Abstract
The rapid development of global COVID-19 pandemic poses an unprecedented challenge to the safety and quality of laboratory diagnostic testing. Little is known about the laboratory surface areas and operation behaviors that may cause potential contamination in SARS-CoV-2 nucleic acid testing. This study aims to provide reference basis for the improvement of laboratory disinfection programs and personal operating protocols. In this study, we compared the qRT-PCR and ddPCR in detecting of residual virus that existed on the object surfaces from sample transportation and reception related facilities, testing related instruments, personal protective equipment and other facilities in nucleic acid testing laboratory. All samples were negative by qRT-PCR, in contrast, 13 of 61 samples were positive for SARS-CoV-2 by ddPCR. The areas with highest density of SARS-CoV-2 nucleic acid were the outer gloves of operator A (37.4 copies/cm2), followed by door handle of 4 °C refrigerator (26.25 copies/cm2), goggles of operator A (22.16 copies/cm2), outer cover of high speed centrifuge (19.95 copies/cm2), inner wall of high speed centrifuge (14.70 copies/cm2) and others. We found that all the positive objects were directly or indirectly contacted by the operator's gloved hands, suggesting that hands contact was the main transmission pathway that led to laboratory environmental contamination. In summary, ddPCR has an advantage over qRT-PCR in tracing laboratory contamination. We evaluated the risk areas and operation behaviors that may easily cause contamination, and provided recommendation for improving the laboratory disinfection programs and personal operating specifications.
Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; BSL-2, biosafety second-level laboratory; qRT-PCR, real-time quantitative PCR; ddPCR, droplet digital PCR
Keywords: SARS-CoV-2, Nucleic acid residue, Laboratory environment, Contamination, ddPCR
Graphical abstract
Hands contact was the main transmission pathway that led to laboratory environmental contamination, during testing on SARS-CoV-2 in BSL-2.
1. Introduction
COVID-19 is highly infectious and causes relatively high mortality especially among the elderly and people with underlying conditions (Boccia et al., 2020; Studdert and Hall, 2020; Liu et al., 2020a; Koff and Williams, 2020). According to the World Health Organization, as of June 16, 2020, the COVID-19 pandemic has spread to 216 countries areas or territories, and resulted in 7,941,791 confirmed cases with 434,796 deaths. SARS-CoV-2 as the causative pathogen of the COVID-19 outbreak was first sequenced and identified by Chinese scientists in early January 2020 (Tian et al., 2020; ICTV, 2020; Li et al., 2020).
Nucleic acid detection of SARS-CoV-2 is the main method for confirming cases of COVID-19. The detection work needs to be carried out in the negative pressure BSL-2, and operators should wear proper personal protective equipment (Ong et al., 2020).
The SARS-CoV-2 is transmitted mainly through human respiratory droplets and contact. Due to the lack or improper use of personal protective equipment, in many countries including China, a large number of healthcare workers on the front line have been infected with SARS-CoV-2 (Ranney et al., 2020; Yu et al., 2020).
Effective disinfection of the environment for nucleic acid testing laboratory and good operation habits are essential to ensure the detection quality and personal safety. The operator needs to understand the possible contamination areas of a nucleic acid testing laboratory to take appropriate disinfection measures. However, until now, little is known about the laboratory surface areas and operation behaviors that may cause potential contamination in SARS-CoV-2 nucleic acid testing. In this study, we aimed to 1) determine the concentration of SARS-Cov-2 present on the object surfaces and personal protective equipment after the nucleic acid test, 2) identify the risk areas and operation behaviors that may cause contamination, and 3) provide reference basis for the targeted formulation of laboratory disinfection programs and personal operating specifications.
2. Methods
2.1. Sample collection
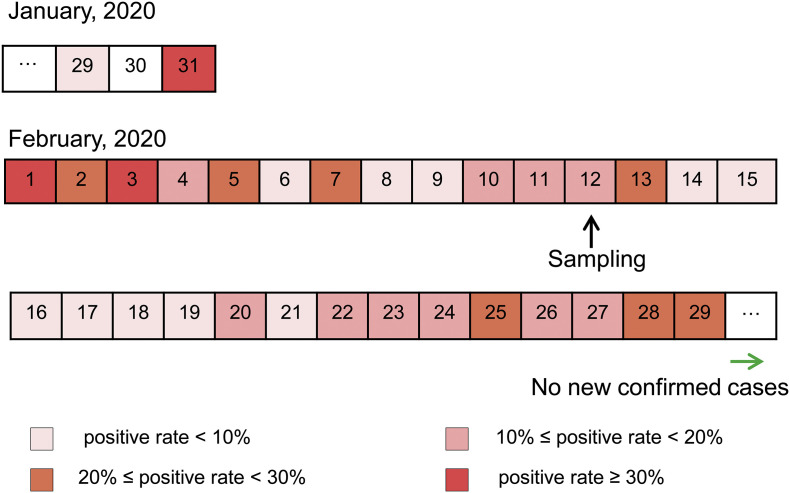
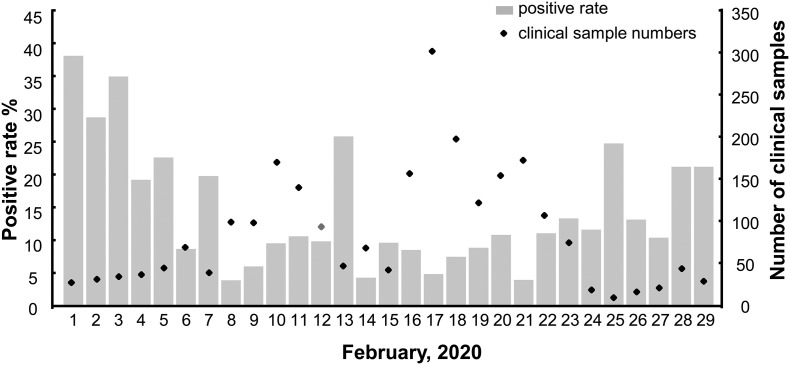
Taihe Hospital is a Grade A Class Three general hospital in Shiyan City, Hubei Province, China. The testing team carried out nucleic acid detection of SARS-CoV-2 since January 23, 2020. Screening of suspected COVID-19 cases was mainly in February and no new cases were confirmed since March 1 (Fig. 1 ). In February, the average daily sample size and positive rate were 86 and 10.57%, respectively. The sampling of nucleic acid testing laboratory environment was conducted on February 12, 2020, on which day the sample size and positive rate was close to the average (Fig. 2 ).
Fig. 1.
Timeline of the positive rate of nucleic acid test for clinical samples. Dates filled in different colors show indicated daily positive rates. The black arrow indicates the date we collected environmental samples in the nucleic acid testing laboratory and the green arrow indicates that there are no new confirmed cases from that day. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Total number and the positive rate of nucleic acid test for clinical samples in February 2020. Diamonds indicate the total number of the testing samples collected clinically. Gray boxes represent the percentages of positive rates.
After the SARS-CoV-2 nucleic acid test for clinical case samples, four types of samples from the nucleic acid testing laboratory, including sample transportation and reception related facilities, testing related instruments, personal protective equipment, and other facilities, were collected. A total of 61 samples were shown in Table 1 .
Table 1.
List of sample information and detection results.
| Sample types | Sample collection points | Sampling area (cm2) | Detected by qPCR (CT) | Detected by ddPCR |
|
|---|---|---|---|---|---|
| Copies/reaction | Copies/cm2 | ||||
| Sample transportation and reception related facilities | Door handle | 60 | − | 0 | 0 |
| Upper elevator button | 10 | − | 0 | 0 | |
| Button in elevator | 10 | − | 0 | 0 | |
| Down elevator button | 10 | − | 0 | 0 | |
| Handle of sample transport box A | 110 | − | 1.8 | 0.86 | |
| Inner wall of sample transport box A | 100 | − | 0 | 0 | |
| Handle of sample transport box B | 110 | − | 0 | 0 | |
| Inner wall of sample transport box B | 100 | − | 0 | 0 | |
| Handle of sample transport box C | 110 | − | 0 | 0 | |
| Inner wall of sample transport box C | 100 | − | 5 | 2.63 | |
| Handle of sample transport box D | 110 | − | 0 | 0 | |
| Inner wall of sample transport box D | 100 | − | 0 | 0 | |
| Sample handover record book | 100 | − | 0 | 0 | |
| Outside door handle of transfer window | 45 | − | 0 | 0 | |
| Inner wall of transfer window | 100 | − | 0 | 0 | |
| Inside door handle of transfer window | 45 | − | 0 | 0 | |
| Testing related instruments | Button of BSC | 15 | − | 0 | 0 |
| Door handle of BSC | 112 | − | 1.8 | 0.84 | |
| Cleaning area of BSC countertop | 100 | − | 0 | 0 | |
| Operation area of BSC countertop | 100 | − | 0 | 0 | |
| Contaminated area of BSC countertop | 100 | − | 0 | 0 | |
| Inner wall of BSC | 100 | − | 0 | 0 | |
| Inside top of BSC | 100 | − | 0 | 0 | |
| 1 mL pipette | 190 | − | 3.2 | 0.88 | |
| 200 μL pipette | 190 | − | 4.2 | 1.16 | |
| 10 μL pipette | 190 | − | 1.8 | 0.50 | |
| Metal bath | 100 | − | 0 | 0 | |
| Outer cover of high speed centrifuge | 100 | − | 38 | 19.95 | |
| Inner cover of high speed centrifuge | 100 | − | 0 | 0 | |
| Rotor of high speed centrifuge | 100 | − | 0 | 0 | |
| Inner wall of high speed centrifuge | 100 | − | 28 | 14.70 | |
| Cover of handheld centrifuge | 100 | − | 0 | 0 | |
| Door handle of 4 °C refrigerator | 120 | − | 60 | 26.25 | |
| Interior of 4 °C refrigerator | 100 | − | 0 | 0 | |
| Door handle −20 °C refrigerator | 120 | − | 0 | 0 | |
| Interior of −20 °C refrigerator | 100 | − | 0 | 0 | |
| Autoclave handle | 100 | − | 0 | 0 | |
| Inner wall of autoclave | 100 | − | 0 | 0 | |
| basket in autoclave | 100 | − | 0 | 0 | |
| Eye washer | 96 | − | 0 | 0 | |
| Faucet | 70 | − | 0 | 0 | |
| Personal protective equipment | Outer gloves of operator A | 73 | − | 52 | 37.4 |
| Outer gloves of operator B | 73 | − | 2.2 | 1.58 | |
| Goggles of operator A | 90 | − | 38 | 22.16 | |
| Goggles of operator B | 90 | − | 0 | 0 | |
| Protective clothing of operator A | 100 | − | 0 | 0 | |
| Protective clothing of operator B | 100 | − | 0 | 0 | |
| Safety Shoes of operator A | 100 | − | 0 | 0 | |
| Safety Shoes of operator B | 100 | − | 0 | 0 | |
| Protective mask of operator A | 70 | − | 7 | 5.25 | |
| Protective mask of operator B | 70 | − | 0 | 0 | |
| Inner gloves of operator A | 73 | − | 0 | 0 | |
| Inner gloves of operator B | 73 | − | 0 | 0 | |
| Surgical hats of operator A | 100 | − | 0 | 0 | |
| Surgical hats of operator B | 100 | − | 0 | 0 | |
| Other facilities | Contaminated area floor | 100 | − | 0 | 0 |
| Contaminated area table | 100 | − | 0 | 0 | |
| Semi-contaminated area floor | 100 | − | 0 | 0 | |
| Clean area floor | 100 | − | 0 | 0 | |
| Chair A | 100 | − | 0 | 0 | |
| Chair B | 100 | − | 0 | 0 | |
BSC, biological safety cabinet; −, negative.
If the surface area of an object exceeded 100 cm2, the sampling area of the object surface was limited to 100 cm2, otherwise the entire surface was sampled. A 5 cm × 5 cm standard specification board was placed on the surface of the object, then a sterile cotton swab soaked with viral transport medium (Yocon, Cat: MT0301) was used to wipe the specification plate for 5 times. After four specification board areas were sampled continuously, the cotton swab was cut off from the hand contact part and put into a test tube containing 3.5 mL of viral transport medium. For small objects such as door handles, faucets, and pipettes, a sterile cotton swab soaked with viral transport medium was used to wipe the entire surface, and the surface areas were estimated.
2.2. Analytical methods
qRT-PCR and ddPCR were applied to detect SARS-CoV-2 simultaneously.
2.2.1. RNA extraction
After collection, all samples were processed immediately in the BSL-2 of Taihe Hospital. The sample tubes were gently shaken for 1 min to elute the virus into preservation solution. RNA was extracted from the elution using the Viral Nucleic Acid Isolation Kit (Bioperfectus, Cat: SDK60102) according to the manufacturer's instruction.
2.2.2. qRT-PCR
qRT-PCR was carried out using the Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (Sansure Biotech, Cat: 20203400064) for amplifying specific genes (ORF1ab and N). The amplification reaction has a total volume of 25 μL containing 10 μL RNA template. The reaction conditions were: 50 °C 30 min for reverse transcription, then 95 °C 1 min for pre-denaturation, followed by 45 cycles of 95 °C 15 s for denaturation and 60 °C 30 s for annealing, extension and fluorescent signals collection. Cycle threshold (Ct) ≤ 40 was interpreted as positive, and Ct > 40 as negative.
2.2.3. ddPCR
The primers specific for the ORF1ab and N gene targeting the SARS-CoV-2 were adopted from Chinese center for disease control and prevention (CDC). The ddPCR was performed on the Bio-rad QX200 system with manufacturer's instructions. The reaction mixtures (20 μL) contained 5 μL of 2× One-Step RT-ddPCR Advanced Kit for Probes (Bio-rad, 1864021), 2 μL of reverse transcriptase, 1 μL of 300 mM DTT, 900 nM of target primers, 250 nM of probe, and 10 μL of extracted RNA. For the detection and quantitative enumeration of SARS-CoV-2, the mixture was transferred into the DG8 cartridge with 70 μL of droplet generation oil for probes (Bio-rad, 1863005) to generate droplets. It took about 2 min to generate a set of eight processed samples by the using QX200TM Droplet Generator. Then the PCR reaction was performed using the following cycling protocol: 42–50 °C 60 min for reverse transcription, 95 °C 10 min for enzyme activation, 40 cycles of 95 °C 30 s for denaturation, 60 °C 1 min for annealing and extension, and 98 °C 10 min for enzyme deactivation. The fluorescence was acquired by QX200TM Droplet Reader after amplification and the output data were analyzed using Quanta Soft TM analysis software. The detection threshold and positive samples were determined by the negative and positive control.
3. Results and discussion
3.1. SARS-CoV-2 RNA residues on object surfaces
The SARS-CoV-2 test results of object surface samples from nucleic acid detection laboratory were shown in Table 1. Test results for all samples (n = 61) were negative by qRT-PCR. In contrast, 13 out of 61 samples were positive by ddPCR. The highest concentration of SARS-CoV-2 RNA molecules was from outer gloves of operator A (37.4 copies/cm2), followed by the door handle of 4 °C refrigerator (26.25 copies/cm2), goggles of operator A (22.16 copies/cm2), outer cover of high speed centrifuge (19.95 copies/cm2), inner wall of high speed centrifuge (14.7 copies/cm2), protective mask of operator A (5.25 copies/cm2), inner wall of sample transport box C (2.63 copies/cm2), outer gloves of operator B (1.58 copies/cm2), 200 μL pipette (1.16 copies/cm2), 1 mL pipette (0.88 copies/cm2), 10 μL pipette (0.5 copies/cm2), door handle of biological safety cabinet (0.84 copies/cm2) and handle of sample transport box A (0.86 copies/cm2) (Table 1). All the positive objects were directly or indirectly contacted by the operator's gloved hands.
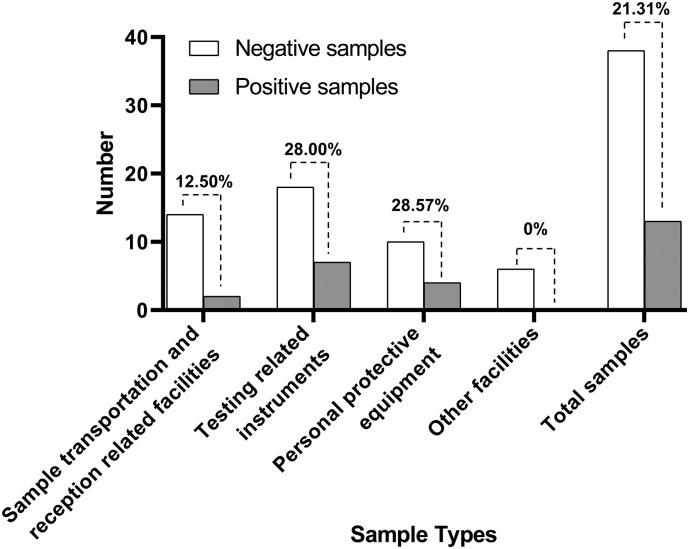
3.2. Positive rate of different sample types
The results of ddPCR detection for SARS-CoV-2 by sample type are shown in Fig. 3 . While the overall positive rate was 21.31%, personal protective equipment had the highest positive rate of 28.57%. Lower rates were found in testing related instruments (28%) and sample transportation and reception related facilities (12.5%). All six samples belonging to other facilities were negative for SARS-CoV-2 by ddPCR.
Fig. 3.
Positive rate of different sample types. The number on the dotted line represents the positive rate.
3.3. Analysis of causes of laboratory contamination and suggestions for improvement
How to ensure the safety of medical staff as much as possible is one of the key factors for the prevention and control of COVID-19. Nucleic acid detection of SARS-CoV-2 currently is the most accurate and direct method for the diagnosis of COVID-19 and has been widely used by most countries in the world (Corman et al., 2020; Yan et al., 2020). In addition to ensuring adequate access to personal protective equipment, thorough disinfection of the BSL-2 and proper use of personal protective equipment are very important to protect operators from infection (Chen et al., 2020).
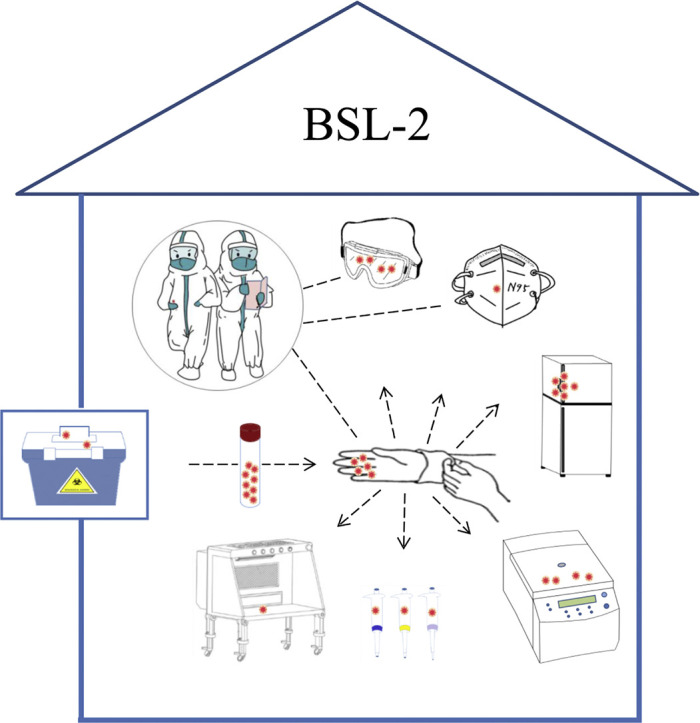
In this study, all objects in nucleic acid detection laboratory that tested positive for SARS-CoV-2 were directly or indirectly contacted by the operator's gloved hands. The outer gloves of operator also showed the highest viral RNA concentration. These results supported the notion that hand contact transmission was the main way of laboratory environmental contamination.
The second highest concentration of SARS-CoV-2 was found on the door handle of 4 °C refrigerator, while the door handle of −20 °C refrigerator was negative. The upper part of the refrigerator was 4 °C for storing samples temporarily, and the lower part was −20 °C for storing reagents. After an initial test, clinical samples were usually packaged and temporarily stored in a refrigerator at 4 °C for several hours to wait for the decision to test again or destroy. The concave of refrigerator door handle has a shadow area that couldn't be irradiated by ultraviolet rays, and the SARS-CoV-2 might be concentrated there. In order to solve this problem, the operators were recommended to wipe the refrigerator door handle with disinfectant to kill the virus after each detection immediately.
The high-speed centrifuge was another instrument that was easily contaminated. In this research, both the outer cover and the inner wall were contaminated with SARS-CoV-2. After detection, the outer cover of the centrifuge was often closed by operators, and disinfected through disinfectant wipe and ultraviolet radiation. However, the inner wall of the centrifuge was usually ignored. It was suggested that the inner wall should be disinfected in the same way to eliminate the virus.
Pipettes of various ranges were the most frequently used instruments in nucleic acid detection of SARS-CoV-2. Therefore, it was not surprising to detect SARS-CoV-2 from the pipette surfaces. From high to low, the virus concentration on the surfaces was 200 μL pipette, 1 mL pipette, and 10 μL pipette, and this order was consistent with the order of the pipette use during nucleic acid extraction.
Nucleic acid detection for SARS-CoV-2 usually requires two laboratory technicians to operate together. They examine each other's personal protective equipment, check sample information, and cooperate with each other during operations. Different operating habits will cause different levels of contamination in personal protective equipment. In this study, the protective mask, goggles and outer gloves of operator A were positive for SARS-CoV-2, while for operator B, SARS-CoV-2 could only be detected from the outer gloves. Our subsequent investigation indicated operator A's mask and goggles were not worn properly, causing goggles to become fogged which affected the sight. Therefore, operator A had to adjust the mask and goggles repeatedly with gloved hands during operation. As a result, the mask and goggles of operator A were contaminated by SARS-CoV-2 from the outer gloves. This indicated that it is very important to wear personal protective equipment correctly before entering into BSL-2. During the operation, one should avoid using gloved hands to touch one's own masks, goggles, and other personal protective equipment.
3.4. Advantage of ddPCR in tracing laboratory contamination and limitation of this study
In this study, we analyzed the qRT-PCR results of clinical samples and found that the average Ct value of human endogenous reference gene and viral target gene were 21.78 and 31.84, respectively. For object surface detection, 46 out of 61 samples tested positive for human endogenous reference gene by qRT-PCR, with the average Ct value being 38.36, which nearly approached the threshold (40) of the kit. It indicated that the amount of contaminated samples on the surface of the object was extremely small. Moreover, positive rate of clinical samples and the proportion between human endogenous reference gene and viral target gene would make it almost impossible to detect viral gene by qRT-PCR, which was supported by the results of this study with all samples testing negative for SARS-CoV-2. In contrast, 21.31% of samples were positive for SARS-CoV-2 with ddPCR, which suggested that ddPCR was more sensitive (Hindson et al., 2013; Liu et al., 2020b) and has broad application prospects in detecting extremely small amounts of samples.
The culture of SARS-CoV-2 is required to be conducted in a BSL-3 according to the biosafety requirements. There was only BSL-2 facility in this hospital, a situation likely to be true in most inpatient hospitals globally, which precluded the determination of whether the detected SARS-CoV-2 was still alive. Nevertheless, the test results could still show the high-risk areas contaminated by SARS-CoV-2 in nucleic acid detection operation, which indicate that all the operators need to develop good operating habits and thoroughly disinfect the laboratory without blind areas after the experiment. Fortunately, during the entire COVID-19 outbreak, all the operators of nucleic acid testing in this hospital benefited from good laboratory conditions and proper use of personal protective equipment and no laboratory-acquired infections were found.
CRediT authorship contribution statement
Jun Lv:Conceptualization, Methodology, Writing - original draft.Jin Yang:Funding acquisition, Investigation, Formal analysis.Juan Xue:Software, Data curation.Ping Zhu:Investigation, Visualization.Lanfang Liu:Resources, Validation.Shan Li:Project administration, Writing - review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgment
This work was supported by the National Key Research and Development Programs of China 2018YFA0508000, Fundamental Research Funds for the Central Universities 2662017PY011, 2662018PY028, 2662019YJ014, Talent funding RCQD002 from Taihe Hospital to SL.
Editor: Kevin V. Thomas
References
- Boccia S., Ricciardi W., Ioannidis J.P.A. What other countries can learn from Italy during the COVID-19 pandemic. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.1447. [DOI] [PubMed] [Google Scholar]
- Chen Q., Liang M., Li Y., Guo J., Fei D., Wang L., et al. Mental health care for medical staff in China during the COVID-19 outbreak. Lancet Psychiatry. 2020;7:e15–e16. doi: 10.1016/S2215-0366(20)30078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson C.M., Chevillet J.R., Briggs H.A., Gallichotte E.N., Ruf I.K., Hindson B.J., et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods. 2013;10:1003–1005. doi: 10.1038/nmeth.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICTV The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff Wayne C., Williams Michelle A. Covid-19 and immunity in aging populations — a new research agenda. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney Megan L., Griffeth Valerie, Jha Ashish K. Critical supply shortages — the need for ventilators and personal protective equipment during the Covid-19 pandemic. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- Studdert David M., Hall Mark A. Disease control, civil liberties, and mass testing —calibrating restrictions during the Covid-19 pandemic. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2007637. [DOI] [PubMed] [Google Scholar]
- Tian H.Y., Liu Y.H., Li Y.D., Wu C., Chen B., Kraemer Moritz U.G. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368(6491):638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Chang L., Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): current status, challenges, and countermeasures. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Ding N., Chen H., Liu X.J., He W.J., Dai W.C., et al. Infection control against COVID-19 in departments of radiology. Acad. Radiol. 2020;27:614–617. doi: 10.1016/j.acra.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]