Abstract
Corona Virus Disease 2019 (COVID-19) pandemic is rapidly spreading all over the world. Excessive immune responses trigger life-threatening cytokine release syndrome (CRS) which can result in overproduction of pro-inflammatory cytokines including tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and IL-1β with different pro-inflammatory roles. Anecdotal evidence suggests that the modulation of systemic immune responses may have a potential role in the treatment of patients with COVID-19. Given the importance of the issue and the lack of therapeutic treatment or vaccine; anti-cytokine therapy such as IL-6, TNFα and IL-1 antagonists have been suggested for the alleviation of hyper-inflammation status in these patients. In this mini-review, we addressed the inflammatory pathways of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its relationship with the host cytokine storm. Furthermore, the proposed therapeutic options to reverse hyper-inflammation in infected patients were mentioned.
Key Words: COVID-19, SARS-CoV-2, Cytokine storm, IL-6, TNFα, IL-1β
Introduction
Prevalence, Taxonomy and Structural Biology of SARS-CoV-2
In December 2019, coronavirus disease 2019 (COVID-19) outbreak commenced in Wuhan, Hubei province, China and spread rapidly to other provinces in China and several countries all over the world (1). This infectious disease has been identified by the World Health Organization (WHO) as a global pandemic. Based on the WHO declaration on January 30, 2020, the outbreak of COVID-19 is a global health emergency of international concerns due to its capability of rapid human-to-human transmission (2). The mortality rate of COVID-19 is terribly growing worldwide. As of April 16, 2020, this severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected a total of 2,060,927 confirmed cases with 134,354 deaths in more than 200 countries and territories (3).
SARS-CoV-2 is a novel β-coronavirus that belongs to the subfamily Orthocoronavirinae in the family of Coronaviridae, in the order of Nidovirales. The SARS-CoV-2 is an enveloped positive-sense single-stranded RNA (+ssRNA) virus with 4 major proteins in its structure, including the spike (S) protein (which mediates attachment to the host receptor and subsequent fusion of the virus and cell membrane), the membrane (M) protein, the envelope (E) protein, and the nucleocapsid (N) protein (4). Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are other β-coronavirus widespread epidemics in 2002 and 2012, respectively (5). Based on recent pieces of evidence, SARS-CoV-2 has approximately 79–82% similarity to human SARS-CoV genome at the nucleotide sequences. It has been supposed that the SARS-CoV-2 virus, similar to SARS CoV, uses the angiotensin-converting enzyme 2 (ACE2) as the receptor for entering host cells (6). ACE2 receptors are expressed in the membrane of various cells in the human body, including type II alveolar epithelial cells of the lung (7). Accordingly, it is expected that the bilateral diffuse alveolar injury with cytodiagnosis of myxoid fibroma exudate is the first pathological finding of COVID-19 (8). Furthermore, kidney, intestine, heart, and blood vessels are the other organs expressing ACE2 receptor, and this may describe why some patients with COVID-19 (∼46%) experience renal, gastrointestinal, and cardiovascular problems (9,10). The spike glycoprotein (S) mediates virus entry via binding to the host cell's ACE2 receptor and membrane fusion (11). It is supposed that the interaction of the viral particle with specific proteins on the host cell surface and entering of the virus to human cells are the main triggers, which initiate infection and inflammatory cascade through various mechanisms with consequent release of pro-inflammatory cytokines (6,12). To the best of our knowledge the main clinical features of patients with COVID-19 consist of a) high concentration of inflammatory parameters such as C-reactive protein (CRP) and pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNFα), etc.; b) Infiltration of immune cells to the lung lesion mostly monocytes and macrophages; c) Destruction of the immune system owing to atrophy of spleen and lymph nodes; d) Reduction of lymphocytes (lymphopenia) in lymphoid organs; and ultimately; e) Vasculitis, hypercoagulability, and multiple organs damage (13).
The term “cytokine release syndrome” (CRS) or cytokine storm syndrome (CSS) was defined as a systemic inflammatory response, which can occur by a variety of factors, including infections and certain drugs (14). In this review, we addressed the inflammatory pathways of SARS-CoV-2 and its relationship with the host cytokine storm. Furthermore, the proposed therapeutic options to reverse hyper-inflammation in infected patients were mentioned. The understanding of molecular mechanisms involved in this viral infection provides new insights into the more appropriate management of COVID-19.
Inflammatory Pathways of SARS-CoV-2
Inflammation is the immediate body's defense against infection or trauma injury; however, it can be considered a double-edged sword. Inflammation with activation of both innate and adaptive immune responses can enhance host immunity against infection, while excessive immune responses following some pathogens such as the influenza virus, trigger life-threatening CSS in the host, which can result in excessive production of pro-inflammatory cytokines and eventually, leads to death (15,16). Cytokines are a group of secreted proteins that are released by some cells with specific effects on the development, differentiation, and regulation of immune cells (17).
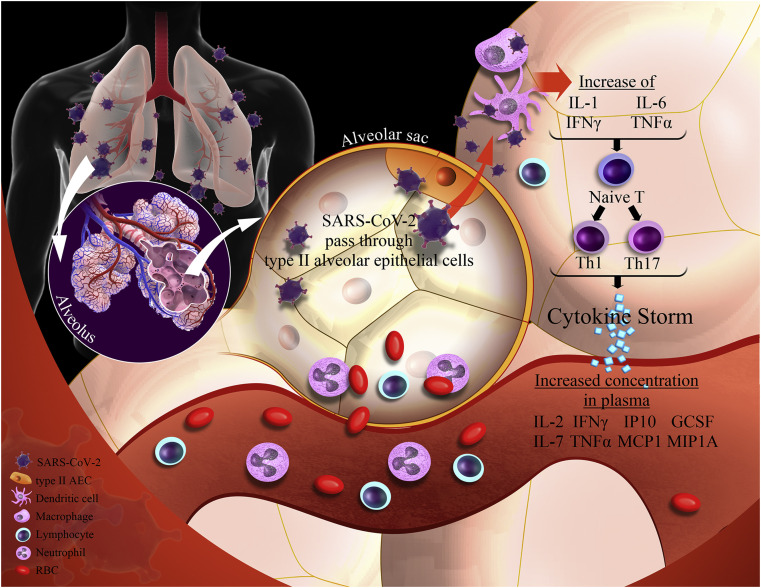
According to recent studies, SARS-CoV-2 stimulates innate and adaptive immune systems after binding to type II alveolar epithelial cells of the lung; the over-stimulated response of the immune system may cause more damage to the host cells than the SARS-CoV-2 as a foreign invader (18). Toll-like receptors (TLRs), a subfamily of pattern recognition receptors (PRRs), can recognize “danger signals” such as SARS-CoV-2 viruses in the extracellular milieu and endosomes and mediate the inflammatory signaling cascade that leads to the activation and production of inflammatory cytokines (19). Inflammasomes are multi-protein cytosolic molecular platforms comprised of caspase-1, Nod-like receptors (NLRs; i.e., NLRP1, NLRP3, and NLRC4) and apoptosis-associated speck-like protein containing a CARD (Caspase activating and recruitment domain or ASC) adaptor protein (20). The cleavage and secretion of pro-IL-1β and pro-IL-18 into bioactive cytokines, caused by the activation of the NLRP3 inflammasome, is a powerful determinant in the inflammatory status (21). On the other hand, TLR activation following a viral infection can induce the production of IL-6 by monocytes and macrophages. TLRs, TNFα, and IL-1β are known as the main stimulators for the synthesis of IL-6 (22). IL-6 is the main regulator of T cells. It can promote the development and function of Th17 cells (a population of CD4+ T helper cells), which may serve as pro-inflammatory self-reactive T cells (23). In addition, IL-6 can induce the production of some acute phase proteins such as CRP (24). Figure 1 shows the proposed pathway for inducing cytokine storm following SARS-Cov-2 infection.
Figure 1.
A proposed pathway for inducing cytokine storm following SARS-Cov-2 infection. SARS CoV-2 virus uses the angiotensin-converting enzyme 2 (ACE2) as the receptor for entering the cells. After entering to type II alveolar epithelial cells of the lungs, SARS CoV-2 triggers life-threatening cytokine release in the host, which can result in excessive levels of pro-inflammatory cytokines production including IL-6, TNF-α and IL-1β. Type II AEC, type II alveolar epithelial cells; RBC, red blood cells; IL, interleukin; TNF α, tumor necrosis factor α; IFN-γ, interferon-gamma; GCSF, granulocyte-colony stimulating factor; IP-10, interferon γ-induced protein; MCP1, monocyte chemo-attractant protein 1; MIP, macrophage inflammatory proteins; Th, T helper.
The results of a recent meta-analysis of nine studies showed that the IL-6 levels were significantly increased in COVID-19-infected patients with severe conditions (SMD = 0.71, 95%CI –0.31 to 1.12, p = 0.0005) (25). Ruan and colleagues showed the elevated level of IL-6 in critically ill individuals with COVID-19 (26). Similarly, other reports also established the increased level of IL-6 in patients with confirmed COVID-19 pneumonia and it has been shown that the level of IL-6 has a positive correlation with the severity of the disease (4,27,28).
In another study, Huang C, et al. (29) reported that the plasmatic concentration of IL-2, IL-7, interferon-gamma (IFN-γ), granulocyte-colony stimulating factor (GCSF), interferon γ-induced protein 10 kDa (IP-10), monocyte chemo-attractant protein 1 (MCP1), macrophage inflammatory proteins (MIP), and TNF-α were higher in 41 admitted severe patients with confirmed COVID-19 in Wuhan. Interestingly, they found that SARS-CoV-2 infection also initiated an increased secretion of Th2-related cytokines such as IL-4 and IL-10 for suppressing hyper-inflammation. The same researchers in another multicenter cohort study reported an increased concentration of IL-6 in non-survival group of patients with COVID-19 (30).
Interleukin-1 family with different pro-inflammatory roles has been considered in patients with COVID-19 due to their important role in CSS symptoms, including fever, edema, and finally, organ dysfunction or death. Interestingly, Huang C, et al. reported an elevated level of IL-1β in severe patients with COVID-19 (29).
Suggested Therapeutic Options to Reverse Hyper-Inflammation in Patients with COVID-19
Modulation of systemic immune responses may have a potential role in the treatment of patients with COVID-19. Given the importance of the issue and the lack of a vaccine or proven effective therapy, anti-cytokine therapy such as IL-6, TNFα and IL-1 antagonists have been suggested for the alleviation of hyper-inflammation as the main leading cause of severe adult respiratory distress syndrome (ARDS) in patients with COVID-19 (31,32).
Importantly, some clinical findings in China have shown that Tocilizumab (a monoclonal anti-IL-6 receptor) can significantly improve oxygenation and may be considered a promising therapeutic option in patients with COVID-19 who are at the risk of cytokine storm (33). Another small sample clinical trial in China has indicated good efficacy of Tocilizumab treatment in severe COVOD-19 patients (34). A case report from France reported successful treatment of Covid-19-related respiratory failure using Tocilizumab in a patient with cancer (35). According to previous studies, Tocilizumab can reduce the affinity of IL-6R to IL-6 by binding to IL-6R and soluble IL-6R, thereby inhibiting the CSS (18). Tocilizumab as a recombinant humanized monoclonal antibody has been approved for the treatment of patients with severe CSS (36,37).
Anakinra (recombinant interleukin-1 receptor antagonist: IL-1Ra) is another therapeutic option with IL-1 blockade properties. A group of American researchers suggested that continuous intravenous Anakinra infusions might have significant survival benefits owing to possibly reversing the cytokine storm in patients with COVID-19 (38). Previously, Shakoory B, et al. (39) claimed that the administration of Anakinra intravenously at a dose of 2.0 mg/kg/h for 72 h could significantly reduce the 28 d mortality in 484 patients with severe sepsis. Though the exact role of IL-1 in the pathogenesis of CSS is unclear, it seems that IL-1 receptor blockade may aid in better control of the inflammatory process. According to the previous reports, Anakinra is a biopharmaceutical drug with a wide therapeutic range, and high safety (40). Currently, Anakinra has been approved by the food and drug administration (FDA) for the treatment of a variety of auto-immune diseases, including rheumatoid arthritis as well as neonatal-onset multisystem inflammatory disease (41).
TNFα is another main cytokine in many inflammatory diseases. The elevated level of TNFα in blood and tissues of patients with COVID-19 has been indicated in previous reports (42,43). The potential role of anti-TNF therapy has been considered recently for reducing inflammation in COVID-19. Some anti-TNF antibodies such as infliximab or adalimumab have been suggested for modulating hyper-inflammatory responses in patients with COVID-19. Based on clinical recent studies, TNF blockade, as an amplifier of inflammation, causes a rapid reduction in IL-6 and IL-1 concentrations (44,45). Feldmann and colleagues have postulated that a single infusion of anti-TNF antibody in the early stage of infection can be effective in the treatment of patients with COVID-19 (42). However, the accuracy of this hypothesis must be confirmed by clinical trials.
Conclusion
Based on available evidence, targeting the cytokine storm should be considered in the current COVID-19 outbreak in patients with severe disease for alleviation patients’ inflammatory status. However, future clinical trials are required to gain more definite conclusion regarding the potential mechanistic connections between blockade of inflammatory cytokines (i.e. IL-6, IL-1 and TNFα) and COVID-19 management in severe patients.
Conflict of Interest
No potential conflict of interest was disclosed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors would like to thank the Cardiovascular Research Center of Tabriz University of Medical Sciences.
(ARCMED_2020_527)
References
- 1.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020:e201017. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R., Pei S., Chen B. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH . 2020. Coronavirus disease 2019 (COVID-19): situation report, 72. [Google Scholar]
- 4.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M., Safavi-Naeini P., Solomon S.D. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y.-Z., Holmes E.C. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L., Mu M., Ren H.G. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belouzard S., Millet J.K., Licitra B.N. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan Y., Shang J., Graham R. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W., Zhao Y., Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude S.L., Barrett D., Teachey D.T. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Elia R.V., Harrison K., Oyston P.C. Targeting the “cytokine storm” for therapeutic benefit. Clin Vaccine Immunol. 2013;20:319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimabukuro-Vornhagen A., Gödel P., Subklewe M. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Shea J.J., Ma A., Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C., Wu Z., Li J.-W. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K., Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 21.Strowig T., Henao-Mejia J., Elinav E. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 22.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Rodríguez N., Apostolidis S.A., Fitzgerald L. Pro-inflammatory self-reactive T cells are found within murine TCR-αβ+ CD4− CD8− PD-1+ cells. Eur J Immunol. 2016;46:1383–1391. doi: 10.1002/eji.201546056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wypasek E., Undas A., Sniezek-Maciejewska M. The increased plasma C-reactive protein and interleukin-6 levels in patients undergoing coronary artery bypass grafting surgery are associated with the interleukin-6− 174G> C gene polymorphism. Ann Clin Biochem. 2010;47:343–349. doi: 10.1258/acb.2010.090305. [DOI] [PubMed] [Google Scholar]
- 25.Ulhaq Z.S., Soraya G.V., Ulhaq Z.S. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan Q., Yang K., Wang W. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;13:e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan S., Yi Q., Fan S. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) Medrxiv. 2020 doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- 29.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo P., Liu Y., Qiu L. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michot J.-M., Albiges L., Chaput N. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020;31:961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maude S.L., Frey N., Shaw P.A. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler U., Jensen M., Manzke O. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 38.Adam M.L., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020;2:276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakoory B., Carcillo J.A., Chatham W.W. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granowitz E.V., Clark B., Mancilla J. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991;266:14147–14150. [PubMed] [Google Scholar]
- 41.Eloseily E.M., Weiser P., Crayne C.B. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 42.Feldmann M., Maini R.N., Woody J.N. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong J., Dong H., Xia S.Q. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv. 2020 doi: 10.1101/2020.02.25.20025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charles P., Elliott M.J., Davis D. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-α therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–1528. [PubMed] [Google Scholar]
- 45.Feldmann M., Maini R.N. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]