Abstract
Purpose
Mitigation strategies to balance the risk of coronavirus disease 2019 (COVID-19) infection against oncologic risk in patients with breast cancer undergoing radiation therapy have been deployed. To this end, shorter hypofractionated regimens have been recommended where appropriate, with prioritization of radiation therapy by oncologic risk and omission or deferral of radiation therapy for lower risk cases. Timely adoption of these measures reduces COVID-19 risk to both patients and health care workers and preserves resources. Herein, we present our early response and adaptation of breast radiation therapy utilization during the COVID-19 pandemic at a large academic cancer center in Canada.
Methods and Materials
A state of emergency was announced in Ontario on March 17, 2020, owing to the COVID-19 pandemic. Emergency guidelines were instituted. To examine our response, the number of weekly breast radiation therapy starts, type of breast radiation therapy, and patient age were compared from March 1 to April 30, 2020 to the same period in 2019.
Results
After the declaration of emergency in Ontario, there was a decrease of 39% in radiation therapy starts in 2020 compared with 2019 (79 vs 129, P < .001). There was a relative increase in the proportion of patients receiving regional nodal irradiation (RNI) in 2020 compared with 2019 (46% vs 29%, respectively), with the introduction of hypofractionated RNI in 2020 (27 of 54 cases, 50%). A smaller proportion of patients starting radiation therapy were aged >50 years in 2020, 66% (78 of 118) versus 83% (132 of 160) in 2019, P = .0027.
Conclusions
A significant reduction in breast radiation therapy starts was noted during the early response to the COVID-19 pandemic, with prioritization of radiation therapy to patients associated with higher oncologic risk requiring RNI. A quick response to a health care crisis is critical and is of particular importance for higher volume cancer sites where the potential effect on resources is greater.
Introduction
In Canada and around the world, health care providers are doing extraordinary work to mitigate the transmission of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2.1 Early data suggest that mortality from the virus ranges from 1% to 4% in the overall population,2 with more severe disease and higher death rates among patients with comorbidities and older age.3, 4, 5 In addition, the risk of infection has been reported to be approximately 2-fold higher in patients with cancer than the normal population.3,6,7 Therefore, in the management of patients with cancer, oncologists must weigh the risk of death and morbidity from COVID-19 against the benefit of cancer therapy when there is the necessity to decrease patient visits to cancer centers and the potential reduction of health care worker availability due to illnesses.8,9 Consequently, general measures to mitigate COVID-19 transmission to patients with cancer receiving radiation therapy have been already described.10
Patients with breast cancer constitute one of the largest groups of patients at most cancer centers, and guidelines for prioritization and multidisciplinary breast cancer treatment have been developed to assist management decisions during the COVID-19 pandemic.11, 12, 13 The majority of patients with breast cancer require radiation therapy as part of their overall management, and breast cancer represents approximately 25% of all cases treated with radiation therapy in Ontario.13 Therefore, reducing patient visits to cancer centers at different parts of the COVID-19 pandemic curve is important. Key strategies to mitigate COVID-19 infection in patients requiring breast radiation therapy include the shortening of overall treatment time using hypofractionated (HF) regimens,14 delay of radiation therapy initiation in those with lower oncologic risk, or radiation therapy omission for older lower risk patients or those with comorbidities, with hormone therapy (ET) as an alternative.13,15 The effectiveness of these mitigation strategies is dependent on the rapid adoption of these measures. Herein, we describe the experience of early adoption of such practices during the COVID-19 pandemic in a large academic radiation medicine program.
Methods and Materials
This study is an evaluation project of adjuvant breast radiation therapy delivery and use at our institution during a 9-week period spanning March 1 to April 30, 2020, compared with the same period in 2019 (institutional waiver 20-0464). Data collection was performed according to our standard work processes and included the monitoring of weekly breast radiation therapy starts, type of radiation therapy delivered, and patient age.
In response to the COVID-19 pandemic, a state of emergency was declared in Ontario on March 17, 2020. Our institution followed the principles of the COVID-19 prioritization and breast radiation therapy planning guidelines in accordance with provincial and international guidelines.13,15 Patients were prioritized for breast radiation therapy by higher oncologic risk: the high-risk category included patients with locally advanced or pT3-4/pN2-3 disease and residual nodal after neoadjuvant chemotherapy; intermediate risk included estrogen receptor (ER) positive and pN1a disease and complete pathologic response after neoadjuvant chemotherapy; and low risk included ductal carcinoma in situ and early stage invasive disease.
Consideration of ET only and omission of radiation therapy was made for women aged >70 years with completely excised (minimum margin of 1 mm) low-risk invasive disease (pT1/pN0, grades 1 or 2, lymphovascular invasion negative, ER positive, human epidermal growth factor receptor 2 negative, without extensive intraductal component). For patients >55 years with ductal carcinoma in situ measuring <2.5 cm, grades 1 or 2, and minimum margin of 1 mm, radiation therapy omission was also considered. After breast conserving surgery, radiation therapy delays up to 20 weeks were considered for patients with low-intermediate risk invasive disease (pT1-2/pN0) or ductal carcinoma in situ, with systemic therapy, and up to 12 weeks without systemic therapy.16, 17, 18 Some low-risk patients eligible for ET received neoadjuvant ET while waiting for their breast surgery and adjuvant radiation therapy. Patient comorbidities and performance status were also considered in the decision-making for radiation therapy deferral or omission. Weekly multidisciplinary case conference, and weekly radiation therapy quality assurance rounds assisted with consensus-building and treatment decisions.
Other key mitigation strategies included adoption of HF regimens to shorten treatment time and minimize visits to the cancer center. Specifically, preference was given to HF regimens of 40.05 Gy in 15 fractions daily for breast radiation therapy, including regional nodal irradiation (RNI), compared with conventional fractionation of 50 Gy in 25 fractions.19 In addition, after the publication of the UK Faster radiotherapy for breast cancer patients (FAST)-Forward (A multicentre, non-inferiority, randomized, phase 3 clinical trial testing a 1-week course of whole breast radiotherapy against a standard 3-week schedule in terms of local tumor control and late adverse effects in patients with early breast cancer) trial,20 26 Gy in 5 fractions daily for whole breast irradiation (WBI), or partial breast irradiation (PBI) was included as an option to 40.05 Gy/15 fractions or 42.40 Gy/16 fractions for suitable patients. For patients eligible for a boost (margins less <1 mm, patient age <40 years, or <50 years with high-risk features of lymphovascular invasion, ER negative, or grade 3 disease), the majority received 10 Gy/4 fractions. For the purpose of this study, the number of new patients starting radiation therapy was monitored; therefore, delivery of a boost was not considered an additional radiation therapy course. To approximate the overall effect on radiation therapy capacity, the total number of treatment visits during this period was also measured and compared with 2019.
For statistical analyses, the exact Poisson test was used to assess equality of 2 rates, and Pearson χ2 test for equality of 2 proportions. All tests were 2-sided, and a P value less than 0.05 was considered statistically significant.
Results
There was a total of 118 breast radiation therapy starts during the 9-week period after March 1, 2020, compared with 160 starts in the same period in 2019 (Fig 1). An initial drop in the number of breast radiation therapy starts was noted within the first 2 weeks after implementation of our emergency guidelines in mid-March (March weeks 3 and 4) compared with 2019, from 43 to 33 (exact Poisson test P = .10), representing a 24% decrease (Fig 1). A significant reduction in radiation therapy starts of 54% (from 34 to 16, 2019 vs 2020, exact Poisson test P < .001) was observed in the following 2 weeks (March week 5, April week 1; Fig 1). Thereafter in April 2020, the number of radiation therapy starts continued to remain lower than in 2019. There was a total decrease of 39% in radiation therapy starts in 2020 compared with 2019 (79 vs 129, exact Poisson test P < .001) measured from the beginning of the third week in March (Fig 1). During the 9-week period in 2020, there were 4 patients with confirmed COVID-19 infections who required a 2-week treatment delay.
Figure 1.
Number of weekly breast radiation therapy starts comparing the 9-week periods of March 3 to May 4, 2019, versus March 1 to May 2, 2020. The beginning of week 3 in March 2020 coincided with the declaration of state of emergency in Ontario.
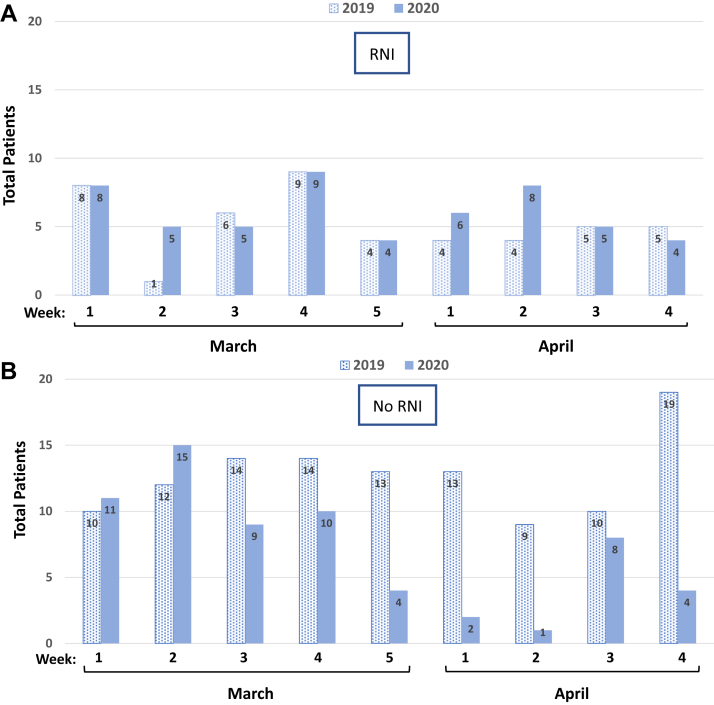
When the types of breast radiation therapy plans were evaluated during the 9-week period, a relative increase of 17% in the number of radiation therapy plans that included RNI was noted in 2020 compared with 2019; 46% (54 of 118) versus 29% (46 of 160) of all radiation therapy starts, respectively (χ2 test P = .005; Fig 2A, Table 1). Before the COVID-19 pandemic, the standard regimen for RNI used at our center was 50 Gy in 25 fractions, with HF RNI introduced during the pandemic to shorten RT. We observed a dramatic rise in the use of the 4-field HF RNI regimen during the 9-week period in 2020 (27 of 54 cases, 50%), which corresponded to 270 fewer treatment visits (Table 1). The majority (26 of 27 patients; 96%) receiving HF RNI began radiation therapy from the end of March 2020, approximately 2 weeks after the COVID-19 prioritization guidelines were implemented (Table 1). In comparison, all radiation therapy plans that included RNI in 2019 during the same period used conventional 2 Gy daily fractionation regimen of 50 Gy in 25 fractions (Table 1).
Figure 2.
Type of breast radiation therapy starts per week comparing the 9-week periods of March 3 to May 4, 2019, versus March 1 to May 2, 2020. (A) Regional nodal irradiation (RNI) starts per week compared for 2019 and 2020. (B) Radiation therapy starts per week without comprehensive RNI (no RNI), including whole breast irradiation, partial breast irradiation, chestwall or high-tangent plans for 2019 and 2020.
Table 1.
Type of breast RT per week comparing the periods of March 3 to May 4, 2019, (top panel) versus March 1 to May 2, 2020 (bottom panel)
| Breast RT |
RNI |
WBI/CW/HT |
PBI |
|||
|---|---|---|---|---|---|---|
| 2019 | CF (25f) | HF (15-16f) | CF (25f) | HF (15-16f) | CF (25f) | HF (15-16f) |
| March week 1 | 8 | 8 | 2 | |||
| 2 | 1 | 9 | 3 | |||
| 3 | 6 | 12 | 2 | |||
| 4 | 9 | 1 | 9 | 4 | ||
| 5 | 4 | 1 | 10 | 2 | ||
| April week 1 | 4 | 11 | 2 | |||
| 2 | 4 | 1 | 8 | |||
| 3 | 5 | 7 | 1 | 2 | ||
| 4 | 5 | 2 | 14 | 3 | ||
| Total | 46 | 0 | 5 | 88 | 1 | 20 |
| 2020 | ||||||
| March week 1 | 7 | 1 | 11 | |||
| 2 | 5 | 13 | 2 | |||
| 3 | 5 | 6 | 3 | |||
| 4 | 8 | 1 | 10 | |||
| 5 | 4 | 3 | 1 | |||
| April week 1 | 1 | 5 | 2 | |||
| 2 | 1 | 7 | 1 | |||
| 3 | 5 | 7 | 1 | |||
| 4 | 4 | 4 | ||||
| Total | 27 | 27 | 0 | 57 | 0 | 7 |
Abbreviations: CF = conventional fractionation; CW = chestwall; f = fraction; HF = hypofractionation; HT = high tangent; PBI = partial breast irradiation; RNI = regional nodal irradiation; RT = radiation therapy; WBI = whole breast irradiation.
For node negative or breast cancer patients not requiring comprehensive RNI (WBI, PBI, chestwall, and high-tangent plans), there was a significant decrease in breast radiation therapy starts during the 9-week period in 2020 compared with 2019; 54% (64 of 118) versus 71% (114 of 160) of overall cases, respectively (χ2 test P = .005; Fig 2B, Table 1). There were only 2 PBI starts after week 3 in March 2020 compared with 13 in the same period in 2019 (Table 1). There was a shift in the use of WBI regimen of 42.40 Gy/16 fractions to 40.05 Gy/15 fractions observed in early April 2020 (Table 2). The abbreviated 26 Gy in 5 fractions daily regimen for WBI or PBI was introduced after the first week of May 2020, coinciding with the publication of the FAST-Forward trial20; no radiation therapy starts with this regimen were noted before May 2, 2020. The proportion of boosts delivered in 2020 was 40% (47 of 118) compared with 48% (77 of 160) in 2019, corresponding to 120 fewer treatment visits (χ2 test P = .21; Table 3).
Table 2.
Weekly breast RT regimens compared over the periods of March 3 to May 4, 2019, (top panel) versus March 1 to May 2, 2020 (bottom panel)
| WBI/CW/HT Breast RT |
CF |
HF |
||
|---|---|---|---|---|
| 2019 | 50 Gy/25f | 42.56 Gy/16f | 42.4 Gy/16f | 40.05 Gy/15f |
| March week 1 | 1 | 6 | 1 | |
| 2 | 8 | 1 | ||
| 3 | 12 | |||
| 4 | 1 | 1 | 7 | 1 |
| 5 | 1 | 10 | ||
| April week 1 | 11 | |||
| 2 | 1 | 8 | ||
| 3 | 7 | |||
| 4 | 2 | 12 | 2 | |
| Total | 5 | 2 | 81 | 5 |
| 2020 | ||||
| March week 1 | 10 | 1 | ||
| 2 | 13 | |||
| 3 | 6 | |||
| 4 | 10 | |||
| 5 | 1 | 2 | ||
| April week 1 | 2 | |||
| 2 | 1 | |||
| 3 | 7 | |||
| 4 | 1 | 3 | ||
| Total | 0 | 1 | 40 | 16 |
Abbreviations: CF = conventional fractionation; CW = chestwall; f = fraction; HF = hypofractionation; HT = high tangent; RT = radiation therapy; WBI = whole breast irradiation.
Table 3.
Comparison of the total number of fractions (no. fx) per week for breast RT initiated during the 9-week period of evaluation, 2019 (top panel) versus 2020 (bottom panel)
|
2019 |
#Fx |
#Fx |
No. of boost | No. of Tx |
|---|---|---|---|---|
| (with boost) | (without boost) | |||
| March week 1 | 398 | 358 | 9 | 18 |
| 2 | 237 | 213 | 6 | 13 |
| 3 | 414 | 372 | 10 | 20 |
| 4 | 513 | 453 | 13 | 23 |
| 5 | 337 | 315 | 5 | 17 |
| April week 1 | 339 | 306 | 7 | 17 |
| 2 | 291 | 253 | 9 | 13 |
| 3 | 323 | 292 | 7 | 15 |
| 4 | 488 | 442 | 11 | 24 |
| Total | 3340 | 3004 | 77 | 160 |
|
2020 |
#Fx |
#Fx |
No. of boost | No. of Tx |
|---|---|---|---|---|
| (with boost) | (without boost) | |||
| March week 1 | 390 | 366 | 6 | 19 |
| 2 | 411 | 363 | 10 | 20 |
| 3 | 286 | 266 | 5 | 14 |
| 4 | 413 | 375 | 9 | 19 |
| 5 | 125 | 121 | 1 | 8 |
| April week 1 | 150 | 130 | 5 | 8 |
| 2 | 149 | 145 | 1 | 9 |
| 3 | 220 | 195 | 6 | 13 |
| 4 | 139 | 122 | 4 | 8 |
| Total | 2283 | 2083 | 47 | 118 |
Comparisons included the total number of fractions with and without boost, and the total number of RT courses (Tx) and the number of boosts delivered. Abbreviations: RT= radiation therapy; Tx = treatment.
In general, older patients with breast cancer are more vulnerable to COVID-19 related morbidity and mortality.21 Therefore, the proportion of breast radiation therapy starts for those aged <50 years versus >50 years during the 9-week period was compared for 2019 and 2020. In 2019, a smaller proportion of patients starting radiation therapy were less than 50 years of age; 18% (28 of 160) versus 34% (40 of 118) in 2020 (Fig 3A). In turn, more patients in 2019 were greater than 50 years; 83% (132 of 160) versus 66% (78 of 118) in 2020 (χ2 test P = .0027; Fig 3B). From the beginning of April, there was also a significant difference based on age in 2019 compared with 2020, with breast radiation therapy starts for patients under 50 years at 13% (9 of 69) in 2019 versus 37% (14 of 38) in 2020, and for those 50 years or greater, 87% (60 of 69) in 2019 versus 63% (24 of 38) in 2020 (χ2 test P = .0087; Fig 3A and B).
Figure 3.
Weekly breast radiation therapy starts comparing the 9-week periods of March 3 to May 4, 2019, and March 1 to May 2, 2020, for (A) age <50 years and (B) age >50 years.
Based on the number of radiation therapy starts and the total number of fractions per course (inclusive of boost), there was an overall reduction of 45% in the number of visits for breast radiation therapy from 2705 to 1482, measured from mid-March in 2019 and 2020, respectively (Table 3).
Discussion
The COVID-19 pandemic has posed challenges for the delivery of cancer care, with increased risk for vulnerable patients with cancer. The safe delivery of care during the pandemic has been guided by prioritization of oncologic risk and by mitigation strategies to minimize the risk of COVID-19 infection. Rapid deployment of these measures is pivotal to a radiation medicine program, particularly for high volume sites such as breast cancer.
We were able to rapidly implement changes to significantly reduce breast radiation therapy starts and decrease the overall number of radiation therapy visits to our center, after the declaration of emergency in Ontario. Patients with higher oncologic risk were prioritized for breast radiation therapy, as judged by the receipt of RNI, which is typically reserved for those with locally advanced or node positive disease. Overall, there was a substantial increase in the proportion of radiation therapy starts that included RNI in 2020 compared with 2019, and a concomitant proportional reduction in the delivery of radiation therapy that excluded comprehensive RNI, usually delivered to patients with node negative or lower risk disease. Collectively, these results suggest that higher risk breast cancer patients were prioritized for breast radiation therapy as a consequence of the adoption of the COVID-19 pandemic guidelines.
There was also a significant decrease in proportion of radiation therapy starts for older patients in 2020 compared with 2019. This finding is consistent with the recommendation to preferentially defer or omit radiation therapy in older women who are eligible for ET. It is also possible that the decrease in radiation therapy starts were at least in part due to patient comorbidities, more likely to be associated with older than younger patients. Very few patients required a treatment delay during the 9-week period due to COVID-19 infection.
The introduction of HF regimens to shorten breast radiation therapy resulted in a considerable reduction in the number of patient visits during the 9-week period in 2020 compared with 2019. Likely we will continue to see additional gains with the regimen of 26 Gy/5 fractions, which became available as an option for some patients in May 2020 at our center. As a result of decreasing the overall number of treatments, there is less risk to patients and to health care workers, reduced pressure on resources, and physical distancing is facilitated. As the availability of breast cancer surgeries and breast cancer screening programs increase in parallel to a decreased risk of COVID-19, referrals for breast radiation therapy are expected to increase. Therefore, these HF regimens will likely continue to remain an important mitigation strategy for breast radiation therapy in the coming months. With the added possibility of a second wave of COVID-19,22 there will be a need for flexibility and to nimbly adapt to the pandemic peaks and surges.
This study describes for the first time the experience and adaptation of breast radiation therapy use at a large academic center in North America during the initial response to the COVID-19 pandemic. There have been several reports to date outlining recommendations for the prioritization and treatment of various cancer sites. However, very few reports describe the experience at the cancer center from a radiation oncology perspective. In a recent retrospective study chronicling the early experience from a large radiation oncology department in New York City during March, 2020, there was a median reduction of 30% for all disease sites for patients on treatment, although the breast site demonstrated a small increase.23 In our study, breast radiation therapy use was significantly reduced, which facilitated the redistribution of resources to other cancer sites more dependent on primary radiation therapy, such as head and neck.
We acknowledge the limitations of this study, which was retrospective and conducted at a single center. In addition to specific radiation therapy mitigation strategies, the introduction of virtual patient visits, use of personal protective equipment, and physical distancing have been important strategies to minimize COVID-19 risk. Furthermore, during this period we did not specifically capture the number of radiation therapy deferrals, cases with radiation therapy omission, patient comorbidities, patient preference for radiation therapy delay, or surgical delays. However, these factors would have primarily effected older patients, patients with lower risk disease, or patients with clinical comorbidities, and to a lesser extent in those with high-risk disease. Indeed, there was a proportional increase in breast radiation therapy starts in 2020 compared with 2019 for higher risk disease requiring RNI.
Conclusions
We describe a rapid response to the COVID-19 prioritization and mitigation strategies for breast radiation therapy at a large academic cancer center. Understanding the timeliness and effectiveness of the response assists in directing resources and planning during the pandemic. These results and other experiences will further assist in the ongoing management of the COVID-19 pandemic and of possible future health care crises.
Footnotes
Sources of support: none.
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at: Accessed May 26, 2020.
- 2.Guan W.-J., Ni Z.Y., Hu Y., Liang W.H. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Ronghui D. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai A.G., Pasea L., Banerjee A. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. https://www.researchgate.net/publication/340984562 Available at: Accessed May 26, 2020.
- 6.Wang D., Hu B., Hu C. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. Online ahead of print. Accessed March 25, 2020. [DOI] [PMC free article] [PubMed]
- 8.Mayor S. COVID-19: Impact on cancer workforce and delivery of care. Lancet Oncol. 2020;21:633. doi: 10.1016/S1470-2045(20)30240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunstein LZ, Gillespie EF, Hong L, et al. Breast radiotherapy under COVID-19 pandemic resource constraints: Approaches to defer or shorten treatment from a comprehensive cancer center in the United States. Adv Radiat Oncol. 2020;5:582-588. [DOI] [PMC free article] [PubMed]
- 10.Simcock R., Thomas T.V., Estes C. COVID-19: Global radiation oncology's targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietz J.R., Moran M.S., Isakoff S.J. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181:487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curigliano G., Cardoso M.J., Poortmans P. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ontario Health COVID-19 health system response materials. https://www.ontariohealth.ca/COVID-19 Available at: Accessed May 26, 2020.
- 14.Al-Rashdan A, Roumeliotis M, Quirk S, et al. Adapting radiotherapy treatments for breast cancer patients during the COVID-19 pandemic: Hypo-fractionation and accelerated partial breast irradiation to address World Health Organization recommendations. Adv Radiat Oncol. 2020;5:575-576. [DOI] [PMC free article] [PubMed]
- 15.Coles C.E., Aristei C., Bliss J. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivotto I.A., Lesperance M.L., Truong P.T. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol. 2009;27:16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 17.Shurell E., Olcese C., Patil S. Delay in radiotherapy is associated with an increased risk of disease recurrence in women with ductal carcinoma in situ: Risk of IBTR with RT delay in DCIS. Cancer. 2018;124:46–54. doi: 10.1002/cncr.30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson P., Cole B.F., Colleoni M. Timing of radiotherapy and outcome in patients receiving adjuvant endocrine therapy. Int J Radiat Oncol Biology Phys. 2011;80:398–402. doi: 10.1016/j.ijrobp.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 20.Brunt A.M., Haviland J.S., Wheatley D.A. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desideri I, Pilleron S, Battisti NML, et al. Caring for older patients with cancer during the COVID-19 pandemic: A Young International Society of Geriatric Oncology (SIOG) global perspective [e-pub ahead of print]. J Geriatr Oncol.https://doi.org/10.1016/j.jgo.2020.05.001. Accessed May 10, 2020. [DOI] [PMC free article] [PubMed]
- 22.Xu S., Li Y. Beware of the second wave of COVID-19. Lancet. 2020;395:1321–1322. doi: 10.1016/S0140-6736(20)30845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckstein M, Skubish S, Smith K, et al. Experiencing the surge: Report from a large New York radiation oncology department during the COVID-19 pandemic. Adv Radiat Oncol. 2020;5:610-616. [DOI] [PMC free article] [PubMed]