Abstract
Background
Since December 2019, when it first occurred in Wuhan, China, coronavirus disease 2019 (COVID-19) has spread rapidly worldwide via human-to-human transmission. We aimed to describe the epidemiological and demographic features of COVID-19 outside Wuhan.
Methods
A single-center case series of 136 consecutive (from January 16 to February 17, 2020) patients with confirmed COVID-19 hospitalized in The First People's Hospital of Jingzhou, China, was retrospectively analyzed. Outcomes were followed up until February 19, 2020.
Results
Of the 136 patients (median age, 49 years; interquartile range [IQR], 33–63 years; range, 0.3–83 years), 91 (67%) had been to Wuhan or contacted persons from Wuhan. Forty-five (33.1%) were familial clusters. The median incubation period was 6 days (IQR: 4–11 days). All children had an exact exposure history, family members with COVID-19, and “Mild/Moderate” symptoms at admission. Among the 64 elderly patients, 14 (21.9%) had no exposure history, and 43 (67.2%) had a chronic illness. All 11 (8.1%) “Severe/very severe” illness at onset cases and 5 (3.7%) fatal cases were elderly patients. The duration from symptom onset to admission was positively correlated with the duration from symptom onset to endpoint. Overall, patients with a longer incubation period had more severe outcomes.
Conclusion
As high-risk susceptible groups, strong protection should be implemented for children and the elderly. Universal screening should be performed for people with a clear exposure history, even lacking apparent symptoms. Given the rapid progression of COVID-19, people should be admitted quickly following symptom onset.
Keywords: Coronavirus disease 2019, Epidemiology, Incubation period, High-risk susceptible people, China
Introduction
In December 2019, a pneumonia of unknown origins was initially detected in the residents of Wuhan (Hubei, China) [1]. The main clinical manifestation of these cases was fever, and a few patients had dyspnea. In a news conference on January 21, 2020, Professor Zhong Nanshan announced that the disease was severe, transmissible from person to person, and seemed to cause clusters of disease in health-care workers [2]. A noval coronavirus was than isolated from the respiratory epithelium of patients, and the new coronavirus was officially named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. The disease prompted the declaration of a public health emergency of international concern, the highest possible level of alarm, by the World Health Organization (WHO) on January 31, 2020. The disease was officially named coronavirus disease 2019 (COVID-19) on February 11, 2020 [4]. Some studies have found that SARS-CoV-2 has the potential to severe acute respiratory syndrome coronavirus (SARS-CoV) which caused an outbreak in China in 2002–2003 [5] and Middle East respiratory syndrome (MERS) which caused an outbreak in the Kingdom of Saudi Arabia in 2012 [6]. Some research have revealed that the original source of SARS-CoV and MERS were bat, civet cats and camels respectively. However, the origin of SARS-CoV-2 was not completely clear, and some studies suggest that the source is bats or pangolins [5]. So the covid-outbreak means that human consumption on wildlife should be limited to prevent zoonotic infections.
COVID-19 has rapidly spread from Wuhan to other areas throughout China, with its spread exacerbated by traditional family gatherings for Chinese lunar New Year from January 24 to January 31, 2020. As of May 13, 2020, the ongoing outbreak of COVID-19 had caused 84,458 confirmed cases and 4644 deaths in China [7]. The COVID-19 outbreak has also spread worldwide, with an increasing number of travel-associated cases confirmed in multiple countries in South-East Asia, North America, and Europe, and has given rise to the first pandemic of the 21st century [8]. Until May 13, 2020, the WHO had recorded 4,004,390 confirmed COVID-19 cases out of China and attributed 278,509 deaths to the disease [9]. Although SARS-CoV-2 was identified as the infectious agent causing COVID-19 on January 9, 2020, the origin of this pandemic remains unclear [10]. Wuhan was the first city to find cases in China, but the latest reports find that Wuhan was not necessary the first place in the world to find COVID-19[11].
A few epidemiological findings and clinical presentations of COVID-19 from the initial cases in China have been described [12], [13], [14], [15], [16], [17]. Here, the epidemiological data of hospitalized patients from the early phase of the COVID-19 outbreak during January 16 to February 17, 2020 in Jingzhou, a neighboring city of Wuhan, are reported. The objective of this study was to characterize the epidemiology of COVID-19 patients outside the origin location of COVID-19. A better understanding of COVID-19 will allow for improved resource planning and better protection for people around the world.
Materials and methods
Study population
We retrospectively gathered epidemiological data from the clinical records of patients with confirmed COVID-19 in The First People's Hospital of Jingzhou, a neighboring city of Wuhan (Hubei Province, China) from January 16, 2020 to February 17, 2020 (Fig. 1 ). The applied case definitions of a confirmed person with COVID-19 were in accordance with the Diagnosis and Treatment Scheme of COVID-19 (Interim Version 6) released by China's National Heath Commission [7] and complied with the WHO interim guidance [18]. Epidemiological information, including age, gender, city of permanent residence, smoking history, comorbidities, and self-reported symptoms, was collected through brief interviews with each patient. Several investigators interviewed each patient to collect the history of exposure during the two weeks before symptom onset, including any dates and times of visiting Wuhan, contact with people from Wuhan or a family member with respiratory symptoms. The incubation period was defined as the time from exposure to the symptom onset, which was estimated using data from patients who could provide the exact date of close exposure. Based on the general information provided by the patients with confirmed COVID-19, the cases were classified as mild, moderate, severe, or very severe; they were all followed up daily until discharge. All information was recorded on the standard electronic form for medical data collection and reviewed by a team of trained physicians. This study was approved by the hospital ethics committee (42016803 – T).
Fig. 1.
The geographical location of Jingzhou city, Hubei province, China. Hubei province is located in central region of China, and Jingzhou city (cyan) is located in the southern part of Hubei province bordering Wuhan city (crimson) which is considered to be the outbreak area of COVID-19.
Statistical analysis
The categorical variables are presented as the number and percentage of cases, and the continuous variables are expressed as the median and interquartile range (IQR). Chi-square tests and Fisher's exact tests were used to compare the differences among categorical variables. Continuous variables were compared by an independent-samples t-test, and a Kruskal–Wallis test was used to compare variables that do not conform to a normal distribution. The time distribution of the incubation period and duration from symptom onset to admission was drawn as a bar plot. Regression equations were then calculated, and scatter plots with the added fitting curve were drawn. The degree of symptom severity at admission and outcome were grouped according to incubation period, duration from symptom onset to admission, and coexisting condition. A series of Sankey plots were drawn according to group to present the transformation pattern of disease. Chi-square tests and Fisher's exact tests were performed by SPSS 26.0. All other analyses were performed by R 3.6.1.
Results
The study population included 136 hospitalized patients with COVID-19, and the details are presented in Table 1 . The median age was 49 years (IQR, 33–63 years; range, 0.28–85 years), 68 (50%) of the patients were men, and 15 (11%) of the patients were smokers. Among the 136 patients, 98 (72.1%) resided in Jingzhou, 35 (25.7%) in Wuhan, and 3 (2.2%) in other cities of Hubei province, China. In terms of exposure history, 61 (44.9%) had visited or resided in Wuhan, 30 (22.1%) had come into contact with a person from Wuhan, 22 (16.2%) had come into contact with a family member who had respiratory symptoms, 3 (2.2%) had visited or worked in the hospital, and the remaining 20 (14.7%) had no memory of potential exposure. Of the 136 patients, 45 (33.1%) patients had been exposed to family member with confirmed COVID-19.
Table 1.
Baseline characteristics of patients with COVID-19 in Jingzhou China (hospital admission on January 16 to February 17, 2020).a
| Case number (n) | Proportion of specified item (%) | |
|---|---|---|
| Age, median (IQR), yearsb | 49 | 33–63 |
| Gender | ||
| Male | 68 | 50 |
| Female | 68 | 50 |
| Smoking history | 15 | 11 |
| Permanent city | ||
| Jingzhou | 98 | 72.1 |
| Wuhan | 35 | 25.7 |
| Other | 3 | 2.2 |
| Exposure history | ||
| Visiting or living in Wuhan | 61 | 44.9 |
| Contact with people from Wuhan | 30 | 22.1 |
| Family member with respiratory symptoms | 22 | 16.2 |
| Visiting or work in the Hospital | 3 | 2.2 |
| None of above | 20 | 14.7 |
| Family member with COVID-19 | 45 | 33.1 |
| Coexisting condition | ||
| Hypertension | 31 | 22.8 |
| Cancer | 9 | 6.6 |
| Diabetes | 7 | 5.1 |
| Chronic respiratory disease | 4 | 2.9 |
| Chronic liver disease | 4 | 2.9 |
| Cardiovascular disease | 3 | 2.2 |
| Chronic kidney disease | 2 | 1.5 |
| Other | 34 | 25 |
| Incubation period, median (IQR), daysb | 6 | 4–11 |
| Duration from symptom onset to admission, median (IQR), daysb | 6 | 4–8 |
| Duration from symptom onset to endpoint, median (IQR), daysb | 16 | 14–20 |
| Discharge at the endpoint | 19 | 14 |
| Hospital stay, median (IQR), daysb | 16.4 | 14–19.5 |
| Death at the endpoint | 5 | 3.7 |
Values are no. (%) except as indicated.
Presented as median (IQR), IQR: interquartile range.
Most of the infected patients had underlying diseases, including hypertension (31, 22.8%), cancer (9, 6.6%), and diabetes (7, 5.1%). Thirty-four (25%) patients had undergone recent medical procedures, such as surgery or childbirth. Forty-five (33.1%) patients could provide the exact date of close contact with someone who had respiratory symptoms or was from Wuhan. Based on the data from those patients, the median incubation period was 6 days (IQR: 4–11 days). The median lengths of time from symptom onset to hospital admission and from admission to observation endpoint were 6 days (IQR: 4–8 days) and 16 days (14–20 days), respectively. Of the 136 patients, 19 (14%) were discharged alive after a median hospital stay of 16 days (IQR: 14–19.5 days), and 5 (3.7%) had died.
All 7 (100%) of the cases with pediatric patients were clustered (Table 2 ); the children all had an exact history of exposure to a family member with a confirmed case of COVID-19. All the pediatric patients had a “Mild/Moderate” illness at symptom onset, and 4 (57.1%) had improved sufficiently to be discharged from the hospital at the time of writing. For the pediatric COVID-19 patients, both the duration from symptom onset to hospital admission and that from admission to observation endpoint were shorter than those of the elderly patients with COVID-19.
Table 2.
Epidemiological features of patients with COVID-19 in the three age groups (hospital admission on January 16 to February 17, 2020).a
| Age ≤ 12 | Age 18–49 | Age ≥ 50 | |
|---|---|---|---|
| (n = 7) | (n = 65) | (n = 64) | |
| Age, median (IQR), yearsb | 3 (2.5–7) | 34 (29–42) | 63.5 (56–67.3) |
| Gender | |||
| Male | 2 (28.5) | 31 (47.7) | 35 (54.7) |
| Female | 5 (71.4) | 34 (52.3) | 29 (45.3) |
| Smoking | 0 (0) | 6 (9.2) | 9 (14.1) |
| Exposure | |||
| Yes | 7 (100) | 59 (90.8) | 50 (78.1)c |
| No | 0 (0) | 6 (9.2) | 14 (21.9)c |
| Coexisting condition | |||
| Yes | 0 (0) | 14 (21.5) | 43 (67.2)c |
| No | 7 (100) | 51 (78.5) | 21 (32.8)c |
| Family member with COVID-19 | 7 (100) | 19 (29.2) | 19 (29.7) |
| Incubation period, median (IQR), daysb | – | 6 (2.8–11) | 6 (4–9.5) |
| Duration from symptom onset to admission, median (IQR), daysb | 3 (1.3–4.8) | 6 (4–8) | 6 (3.8–10) |
| Duration from symptom onset to endpoint, median (IQR), daysb | 12 (10.5–15) | 16 (15–20) | 16.5 (13.8–19) |
| Fever | |||
| Yes | 5 (71.4) | 45 (69.2) | 46 (71.9) |
| No | 2 (28.6) | 20 (30.8) | 18 (28.1) |
| Degree of symptom at admission | |||
| Mild/Moderate | 7 (100) | 65 (100) | 53 (82.8)b |
| Severe/Very severe | 0 (0) | 0 (0) | 11 (17.2)b |
| Patient's progress | |||
| No change | 3 (42.9) | 51 (78.5) | 34 (53.1) |
| Improvement | 4 (57.1) | 14 (21.5) | 8 (12.5)c |
| Aggravated | 0 (0) | 0 (0) | 22 (34.4)c |
| Outcome at the endpoint | |||
| Discharge | 4 (57.1) | 10 (15.4) | 5 (7.8)c |
| Mild | 2 (28.6) | 2 (3.1) | 0 (0) |
| Moderate | 1 (14.3) | 53 (81.5) | 34 (53.1)c |
| Severe/Very severe | 0 (0) | 0 (0) | 20 (31.2)c |
| Dead | 0 (0) | 0 (0) | 5 (7.8)b |
Values are no. (%) except as indicated.
Presented as median (IQR), IQR: interquartile range.
Statistically significant compared to the age group 13–49.
Among the 64 patients who were older than 50 years, 35 (54.7%) were men, 14 (21.9%) had no exact history of exposure, and 43 (67.2%) had a chronic medical illness. The proportions of non-exposure history and comorbidities in this group were higher than those in the group of patients aged younger than 50 years (p < 0.05). Of the 136 enrolled patients, all 11 (8.1%) of the cases with “Severe/Very severe” illness at onset and all 5 (3.7%) fatal cases were in older (>50 years old) patients. Furthermore, only 8 (12.5%) of the older patients improved and 5 (7.8%) were discharged from the hospital; these proportions are lower than those of the patients aged younger than 50 years (p < 0.05).
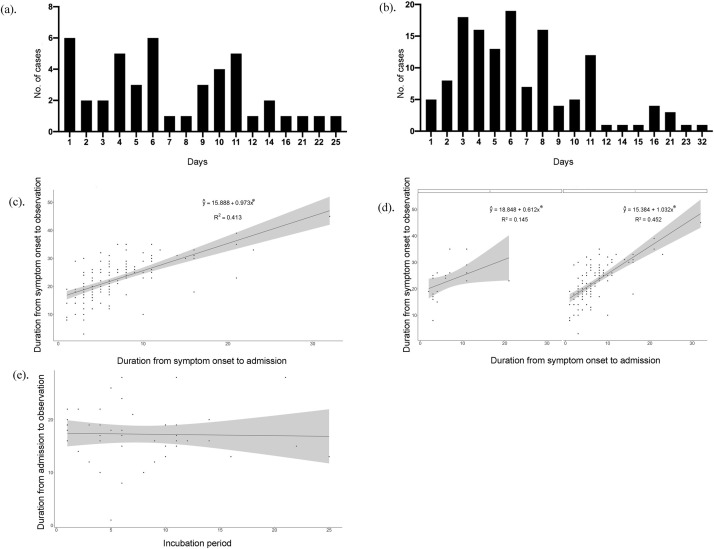
The distribution of incubation period and duration from symptom onset to admission for each patient are presented in Fig. 2 a and b. The minimum and maximum incubation periods were 1 day and 21 days, respectively. The length of time from symptom onset to admission ranged from 1 day to 32 days. The relationship between the duration from symptom onset to admission and the duration from symptom onset to observation endpoint are shown in Fig. 2c and d. An analysis of the data revealed that the duration from symptom onset to admission was positively correlated with the duration from symptom onset to observation endpoint; the regression coefficient was statistically significant (p < 0.05). In the unstratified case, the adjusted R 2 was 0.413. After stratification, the adjusted R 2 was 0.145 with no exposure history; with exposure history, the adjusted R 2 rose slightly to 0.452. Notably, there was no significant relationship detected between the incubation period and the duration from admission to observation endpoint (Fig. 2e).
Fig. 2.
Relationship between different durations of COVID-19. Distribution of incubation period. Distribution of duration from symptom onset to admission. Relationship between duration from symptom onset to admission and duration from symptom onset to the endpoint. Relationship between duration from symptom onset to admission and duration from symptom onset to the endpoint. It was stratified according to whether the patient had a history of exposure, where 0 represented none exposure history and 1 represented one or more history of exposure. Relationship between incubation period and duration from admission to the endpoint *p < 0.05.
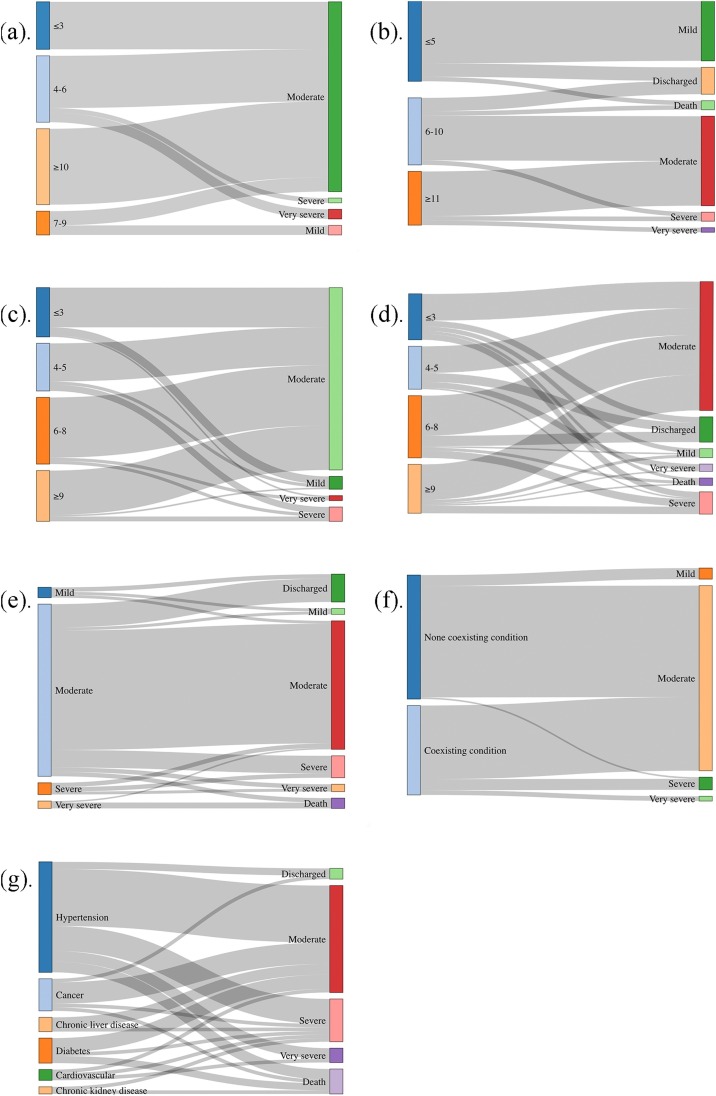
The factors found to influence the degree of symptom severity at admission and outcome at observation endpoint are shown in Fig. 3 . The incubation period of the three patients with “Severe or Very severe” illness at admission was 4–6 days, whereas the two patients with “Mild” illness at admission had an incubation period of 7–9 days. The incubation period of the 17 patients with a “Discharge or Mild” endpoint classification was less than 5 days, while that of the two patients with a “Severe or Very severe” endpoint classification was more than 11 days.
Fig. 3.
Influence factors on degree of symptoms at admission and outcomes at the endpoint. Incubation period (IP) and degree of symptoms at admission: IP was less than 3 days, and there were 10 “Moderate” patients; IP was 4–6 days, there were 11 “Moderate”, 1 “Severe”, and 2 “Very severe” patients; IP was 7–9 days, there were 2 “Mild” and 3 “Moderate” patients; IP was longer than 10 days, and there were 16 “Moderate” patients. Incubation period (IP) and outcomes at the endpoint: IP was less than 5 days, there were 3 “Discharged”, 14 “Mild” and 1 “Death” patients; IP was 6–10 days, there were 3 “Discharged”, 10 “Moderate”, 1“Severe” and 1 “Death” patients; IP was longer than 11 days, and there were 10 “Moderate”, 1“Severe” and 1 “Very severe” patients. Duration from symptom onset to admission (SOA) and degree of symptoms at admission: SOA was less than 3 days, there were 5 “Mild”, 25 “Moderate” and 1 “Very severe” patients; SOA was 4–5 days, there were 2 “Mild”, 24 “Moderate” and 4 “Severe” patients; SOA was 6–8 days, there were 38“Moderate”, 2 “Severe” and 2 “Very severe” patients; SOA was longer than 9 days, and there were 1 “Mild”, 28 “Moderate” and 3“Severe” patients. Duration from symptom onset to admission (SOA) and outcomes at the endpoint: SOA was less than 3 days, there were 4 “Discharged”, 3 “Mild”, 18 “Moderate”, 4 “Severe” and 2 “Death” patients; SOA was 4–5 days, there were 6 “Discharged”, 18 “Moderate”, 1 “Severe” and 4 “Very severe” patients; SOA was 6–8 days, there were 7 “Discharged”, 1 “Mild”, 27 “Moderate”, 5 “Severe” and 2 “Death” patients; SOA was longer than 9 days, and there were 2 “Mild”, 24 “Moderate”, 5 “Severe”, 1 “Very severe” and 1 “Death” patients. Degree of symptoms at admission and outcomes at the endpoint: of 7 “Mild” admission patients, there were 3“Discharged”, 2 “Mild” and 2 “Moderate” at the endpoint; of 118 “Moderate” admission patients, there were 16 “Discharged”, 2 “Mild”, 82 “Moderate”, 12 “Severe”, 3 “Very severe” and 3 “Death” at the endpoint; of 8 “Severe” admission patients, there were 3“Moderate”, 3 “Severe” and 2 “Very severe” at the endpoint; of 3 “Very severe” admission patients, 1“Moderate” and 2 “Death” at the endpoint. With/without coexisting condition and degree of symptoms at admission: of 79 patients without coexisting condition, there were 7 “Mild”, 71 “Moderate” and 1“Severe” at admission; of 57 patients without coexisting condition, there were 47 “Moderate”, 7 “Severe” and 3 “Very severe” at admission. Underlying diseases and outcomes at the endpoint: of 31 patients with hypertension, there were 2“Discharged”, 16 “Moderate”, 7 “Severe”, 3 “Very severe” and 3 “Death” at the endpoint; of 9 patients with cancer, there were 1“Discharged”, 6 “Moderate”, 1 “Severe” and 1 “Death” at the endpoint; of 7 patients with diabetes, there were 4“Moderate”, 1 “Severe” and 2 “Death” at the endpoint; of 4 patients with chronic liver disease, there were 3 “Moderate” and 1 “Severe” at the endpoint; of 3 patients with cardiovascular, there were 1 “Moderate”, 1 “Severe” and 1 “Very severe” at the endpoint; of 2 patients with chronic kidney disease, there were 1 “Very severe” and 1 “Death” at the endpoint.
The duration from symptom onset to admission of the seven patients with “Mild” illness at admission was less than 5 days, whereas that of the seven patients with “Severe or Very severe” illness at admission was more than 6 days. The duration from symptom onset to admission of the 13 patients with a “Discharge or Mild” endpoint classification was less than 5 days, whereas that of the seven patients with a “Severe or Very severe or Death” endpoint classification was more than 9 days.
Most of the patients with a “Mild” illness at admission had a “Discharge or Mild” endpoint. Most of the patients with a “Severe or Very severe” illness at admission had a “Severe or Death” endpoint classification. Most of the patients without a coexisting condition had “Mild” and “Moderate” illness at admission, whereas all the patients with “Severe or Very severe” illness at admission had a coexisting condition.
The cases with a “Severe” endpoint classification had hypertension, cancer, chronic liver disease, diabetes, cardiovascular, or chronic kidney disease. The cases with a “Very severe” endpoint classification had cardiovascular disease or hypertension. The cases with “Death” as the endpoint had chronic kidney disease, diabetes, cancer, or hypertension.
Discussions
In December 2019, a cluster of cases of acute respiratory illness, now known as COVID-19, occurred in Wuhan (Hubei, China). Through person-to-person transmission, COVID-19 has rapidly spread from Wuhan to other areas over the world, and the number of new cases reported outside China exceeded the number of new cases in China for the first time on February 25, 2020 [9]. It is possible that an even greater number of infected patients exist but have not yet been diagnosed because they are still within the viral incubation period or because some patients with COVID-19 experience atypical symptoms in the early stage of the disease. Based on the data from a recent epidemiological survey, more comprehensive information is required to understand the epidemiology of the COVID-19 outbreak, and further plans and policies for intervention strategies will need to be determined according to the current status.
In the present study, the incubation period for COVID-19 ranged from 1 to 25 days; however, for the patients with severe and critical symptoms, this period was 4–6 days, and for patients with mild symptoms, it was 7–9 days. Notably, there was no significant correlation between the disease severity and the incubation period. The incubation period is influenced by the exposure route, age, gender, underlying diseases, and immunity of the patients, factors which themselves may alter the pathogenic capacity of the virus and lead to different symptom severity [19].
The majority of the patients with severe or critical cases of COVID-19 had underlying diseases, whereas the patients with a mild symptom onset had no underlying diseases [20]. At the endpoint of this study, all the patients with chronic kidney disease had died or were in critical condition, half of the diabetic patients had died or were in severe condition, and more than half of those with cardiovascular disease were in severe or critical condition.
At the endpoint of this study, the incubation period was less than 5 days for all the patients with mild cases and for half of the discharged patients, whereas the incubation period for all patients with critical cases and for half of the patients with severe cases was more than 11 days. This finding is different from the findings for patients infected with the related diseases SARS and MERS, in which patients with a shorter incubation period had more severe disease outcomes. This association may be closely related to the pathogenicity and lethality of the virus. Comparing the pathogenicity of SARS-Cov, MERS and SARS-CoV-2, the previous studies have found that the higher virus titers in the early stages of SARS-Cov infection could led to infiltration of multiple inflammatory cells [21], [22]. Subsequent accumulation of cytokines produced by inflammatory cells causes “cytokine storms” and damages lung tissue [23]. For MERS, its tissue tropism is wider than other coronaviruses [24]. It widely expressed on epithelial cells through dipeptidyl peptidase 4 (DPP4) receptors in alveoli, liver, kidney, small intestine and etc. [25]. Therefore, it can also act on the production of cytokines from Multiple immune cells, which in turn induce inflammation and tissue damage [26], [27]. SARS-CoV-2 and SARS-CoV have 79% homology, so the characteristics of the two diseases are similar [28]. SARS-CoV-2 activates innate and adaptive immune responses to cause tissue damage. In severe patients, the level of serum proinflammatory cytokines rises significantly, which lead to “cytokine storm” [29]. High concentrations of cytokines cause extensive damage to various organs, leading to respiratory failure and multiple organ failure. At the same time, some studies suggest that the virus causes damage to immune organs [30]. Previous studies showed that the mortality rates of SARS and MERS infections were 9.6% and 35% [31], respectively, whereas this study found that the mortality rate of SARS-CoV-2 infection was 3.7%, and Zhong's team reported a mortality rate of 1.4% for SARS-CoV-2 infection based on the results of 1099 subjects [10]. It takes longer for the SARS-CoV-2 virus to act in the body and for clinical symptoms to develop, as reproduction and replication must first reach a certain concentration or total amount. Therefore, in terms of preventive measures, it is necessary to screen people who have a clear contact history, even if they have no apparent symptoms because they are still in the incubation period, such that intervention and treatment can be conducted in time. In particular, patients with chronic kidney disease, diabetes, or cardiovascular disease who may have been exposed to SARS-CoV-2 need early screening, diagnosis, and treatment for COVID-19.
The majority of patients with mild symptoms were admitted less than 5 days after symptom onset, whereas most patients with severe or critical symptoms were admitted more than 6 days after symptom onset, or even more than 9 days after symptom onset. The patients with severe or critical symptoms at onset usually had severe, critical, or fatal outcomes, with only a few patients showing improvement and symptom relief. In the present study, we found that the duration from symptom onset to admission was less than 8 days for discharged patients, whereas most patients whose duration from symptom onset to admission was longer than 9 days progressed into severe or critical conditions or even death. This study also found that the longer the duration from symptom onset to admission, the longer the course of illness. It was consistent with previous studies of Ebola Virus Disease (EVD) patients. The duration from symptom onset to hospital admission was positively related to the severity of EVD [32]. Therefore, it is necessary to be admitted to the hospital quickly when clinical symptoms appear, thus shortening the time between symptom onset and admission and increasing the chance of a good outcome. Additionally, timely hospitalization is also an isolation measure that can help prevent the infection of others, shorten the length of required treatment in the hospital, and reduce the overall cost.
All the children in this study were infected by family members, with an obvious familial aggregation in the pediatric cases. The onset symptoms of the pediatric patients were relatively mild, and they were discharged or improved after treatment. In contrast, most of the elderly patients did not have a clear history of exposure and could not record the time of exposure; they were infected unconsciously. The elderly patients largely suffered from underlying diseases and had generally severe onset symptoms with a poor treatment effect. In this study, all the patients who died from COVID-19 infection were over 50 years old. Therefore, as part of the prevention and treatment of COVID-19, it is essential to pay attention to vulnerable and high-risk groups, strengthen the family protection of children, and focus on publicity and education for the elderly to help them take the necessary precautions for minimizing infection risk.
According to WHO regional division, as of May 13, 2020, there were 46,829 cases in Africa, 1,743,717 cases in the Americas, 274,027 cases in Eastern Mediterranean, 1,755,790 cases in Europe, 105,901cases in South-East Asia, and 161,872 cases in the Western Pacific. Among all countries and regions, the United States has the largest number of confirmed patients, which was 1,298,287 cases [9]. In the United States, the first COVID-19 patient was reported on January 19, 2019, and the pandemic trend began in late February. In the early stage, the rapid increase of cases was caused by the (1) increase of imported cases, (2) mass gatherings and travel, (3) lack of effective isolation measures, (4) lack of protection measures in hospitals and communities and (5) the limitation of virus detection [33]. Meanwhile, some researchers believed that the insufficient investment in public health aggravated the progress of the epidemic [34]. In Europe, large-scale epidemics first occurred in Italy, and then quickly spread in Spain, Germany, Britain, France and other places. In the initial stage, the reasons for the emergence of pandemic were very similar to those in the United States. Subsequently, different countries adopted different measures to deal with this public health crisis. For example, Italy blocked cities nationwide [35], and the Britain recommends that citizens isolate themselves to reduce the risk of infection [36]. Europe countries and United States tend to use a mitigation strategy to achieve herd immunization, which has led to a rapid rise in number of confirmed cases and caused supercritical strain on public health system, but it is possible for citizens to gain lasting immunity. Countries in the Asian region have adopted rapid and more severe countermeasures to curb the spread of the virus. In South Korea, after a religious rally in Daegu City led to a large-scale epidemic, the government quickly took action to track infected patients by smartphone application and rigorously tested for viruses [37]. In Japan, the government recommended closing schools, entry ban of citizen from high-risk areas and canceled sports or cultural events. In addition, the habit of wearing masks during the seasonal influenza helped to control the epidemic [38]. In the face of global pandemic of SARS-Cov-2, the strategies in Europe countries and United States and strategies in East Asia have their own advantages. Therefore, strengthening cooperation between countries is essential to control the spread of the epidemic. Avoid patients who have been diagnosed leaving the country and entering other countries. Prohibiting large-scale aggregation events helps reduce the spread of the epidemic in the country. Expanding the scope of polymerase chain reaction (PCR) testing to identify potential infected persons. At the same time, medical assistance to less developed areas such as Africa should be increased to reduce the risk of new outbreak.
This study focused on describing the characteristics of the incubation period, duration from symptom onset to admission, and high-risk susceptible populations for COVID-19 infection. Due to the limited sample size, some associations were not statistically significant. And the sample size of children patients is small, so there may be some limitations on the epidemiological characteristics of children patients’ symptoms. However, the rapid spread of COVID-19 around the world at present has a serious impact on global health, and the findings of this study will provide a basis for the formulation of public health strategies and measures.
Funding
This work was supported by COVID-19 epidemic prevention and control releated research project of China Medical University.
Competing interests
We declare no competing interest.
Ethical approval
This study was approved by the ethics committee of The People's Hospital of Jingzhou (42016830-T).
Authors’ contribution
YX, XZ, LL, WZ, TX, CZ, and YC collected the epidemiological and clinical data and CZ and YZ processed statistical data. YX and XZ were responsible for summarizing all epidemiological and clinical data. MH and WX drafted the manuscript. JZ revised the final manuscript.
Acknowledgments
We thank all patients involved in the study.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X., Tian J., Li G., Li G. Initiation of a new infection control system for the COVID-19 outbreak. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30110-9. [published online February 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu P., Hao X., Lau E.H.Y., Wong J.Y., Leung K.S.M., Wu J.T. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25(3):2000044. doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus disease (COVID-19) outbreak; 2019. https://www.who.int [accessed 16.03.20].
- 5.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):61–69. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Health Commission of the People's Republic of China home page; 2020. http://www.nhc.gov.cn [accessed 13.05.20]. [DOI] [PMC free article] [PubMed]
- 8.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Coronavirus disease (COVID-2019) situation reports; 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [accessed 13.05.20].
- 10.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deslandes A., Berti V., Tandjaoui-Lambotte Y., Alloui C., Carbonnelle E., Zahar J.R. Int J Antimicrob Agents. SARS-COV-2 was already spreading in France in late December 2019. 2020:106006. doi: 10.1016/j.ijantimicag.2020.106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [published online February 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X., Wu X., Jiang X., Xu K., Ying L., Ma C. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa199. [published online February 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.018. [published online February 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [published online February 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance; January 28, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novelcov.pdf [accessed 29.02.20].
- 19.Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020:9. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(9371):1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widagdo W., Raj V.S., Schipper D., Kolijn K., van Leenders G.J.L.H., Bosch B.J. Differential expression of the middle east respiratory syndrome coronavirus receptor in the upper respiratory tracts of humans and dromedary camels. J Virol. 2016;90(9):4838–4842. doi: 10.1128/JVI.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu H., Zhou J., Wong B.H., Li C., Cheng Z.S., Lin X. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454–455:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J., Chu H., Li C., Wong B.H., Cheng Z.S., Poon V.K. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis. 2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui D.S., Azhar I., Madani E., Ntoumi T.A., Kock F., Dar R.O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theocharopoulos G., Danis K., Greig J., Hoffmann A., De Valk H., Jimissa A. Ebola management centre proximity associated with reduced delays of healthcare of Ebola Virus Disease (EVD) patients, Tonkolili, Sierra Leone, 2014–15. PLoS One. 2017;12:e0176692. doi: 10.1371/journal.pone.0176692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuchat A. CDC COVID-19 response team. Public health response to the initiation and spread of pandemic COVID-19 in the United States, February 24–April 21, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):551–556. doi: 10.15585/mmwr.mm6918e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maani N., Galea S. COVID-19 and underinvestment in the public health infrastructure of the United States. Milbank Q. 2020 doi: 10.1111/1468-0009.12463. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebastiani G., Massa M., Riboli E. Covid-19 epidemic in Italy: evolution, projections and impact of government measures. Eur J Epidemiol. 2020;35(4):341–345. doi: 10.1007/s10654-020-00631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haines A., de Barros E.F., Berlin A., Heymann D.L., Harris M.J. National UK programme of community health workers for COVID-19 response. Lancet. 2020;395(10231):1173–1175. doi: 10.1016/S0140-6736(20)30735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.COVID-19 National Emergency Response Center, Epidemiology & Case Management Team, Korea Centers for Disease Control & Prevention Contact transmission of COVID-19 in South Korea: novel investigation techniques for tracing contacts. Osong Public Health Res Perspect. 2020;11(1):60–63. doi: 10.24171/j.phrp.2020.11.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada K., Oka-Ezoe K., Smith D.R. Wearing face masks in public during the influenza season may reflect other positive hygiene practices in Japan. BMC Public Health. 2012;12:1065. doi: 10.1186/1471-2458-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]