb protected mice from sublethal and lethal challenges from H1N1, H5N1, H3N2 and H7N9 strains [44]. Turner et al. reported a similar H7-specific mAb, H7.5, the recognition site of which is located at the precursor interface of HA-Head [45]. With only minimal contact, trimeric dissociation of HA can be induced before exposure to low pH, preventing full HA from exposure to sialic acid and, hence, blocking viral fusion [45].
Within the vestigial esterase domain located between RBS and HA-Stalk, the epitope is highly conserved in IBVs [35] (Fig. 3C). CR8071 binds to the remaining esterase domain. The mAb, like CR8033, can protect mice inoculated with both lineages of IBVs from lethal challenges, albeit with less efficacy [35]. 5A7 can widely neutralize multiple IBVs (1985-2006) in both lineages, with the residual esterase domain as the target. At a low dose of 10 mg/kg, it can still provide effective protection in mice after IBV infection for a certain period of time, making it a good vaccine candidate [36]. 46B8 is a mAb specific to IBV. It could neutralize 11 tested IBVs with IC50 as low as 0.58 nM [46]. By targeting the remaining esterase domain, it prevents the conformational change of HA during the fusion of viral membrane and endosomal membrane induced by low pH, thus inhibiting viral infection [46]. Fifteen mg/kg of 46B8 was an effective dose in protecting mice at 24 and 48 h from all lethal challenges of IBVs tested, and it could be combined with Tamiflu to combat the virus [46]. When tested with the mutant strain, it was found that 46B8 could still protect mice, suggesting that it might have the ability to mediate ADCC [46]. Based on the broad-spectrum of this mAb against IBVs, Lim et al. tested 46B8 (MHAB5553A) in a clinical trial, and subjects were followed up for 120 days after injection of a certain dose. No adverse events were reported, and most of the adverse reactions were mild colds and headaches, demonstrating its good tolerance and pharmacokinetic profiles, thus offering the possibility for the development of therapeutic drugs in patients with severe IBVs [47].
1.2. Stalk
The Stalk is highly conserved compared to the Head, and partial Stalk residues are the same in many IAVs [48]. The Stalk goes through a rearrangement during fusion, and mAbs can block this process to prevent membrane fusion [49] (Fig. 3B) (Table 2).
The VH1-69 gene is the only human heavy-chain gene that encodes two hydrophobic residues at the end of the HCDR 2 loop. As such, the VH1-69 class of mAbs can recognize the hydrophobic groove structure on the Stalk through its own heavy chain, providing extensive inhibition [50,51]. Accordingly, the VH1-69 class of mAbs has been the main force in a broad range of antibodies against HA-Stalk [50,52]. The CR6261 mAb was isolated from a library of phage antibodies constructed from memory B cells of volunteers who had been vaccinated against influenza [53,54]. The mAb has neutralizing activity against H1, H3, H5, H6, H8, and H9 subtypes with IC50 value of 0.12 - >50 μg/ml [53]. The mechanism of action for CR6261 is binding of the conserved hydrophobic pocket of HA-Stalk [35,53,54]. Furthermore, 5 mg/kg of the mAb could protect mice from the lethal challenge of H5N1 and H1N1 viruses [53]. In addition, the team screened another broadly neutralizing human mAb, CR8020, against H1, H3, H4, H5, H7, H9 and H10 subtypes with an IC50 as low as 1.1 μg/ml [55]. The crystal structure shows that CR8020 recognizes a highly conserved site of HA-Stalk near the viral membrane [55]. In vivo, 3 mg/kg of CR8020 could effectively prevent and protect mice from infection with lethal doses of H3N2 and H7N7 [55]. Unlike the CR6261 recognition site, the combination of two mAbs neutralizes most influenza A subtypes [55]. CR8043 acts on epitopes similar to those of CR8020 through different residues and angles, having high affinity with H3 and H10 in vitro. A dose of ≥3 mg/kg CR8043 can protect mice from infection by H3N2 and H7N7 [56]. Human mAb F10, which neutralizes all group 1 IAVs, including H5N1, prevents fusion of the viral membrane with the endosomal membrane by binding the conserved hydrophobic groove of HA-Stalk. Ten mg/kg of F10 prevented infection and treated H5N1-infected mice [57]. CR9114 was isolated from combinatorial display libraries of B cells from volunteers who had been vaccinated against seasonal influenza [35]. It blocked HA pH-induced conformational changes associated with membrane fusion by binding a highly conserved site of HA-Stalk [35]. CR9114 has good cross-reactivity with H1, H3, H7, H9 and IBV HA. Studies have shown that ≥1.7 mg/kg of the mAb CR9114 can protect mice from lethal challenge of several different subtypes of influenza viruses [35]. Human mAb 27F3 isolated by phage display, when combined with the hydrophobic groove of HA-Stalk through its HCDR2 and HCDR3, could widely recognize IAV subtypes from group 1 and group 2 with EC50 from 0.008 to 4.8 μg/ml [52].
A broadly neutralizing mAb, FI6, was isolated from plasma cells of human peripheral blood [58]. This mAb recognizes HA of 16 subtypes (H1-16) and neutralizes IAV of group 1 and group 2 with EC50 values of 10–270 ng/ml [58]. X-ray crystallographic analysis shows that the HCDR3 loop of FI6 binds to a conserved site in the F subdomain of HA-Stalk. Four mg/kg and 15 mg/kg of FI6 prevented H1N1 infection and treated infected mice, respectively, while the mAb protected ferrets against highly pathogenic H5N1 [58]. Three mAbs, 05-2G02, 09-2A06, and 09-3A01, were isolated from plasma cells of volunteers inoculated with the 2009 pandemic H1N1 vaccine and were bound to HA-Stalk by different VH genes through competitive ELISA and sequence homology analysis [59]. The three mAbs neutralized all H1N1 strains tested, with 05-2G02 also having broad neutralizing activity against H3N2 and H5N1 [59]. In the same way, Nakamura et al. isolated two mAbs, 39.29 and 81.39, that neutralized the H1, H2 and H3 subtypes of IAVs tested with IC50 of 1.3–45.1 nM and 0.65–26.3 nM [60]. The crystal structure shows that the epitope of 39.29 is located at the top of HA2 helix A [60]. By competitive ELISA, the epitopes of 81.39 overlapped those of 39.29 in H3-Stalk [60]. Compared with oseltamivir, 45 mg/kg of 39.29 was more effective in treating mice and ferrets infected with H1N1 and H3N2 viruses. Furthermore, a synergistic effect was noted when used in combination with oseltamivir [60]. In phase 2 clinical trials, the combination of the mAb and oseltamivir for treatment of hospitalized patients with severe influenza was discontinued after a mid-term evaluation, although 39.29 (MHAA4549A) proved to be well tolerated and significantly reduced flu-related symptoms [61,62]. MAb 81.39a is a derivative of parental 81.39 that effectively neutralized 16 subtypes of the tested IAVs with EC50 of 0.01–4.9 μg/ml [63]. In a mouse model, a single injection of 15 or 45 mg/kg of 81.39a was used to treat mice infected with H5Nx, H6N1, or H7N9 viruses, and it was demonstrated to inhibit virus replication and weight loss [63]. In addition, 25 mg/kg of 81.39a could effectively reduce virus titer in ferrets infected with H1N1 and alleviate pathological changes in the lungs [63]. MAb 3.1 isolated from the phage display library could effectively neutralize influenza viruses of H1, H2, H5 and H6 subtypes [64]. This mAb is encoded by VH3-30 and binds to the hydrophobic groove through HCDR1 and HCDR3 loops [64]. A single injection of 10 mg/kg of 3.1 provided complete protection and prevented weight loss [64]. CT149 was isolated from patients infected with 2009 pandemic H1N1 and is a broadly neutralizing mAb [65]. The crystal structure indicates that the epitope of CT149 is located in the Stalk’s fusion peptide. It neutralized all tested IAVs in groups 1 and 2 (H1, H2, H3, H7, H9) by inhibiting the low pH-induced, HA-mediated membrane fusion process [65]. Furthermore, it could mediate ADCC and CDC to promote apoptosis in infected cells [65]. During in vivo neutralization experiments, the mAb was effective in treating mice infected with several subtypes of IAVs [65]. VIS410 targeting HA-Stalk and can broadly neutralize the H1, H3, and H5 subtypes of IAVs with EC50 of 0.3–11 μg/ml [66]. In vivo, VIS410 protected mice infected with H3N2 and H7N9. Moreover, a synergistic effect in combination with oseltamivir was demonstrated [66]. Then the mAb was tested in clinical trials in order to evaluate whether treatment with VIS410 would result in decreased incidence and reduce serious disease of high-risk groups, and an epidemic microsimulation model was developed [67]. The results showed that VIS410 is generally safe, has potential to reduce the incidence of disease, can greatly reduce hospitalization rates for people over age 65, and can be recommended as a drug for the treatment or prevention of IAVs [67]. Recently, the safety and tolerability of VIS410 were also evaluated in non-hospitalized patients with IAVs infection, demonstrating that the mAb could effectively relieve symptoms and inhibit viral replication [68]. This provides strong support for the development of vaccines to treat and prevent severe influenza infections [68]. MEDI8852 is a broad-spectrum human mAb effective against all HA subtypes of IAVs with an IC50 range of 0.41–4.05 μg/ml [69]. It is generated by optimizing the potency, breadth, and low somatic mutations of FY1 from the donor’s memory B cells through combining the low-frequency mutation site in the CDRs with the unnecessary somatic cell recovery mutation in the framework [69,70]. MEDI8852 binds through coordinated motion of CDRs to the hydrophobic groove of the fusion domain and most of the fusion peptide [69]. It has higher antibody neutralization breadth and potency than other mAbs, and 25 mg/kg of MEDI8852 was superior to oseltamivir in mouse and ferret models [69,71]. However, in subsequent clinical trials, MEDI8852 produced adverse reactions, and studies of oseltamivir in combination with simple influenza were not able to confirm effective clinical significance owing to the small number of subjects. Therefore, it did not enter the Phase 2 trial [72,73].
2. Monoclonal antibodies targeting NA
Neuraminidase inhibition has two main pathways: one is to bind to NA by antibodies to prevent its direct binding to the active site, and the other is to inhibit the activity of NA by steric hindrance of antibodies bound to HA [74,75]. Although NA has a lower ability to drift antigens, it contributes to the production of broadly cross-reactive antibodies [74,76]. MAbs targeting NA have been isolated from naturally infected influenza patients [74]. MAbs 1000-3B06, 1000-1D05, 294-A-1C02, and 294-A-1D05 target N1, and mAbs 229-1D05, 235-1C02 and 235-1E06 target N2, among which several conserved amino acids on the epitope play a crucial role [74] (Fig. 3D) (Table 3). These mAbs have broad cross-reactivity and in vitro neutralization activity. In vivo, they can protect mice against lethal and sublethal virus dose testing [74]. Unlike vaccination, natural influenza viruses easily induce the body to produce anti-NA antibodies because key sites on the NA of the inactivated vaccine are destroyed, causing non-response of mAbs targeting the NA [74]. Nevertheless, Piepenbrink et al. isolated 6 NA-specific mAbs in subjects vaccinated with the seasonal vaccine, of which 1086C12, 1086F8, 1092D4, 1122C6, and 1092E10 inhibited all IBVs tested in a dose-dependent manner. The 1122C7 mAb only recognized one of them, and the inhibitory effect was not significant [77] (Table 3). With the exception of 1122C7, other mAbs were effective in preventing infection and treating IBVs-infected mice [77].
3. Discussion
Human mAbs targeting conserved sites on HA and NA have been the main focus of anti-influenza drug research. RBS and antigen sites of HA-Head are conserved in the same subtype as a result of their special functions. However, mAbs against this epitope are not very broad-spectrum and can only target one subtype. Because of its highly conserved nature, mAbs targeting HA-Stalk can inhibit multiple subtypes of influenza viruses, thus becoming the basis for a universal vaccine. Research on mAbs targeting NA is far less in-depth than that of HA, even though it might have significant therapeutic potential.
IgG influenza mAbs can mediate ADCC, and they are an effective way to treat influenza. At present, a variety of mAbs can perform this function, such as m826 developed by Yu et al. It protects mice from H7 IAV by mediating ADCC. IBV-specific mAbs 46B8 and C12G6 also mediate ADCC.
Matrix protein 2 (M2e) is another epitope, besides HA and NA. Although the mAbs targeting M2e cannot prevent the virus from binding to the receptor and have no neutralizing effect, virus replication can be inhibited by preventing germination, mediating CDC, mediating ADCC, and other ways. Therefore, IAV and IBV can be potentially inhibited. TCN-031 and TCN-032 were isolated from human B cells. The study showed that the mAbs target the ectodomain of M2e, a highly conserved structure in IAVs [78]. In vivo experiments have shown that TCN-031 and TCN-032 can treat mice infected with H5N1 or H1N1 influenza viruses that they have synergistic effects when combined with oseltamivir in patients [79,78]. Clinical trials have proved that TCN-032 has good tolerance and safety and that it is an effective antibody for the treatment of IAVs [79].
In summary, application of mAbs isolated from humans capable of neutralizing a variety of IAV and IBV subtypes is the most effective way to prevent and treat influenza and is a major direction for future research. However, because of HA and NA antigenic drift, treatment with mAbs often cannot achieve the desired effect. This calls for the further exploration of HA and NA conserved sites and mechanisms of action.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81974302), the Program for Youth Talent of Higher Learning Institutions of Hebei Province (BJ2018045), and Hebei Province’s Program for Talents Returning from Studying Overseas (CN201707).
Contributor Information
Shibo Jiang, Email: shibojiang@fudan.edu.cn.
Fei Yu, Email: shmyf@hebau.edu.cn.
References
- 1.WHO. Influenza (Seasonal). https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). 2020.05.01.
- 2.Centers for Disease C, Prevention. Pandemic influenza, past pandemics. https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html. 2020.05.01.
- 3.Centers for Disease C, Prevention Isolation of avian influenza A(H5N1) viruses from humans--Hong Kong, May-December 1997. MMWR Morb Mortal Wkly Rep. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 4.WHO. Antigenic and genetic characteristics of zoonotic influenza A viruses and development of candidate vaccine viruses for pandemic preparedness. https://www.who.int/influenza/vaccines/virus/characteristics_virus_vaccines/en/. 2020.05.01.
- 5.WHO. Influenza at the human-animal interface. https://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en/. 2020.05.01.
- 6.Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 7.Byrd-Leotis L., Cummings R.D., Steinhauer D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int J Mol Sci. 2017;18:1541. doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W., Li R., Li X., He J., Jiang S., Liu S. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2015;8:6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobusawa E., Aoyama T., Kato H., Suzuki Y., Tateno Y., Nakajima K. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology. 1991;182:475–485. doi: 10.1016/0042-6822(91)90588-3. [DOI] [PubMed] [Google Scholar]
- 10.Tong S., Zhu X., Li Y., Shi M., Zhang J., Bourgeois M. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita M., Krystal M., Fitch W.M., Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988;163:112–122. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]
- 12.Wiley D.C., Skehel J.J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 13.Lee P.S., Yoshida R., Ekiert D.C., Sakai N., Suzuki Y., Takada A. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci U S A. 2012;109:17040–17045. doi: 10.1073/pnas.1212371109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson C.S., Ortega S., Chaves F.A., Clark A.M., Yang H., Topham D.J. Natural and directed antigenic drift of the H1 influenza virus hemagglutinin stalk domain. Sci Rep. 2017;7:14614. doi: 10.1038/s41598-017-14931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weis W., Brown J.H., Cusack S., Paulson J.C., Skehel J.J., Wiley D.C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 16.Matrosovich M.N., Matrosovich T.Y., Gray T., Roberts N.A., Klenk H.D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendoza P., Gruell H., Nogueira L., Pai J.A., Butler A.L., Millard K. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561:479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautam R., Nishimura Y., Pegu A., Nason M.C., Klein F., Gazumyan A. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripp R.A., Haynes L.M., Moore D., Anderson B., Tamin A., Harcourt B.H. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. J Virol Methods. 2005;128:21–28. doi: 10.1016/j.jviromet.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Wan Y., Liu P., Zhao J., Lu G., Qi J. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015;25:1237–1249. doi: 10.1038/cr.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Gonzalez E., Alvarez M.M., Marquez-Ipina A.R., Trujillo-de Santiago G., Rodriguez-Martinez L.M., Annabi N. Anti-Ebola therapies based on monoclonal antibodies: current state and challenges ahead. Crit Rev Biotechnol. 2017;37:53–68. doi: 10.3109/07388551.2015.1114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.S., Adhikari N.K.J., Kwon H.Y., Teo K., Siemieniuk R., Lamontagne F. Anti-Ebola therapy for patients with Ebola virus disease: a systematic review. BMC Infect Dis. 2019;19:376. doi: 10.1186/s12879-019-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broder C.C., Weir D.L., Reid P.A. Hendra virus and Nipah virus animal vaccines. Vaccine. 2016;34:3525–3534. doi: 10.1016/j.vaccine.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corti D., Cameroni E., Guarino B., Kallewaard N.L., Zhu Q., Lanzavecchia A. Tackling influenza with broadly neutralizing antibodies. Curr Opin Virol. 2017;24:60–69. doi: 10.1016/j.coviro.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla-Quirarte H.O., Lopez-Guerrero D.V., Gutierrez-Xicotencatl L., Esquivel-Guadarrama F. Protective antibodies against influenza proteins. Front Immunol. 2019;10:1677. doi: 10.3389/fimmu.2019.01677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachbagauer R., Krammer F. Universal influenza virus vaccines and therapeutic antibodies. Clin Microbiol Infect. 2017;23:222–228. doi: 10.1016/j.cmi.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldmann H. Human monoclonal antibodies: the benefits of humanization. Methods Mol Biol. 2019;1904:1–10. doi: 10.1007/978-1-4939-8958-4_1. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin E., Wang W., McAuliffe J.M., Palmer-Hill F.J., Kallewaard N.L., Chen Z. A broadly neutralizing human monoclonal antibody directed against a novel conserved epitope on the influenza virus H3 hemagglutinin globular head. J Virol. 2014;88:6743–6750. doi: 10.1128/JVI.03562-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekiert D.C., Kashyap A.K., Steel J., Rubrum A., Bhabha G., Khayat R. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489:526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caton A.J., Brownlee G.G., Yewdell J.W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 31.Whittle J.R., Zhang R., Khurana S., King L.R., Manischewitz J., Golding H. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 2011;108:14216–14221. doi: 10.1073/pnas.1111497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt A.G., Therkelsen M.D., Stewart S., Kepler T.B., Liao H.X., Moody M.A. Viral receptor-binding site antibodies with diverse germline origins. Cell. 2015;161:1026–1034. doi: 10.1016/j.cell.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohshima N., Iba Y., Kubota-Koketsu R., Asano Y., Okuno Y., Kurosawa Y. Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J Virol. 2011;85:11048–11057. doi: 10.1128/JVI.05397-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu R., Krause J.C., McBride R., Paulson J.C., Crowe J.E., Jr., Wilson I.A. A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nat Struct Mol Biol. 2013;20:363–370. doi: 10.1038/nsmb.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreyfus C., Laursen N.S., Kwaks T., Zuijdgeest D., Khayat R., Ekiert D.C. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Messling V., Yasugi M., Kubota-Koketsu R., Yamashita A., Kawashita N., Du A. Human monoclonal antibodies broadly neutralizing against influenza B virus. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iba Y., Fujii Y., Ohshima N., Sumida T., Kubota-Koketsu R., Ikeda M. Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses. J Virol. 2014;88:7130–7144. doi: 10.1128/JVI.00420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z., Wang J., Bao L., Guo L., Zhang W., Xue Y. Human monoclonal antibodies targeting the haemagglutinin glycoprotein can neutralize H7N9 influenza virus. Nat Commun. 2015;6:6714. doi: 10.1038/ncomms7714. [DOI] [PubMed] [Google Scholar]
- 39.Xiong X., Corti D., Liu J., Pinna D., Foglierini M., Calder L.J. Structures of complexes formed by H5 influenza hemagglutinin with a potent broadly neutralizing human monoclonal antibody. Proc Natl Acad Sci U S A. 2015;112:9430–9435. doi: 10.1073/pnas.1510816112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogales A., Piepenbrink M.S., Wang J., Ortega S., Basu M., Fucile C.F. A highly potent and broadly neutralizing H1 Influenza-Specific human monoclonal antibody. Sci Rep. 2018;8:4374. doi: 10.1038/s41598-018-22307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J.-G., Ye C., Piepenbrink M.S., Nogales A., Wang H., Shuen M. A broad and potent H1-Specific human monoclonal antibody produced in plants prevents influenza virus infection and transmission in Guinea pigs. Viruses. 2020;12:167. doi: 10.3390/v12020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen C., Chen J., Li R., Zhang M., Wang G., Stegalkina S. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam5752. [DOI] [PubMed] [Google Scholar]
- 43.Yu F., Song H., Wu Y., Chang S.Y., Wang L., Li W. A potent germline-like human monoclonal antibody targets a pH-Sensitive epitope on H7N9 influenza hemagglutinin. Cell Host Microbe. 2017;22:471–483 e5. doi: 10.1016/j.chom.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bangaru S., Lang S., Schotsaert M., Vanderven H.A., Zhu X., Kose N. A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell. 2019;177:1136–1152 e18. doi: 10.1016/j.cell.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner H.L., Pallesen J., Lang S., Bangaru S., Urata S., Li S. Potent anti-influenza H7 human monoclonal antibody induces separation of hemagglutinin receptor-binding head domains. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai N., Swem L.R., Park S., Nakamura G., Chiang N., Estevez A. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat Commun. 2017;8:14234. doi: 10.1038/ncomms14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim J.J., Derby M.A., Zhang Y., Deng R., Larouche R., Anderson M. A phase 1, randomized, double-Blind, placebo-controlled, single-ascending-dose study to investigate the safety, tolerability, and pharmacokinetics of an anti-influenza B virus monoclonal antibody, MHAB5553A, in healthy volunteers. Antimicrob Agents Chemother. 2017;61:17. doi: 10.1128/AAC.00279-17. e00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bullough P.A., Hughson F.M., Skehel J.J., Wiley D.C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 49.Krammer F., Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avnir Y., Watson C.T., Glanville J., Peterson E.C., Tallarico A.S., Bennett A.S. IGHV1-69 polymorphism modulates anti-influenza antibody repertoires, correlates with IGHV utilization shifts and varies by ethnicity. Sci Rep. 2016;6:20842. doi: 10.1038/srep20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang C.C., Venturi M., Majeed S., Moore M.J., Phogat S., Zhang M.Y. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A. 2004;101:2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang S., Xie J., Zhu X., Wu N.C., Lerner R.A., Wilson I.A. Antibody 27F3 broadly targets influenza A group 1 and 2 hemagglutinins through a further variation in VH1-69 antibody orientation on the HA stem. Cell Rep. 2017;20:2935–2943. doi: 10.1016/j.celrep.2017.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Throsby M., van den Brink E., Jongeneelen M., Poon L.L., Alard P., Cornelissen L. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS One. 2008;3 doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ekiert D.C., Bhabha G., Elsliger M.A., Friesen R.H., Jongeneelen M., Throsby M. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekiert D.C., Friesen R.H., Bhabha G., Kwaks T., Jongeneelen M., Yu W. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friesen R.H., Lee P.S., Stoop E.J., Hoffman R.M., Ekiert D.C., Bhabha G. A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci U S A. 2014;111:445–450. doi: 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sui J., Hwang W.C., Perez S., Wei G., Aird D., Chen L-m. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corti D., Voss J., Gamblin S.J., Codoni G., Macagno A., Jarrossay D. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 59.Li G.M., Chiu C., Wrammert J., McCausland M., Andrews S.F., Zheng N.Y. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci Unit States Am. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura G., Chai N., Park S., Chiang N., Lin Z., Chiu H. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe. 2013;14:93–103. doi: 10.1016/j.chom.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 61.McBride J.M., Lim J.J., Burgess T., Deng R., Derby M.A., Maia M. Phase 2 randomized trial of the safety and efficacy of MHAA4549A, a broadly neutralizing monoclonal antibody, in a human influenza A virus challenge model. Antimicrob Agents Chemother. 2017;61:17. doi: 10.1128/AAC.01154-17. e01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim J.J., Deng R., Derby M.A., Larouche R., Horn P., Anderson M. Two phase 1, randomized, double-blind, placebo-controlled, single-ascending-dose studies to investigate the safety, tolerability, and pharmacokinetics of an anti-influenza A virus monoclonal antibody, MHAA4549A, in healthy volunteers. Antimicrob Agents Chemother. 2016;60:5437–5444. doi: 10.1128/AAC.00607-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marjuki H., Mishin V.P., Chai N., Tan M.W., Newton E.M., Tegeris J. Human monoclonal antibody 81.39a effectively neutralizes emerging influenza A viruses of group 1 and 2 hemagglutinins. J Virol. 2016;90:10446–10458. doi: 10.1128/JVI.01284-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wyrzucki A., Dreyfus C., Kohler I., Steck M., Wilson I.A., Hangartner L. Alternative recognition of the conserved stem epitope in influenza A virus hemagglutinin by a VH3-30-encoded heterosubtypic antibody. J Virol. 2014;88:7083–7092. doi: 10.1128/JVI.00178-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Cho M., Shore D., Song M., Choi J., Jiang T. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat Commun. 2015;6:7708. doi: 10.1038/ncomms8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tharakaraman K., Subramanian V., Viswanathan K., Sloan S., Yen H.L., Barnard D.L. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proc Natl Acad Sci U S A. 2015;112:10890–10895. doi: 10.1073/pnas.1502374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wollacott A.M., Boni M.F., Szretter K.J., Sloan S.E., Yousofshahi M., Viswanathan K. Safety and upper respiratory pharmacokinetics of the hemagglutinin stalk-binding antibody VIS410 support treatment and prophylaxis based on population modeling of seasonal influenza A outbreaks. EBioMedicine. 2016;5:147–155. doi: 10.1016/j.ebiom.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hershberger E., Sloan S., Narayan K., Hay C.A., Smith P., Engler F. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: results from a randomized, double-blind, phase-2, placebo-controlled study. EBioMedicine. 2019;40:574–582. doi: 10.1016/j.ebiom.2018.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kallewaard N.L., Corti D., Collins P.J., Neu U., McAuliffe J.M., Benjamin E. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell. 2016;166:596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pappas L., Foglierini M., Piccoli L., Kallewaard N.L., Turrini F., Silacci C. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- 71.Paules C.I., Lakdawala S., McAuliffe J.M., Paskel M., Vogel L., Kallewaard N.L. The hemagglutinin A stem antibody MEDI8852 prevents and controls disease and limits transmission of pandemic influenza viruses. J Infect Dis. 2017;216:356–365. doi: 10.1093/infdis/jix292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallory R.M., Ali S.O., Takas T., Kankam M., Dubovsky F., Tseng L. A phase 1 study to evaluate the safety and pharmacokinetics of MEDI8852, an anti-influenza A monoclonal antibody, in healthy adult volunteers. Biologicals. 2017;50:81–86. doi: 10.1016/j.biologicals.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Ali S.O., Takas T., Nyborg A., Shoemaker K., Kallewaard N.L., Chiong R. Evaluation of MEDI8852, an anti-influenza A monoclonal antibody, in treating acute uncomplicated influenza. Antimicrob Agents Chemother. 2018;62:18. doi: 10.1128/AAC.00694-18. e00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y.Q., Wohlbold T.J., Zheng N.Y., Huang M., Huang Y., Neu K.E. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell. 2018;173:417–429 e10. doi: 10.1016/j.cell.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y.Q., Lan L.Y., Huang M., Henry C., Wilson P.C. Hemagglutinin stalk-reactive antibodies interfere with influenza virus neuraminidase activity by steric hindrance. J Virol. 2019;93:18. doi: 10.1128/JVI.01526-18. e01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandbulte M.R., Westgeest K.B., Gao J., Xu X., Klimov A.I., Russell C.A. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci U S A. 2011;108:20748–20753. doi: 10.1073/pnas.1113801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piepenbrink M.S., Nogales A., Basu M., Fucile C.F., Liesveld J.L., Keefer M.C. Broad and protective influenza B virus neuraminidase antibodies in humans after vaccination and their clonal persistence as plasma cells. mBio. 2019;10:19. doi: 10.1128/mBio.00066-19. e00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grandea A.G., 3rd, Olsen O.A., Cox T.C., Renshaw M., Hammond P.W., Chan-Hui P.Y. Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci U S A. 2010;107:12658–12663. doi: 10.1073/pnas.0911806107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramos E.L., Mitcham J.L., Koller T.D., Bonavia A., Usner D.W., Balaratnam G. Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis. 2015;211:1038–1044. doi: 10.1093/infdis/jiu539. [DOI] [PubMed] [Google Scholar]