Abstract
Patients with moderate to severe acute respiratory distress syndrome (ARDS) benefit from prone positioning. Although the accuracy of esophageal pressure (Pes) to estimate regional pleural pressure (Ppl) has previously been assessed in the supine position, such data are not available in the prone position in ARDS. In six anesthetized, paralyzed, and mechanically ventilated female pigs, we measured Pes and Ppl into dorsal and ventral parts of the right pleural cavity. Airway pressure (Paw) and flow were measured at the airway opening. Severe ARDS [arterial partial pressure of oxygen ()/fraction of inspired oxygen () < 100 mmHg at positive end-expiratory pressure (PEEP) of 5 cmH2O] was induced by surfactant depletion. In supine and prone positions assigned in a random order, PEEP was set to 20, 15, 10, and 5 cmH2O and static end-expiratory chest wall pressures were measured from Pes (PEEPtot,es) and dorsal (PEEPtot,PplD) and ventral (PEEPtot,PplV) Ppl. The magnitude of the difference between PEEPtot,es and PEEPtot,PplD was similar in each position [−3.6 cmH2O in supine vs. −3.8 cmH2O in prone at PEEP 20 cmH2O (PEEP 20)]. The difference between PEEPtot,es and PEEPtot,PplV became narrower in the prone position (−8.3 cmH2O supine vs. −3.0 cmH2O prone at PEEP 20). PEEPtot,PplV was overestimated by Pes in the prone position at higher pressures. The median (1st–3rd quartiles) dorsal-to-ventral Ppl gradient was 4.4 (2.4–6.8) cmH2O in the supine position and −1.5 (−3.5 to +1.1) cmH2O in the prone position (P < 0.0001) and marginally influenced by PEEP (P = 0.058). Prone position narrowed end-expiratory dorsal-to-ventral Ppl vertical gradient, likely because of a more even distribution of mechanical forces over the chest wall.
NEW & NOTEWORTHY In a porcine model of acute respiratory distress syndrome, we found that static end-expiratory esophageal pressure did not change significantly in prone position compared with supine position at any positive end-expiratory pressure (PEEP) tested between 5 and 20 cmH2O. Prone position was associated with an increased ventral pleural pressure and reduced end-expiratory dorsal-to-ventral pleural pressure (Ppl) vertical gradient, likely due to a more even distribution of mechanical forces over the chest wall.
Keywords: acute respiratory distress syndrome, esophageal pressure, pleural pressure, positive end-expiratory pressure, prone position, transpulmonary pressure
INTRODUCTION
After decades of use of esophageal pressure (Pes) to estimate pleural pressure (Ppl), the interest for Pes monitoring has rapidly grown in ICU patients (2) and particularly in those with acute respiratory distress syndrome (ARDS). By partitioning the lung and chest wall contribution to the impairment of respiratory system mechanics (11), this tool has the important implication of allowing for the personalization of ventilation support based on the patient’s own respiratory mechanics (12). Of note, Pes monitoring has been proposed to set positive end-expiratory pressure (PEEP) by using the absolute value of end-expiratory Pes with the goal of reaching a positive end-expiratory transpulmonary pressure that would reflect an open lung (22). Whereas the idea was attractive, with physiological benefits from using the Pes-guided PEEP strategy in an early single-center study (22), it did not result in patient outcome improvement in a subsequent multicenter trial (5). Several reasons may explain this disappointing result. First, the PEEP levels were not different between the two groups of patients enrolled in the multicenter trial (5), unlike the early single-center study where PEEP was on average 6 cmH2O higher in the Pes-guided group (22). Second, the absolute end-expiratory Pes was shown in other studies to be unrelated to lung recruitability (7, 8). Third, Pes can be overestimated by a nonoptimal esophageal balloon inflation, and consequently the transpulmonary pressure (17). However, in pigs with acute lung injury end-expiratory transpulmonary pressure measured from Pes was close to that measured from Ppl in the dependent part of the chest (24), indicating that absolute end-expiratory Pes may inform about regional lung stress. As prone positioning ARDS patients has been shown to improve patient outcome (13), it is important to know how well Pes relates to Ppl in the prone position in ARDS, as this has not previously been investigated. This question is particularly urgent to address in the context of an ongoing COVID-19-induced ARDS in pandemic proportions, to gain better knowledge of Pes monitoring in patients receiving invasive mechanical ventilation. Given that an esophageal probe would be submitted less to compressive forces in the prone position (14), one could hypothesize that end-expiratory Pes, the central component of the Pes-guided PEEP strategy, would better reflect the overall rather than the dorsal Ppl. With this hypothesis in mind, we aimed to assess the relationship between Pes and regional Ppl in supine and prone positions at different PEEP levels in a porcine model of ARDS. Our secondary aim was the effect of prone position on the vertical Ppl gradient.
MATERIALS AND METHODS
Animal preparation.
The experimental protocol was approved and authorized by the “Direction générale de la Santé, Rue Adrien-Lachenal 8, 1207 Geneva, Switzerland”. The number of the protocol was GE/19/19. Female “Grand Blan” white domestic pigs weighting roughly 40 kg were obtained from a farm in Aple (Canton de Vaud, Switzerland). Animals were housed at the University of Geneva Medical School animal facility for 1 or more days before the experiment. They were given food and water ad libitum. On the morning of the experiment, animals were premedicated with 8 mg/kg haloperidol (Janssen-Cilag, Zug, Switzerland), 0.75 mg/kg midazolam (Aguettant, Lyon, France), and 25 µg/kg atropine (Aguettant, Lyon, France) intramuscularly. Animals were placed in the supine position and moved to the operating room, where a 20-gauge catheter (Braun, Sempach, Switzerland) was placed in each ear vein. General anesthesia was induced by sevoflurane 5% administered via a face mask and intravenous 0.1 mg·kg−1·h−1 midazolam. Orotracheal intubation was then performed with a 6.5- to 7.0-mm-inner diameter (ID) tube (Mallinckrodt; Covidien-Medtronic, Münchenbuchsee, Switzerland) by adding intravenous 0.5 mg/kg atracurium (Aspen, Baar, Switzerland) and general anesthesia and paralysis maintained with inhaled sevoflurane 5% (Baxter, Zürich, Switzerland) and intravenous 1 mg·kg−1·h−1 rocuronium (Labatec, Meyrin, Switzerland) continuously. Mechanical ventilation was performed by using a Primus ventilator (Dräger Suisse SA, Liebefeld, Switzerland) in volume-control mode with a tidal volume (Vt) of 6 mL/kg body wt, PEEP 5 cmH2O, inspiratory time 1 s, respiratory rate 22 breaths/min, fraction of inspired oxygen () 21%, and fresh gas flow rate 2.5 L/min. After initiation of mechanical ventilation, sevoflurane was stopped and general anesthesia maintained with 5 mg·kg−1·h−1 propofol (B Braun Medical, Sempach, Switzerland), 12.5 μg·kg−1·h−1 fentanyl (Sandoz, Rotkreuz, Switzerland), 0.1 mg·kg−1·h−1 midazolam, 1 mg·kg−1·h−1 rocuronium, and 2.5 mg·kg−1·h−1 ketamine. Sedation was titrated to Richmond Agitation-Sedation Scale (RASS)-4. A central venous line (Baxter, Zürich, Switzerland) was introduced into an internal jugular vein, and a carotid artery was cannulated for arterial blood pressure monitoring and arterial blood gas measurement (iSTAT; Abbott, Maidenhead, United Kingdom). A Fleish 2 pneumotachograph was used to measure flow, and airway pressure (Paw) was measured at the proximal end of the endotracheal tube. Fluid intake (Ringer acetate; Fresenius Kabi, Oberdorf, Switzerland) was given intravenously at a rate of 4 mL·kg−1·h−1. An 18-gauge catheter was transcutaneously inserted into the bladder (Rüsch; Teleflex, Belp, Switzerland), and urine was drained continuously.
Pes and Ppl measurement.
We used custom-made 10 × 10-cm silicon pleural pouches made from infusion bags (Macopharma, Tourcoing, France) (Supplemental Fig. S1; all Supplemental Material available at https://doi.org/10.6084/m9.figshare.12278342). These were inserted into the right pleural cavity via a single thoracotomy of 11-cm length at the 7th intercostal space (Supplemental Fig. S2) one in dorsal (PplD) and the other in ventral (PplV) position. The same operator (Jean-Pierre Giliberto) was in charge of the surgical procedure. Care was taken to place the pouches in a reproducible fashion. Each pouch was connected to a pressure transducer through a separate orifice opened in the chest wall (Supplemental Fig. S3). The different tissue planes were sutured one by one to prevent any air leak. The remaining air was expelled by performing a manual insufflation twice at 30 cmH2O during closure of the subcutaneous plane. The tubings were securely attached to the parietal muscle and to the skin to avoid any displacement during the prone positioning maneuver (Supplemental Fig. S4). The EKG, tail pulse oximetry (), end-tidal CO2 (ETCO2), Paw, and respiratory airflow were displayed with a vital signs monitor (Datex Ohmeda, Baar, Switzerland). Airflow, Pes, Paw, and Ppl sensors were calibrated before each experiment. All signals were continuously digitized at 1 kHz with an analog/digital interface (LabChart; ADInstruments, Oxford, UK) and recorded on a desktop computer.
Pes was measured with an air-filled balloon catheter (Nutrivent; Sidam, Mirandola, Italy). The correct positioning of the Pes catheter and Ppl pouches was checked by planar radiography (Ziehm Vista Imager, Nuremberg, Germany) (Supplemental Fig. S5). The pressure-volume curve of the esophageal balloon and of each Ppl pouch was recorded at their optimal volume, which was taken as the plateau value (17). At this volume, a Baydur maneuver was performed to check appropriate pressure transmission. Briefly, a regression line was fitted between Pes, PplD, PplV, and Paw. A slope of 0.8–1.2 was considered appropriate (4).
Study protocol.
Once animal preparation was completed and all the measurement probes were in place, the animal was switched to the Servo-I ventilator (Maquet-Getinge, Solna, Sweden) set in volume-control mode, constant flow inflation; Vt 6 mL/kg body wt, respiratory rate 22 breaths/min; PEEP 5 cmH2O; 0.40, inspiratory flow 25 L/min, inspiratory time 0.65 s, and inspiratory pause 10%. Before lung injury, mechanical ventilation was switched to pressure-control mode, PEEP 5 cmH2O, set pressure 23 cmH2O above PEEP, and 1.0. Lung injury was induced by repeated whole lung lavage with 37°C normal saline to deplete surfactant, with a target arterial partial pressure of oxygen () < 100 mmHg on = 1.0. Briefly, a bolus of 1 liter of 0.9% saline warmed at 37°C was instilled via the endotracheal tube after disconnection from the ventilator and poured out immediately. Mechanical ventilation was then resumed. After the induction of lung injury, the animal was randomly allocated to either supine then prone position or the opposite.
In the supine-prone (SP-PP) group, the lungs were recruited for 5 min in pressure-control mode, PEEP 20 cmH2O, 1.0, and set pressure 15 cmH2O above PEEP. Volume control ventilation was resumed with PEEP 20 cmH2O, Vt 6 ml/kg, respiratory rate adjusted to maintain pH between 7.30 and 7.40, and 1.0, and then PEEP decreased by steps of 5 cmH2O, each lasting 5 min, to PEEP 5 cmH2O (PEEP 5). At each step, optimal volume of probes was determined and a Baydur maneuver performed. Then, a 3-s end-expiratory and a 5-s end-inspiratory occlusion were performed by pressing the appropriate knobs on the ventilator. Arterial blood gases were measured on PEEP 5. The animal was then moved to the prone position at PEEP 5. After a 30-min stabilization period, the optimal esophageal balloon and pleural sensor volume was determined. PEEP was increased to 20 cmH2O, the lungs were recruited for 5 min, and PEEP was subsequently decreased to 5 cmH2O with the same measurements as in the supine position at each PEEP level.
In the prone-supine (PP-SP) group, the optimal esophageal balloon and pleural sensor volume was first determined in supine position at PEEP 5 cmH2O. Animals were then turned to the prone position, and after a 30-min stabilization period, optimal volume was determined at PEEP 5 cmH2O. PEEP was increased to 20 cmH2O, the lungs were recruited for 5 min, and PEEP was subsequently decreased to 5 cmH2O as in the SP-PP group. The measurements were repeated at each PEEP level with same consideration for optimal volume and Baydur maneuver as in the other group. Animals were then moved back to the supine position. After a 30-min stabilization period PEEP was increased to 20 cmH2O, the lungs were recruited for 5 min, and PEEP was subsequently decreased as above with the same measurements at each step. At this stage, the optimal volume was that used in the supine position before prone positioning. At the end of the experiment the animals were euthanized by intravenous KCl.
Data analysis.
At each step in each position, we measured the chest wall static pressures (Pes, PplD, and PplV) at both end-expiration (PEEPtot,es, PEEPtot,PplD, and PEEPtot,PplV, respectively) and end-inspiration (Pplat,es, Pplat,PplD, and Pplat,PplV, respectively) during the previously mentioned airway occlusions. From these data, we computed the chest wall driving pressures (DP,es, DP,PplD, and DP,PplV, respectively) by subtracting the corresponding end-expiratory pressures from the end-inspiratory pressures. Chest wall elastance (Est,cw) was obtained by dividing the corresponding DPs by Vt. The dorsal-ventral Ppl gradient was computed as PplD minus PplV at end-expiration.
Values are presented as medians (1st–3rd quartiles). The primary end point was the effect of position and PEEP on the three different measurements of chest wall static pressures: PEEPtot,es, PEEPtot,PplD, and PEEPtot,PplV. We performed linear regressions between end-expiratory and end-inspiratory static Pes and Ppl over all PEEP levels in each pig in the supine and then prone position and used the Spearman rank correlation coefficient. Bland–Altman plots of difference against mean were drawn to assess the agreement between variables in each position. We used linear mixed-effects models in which position, nominal PEEP, and site of chest wall static pressure measurement (Pes, PplD, and PplV) were the fixed-effect factors and the pig the factor with a random effect. The reference was Pes and PEEP 20 cmH2O in supine position. In case of interaction between PEEP and position, we developed a specific model for each position in which the effect of PEEP and site of measurement was tested. The significance of the fixed effects was tested by ANOVA, and, if significant, values were compared to the reference with the Dunnett’s test. The validity of the models was tested by inspection of the residuals against fitted values by position plots and the normal distribution of the residuals. The assumption of normality of the distribution of random effect factor was also tested. Statistical analysis was performed by Rstudio software (version 1.0.153; RStudio, Inc.). A P value < 0.05 was deemed statistically significant.
RESULTS
Lung injury.
Six female pigs were enrolled, with three receiving the SP-PP sequence and three the PP-SP sequence. Weight was 41 (41–42) kg, Vt (6 mL/kg) 250 (250–258) mL, respiratory rate 24 (22–26) breaths/min, and inspiratory flow 26 (25–28) L/min. At the time of ARDS at PEEP 5 cmH2O in supine position, / was 67 (61–77) mmHg, arterial partial pressure of carbon dioxide () 63 (50–63) mmHg, pH 7.31 (7.30–7.37), and lactate 0.7 (0.4–1.0) mmol/L.
Pleural and esophageal pressures.
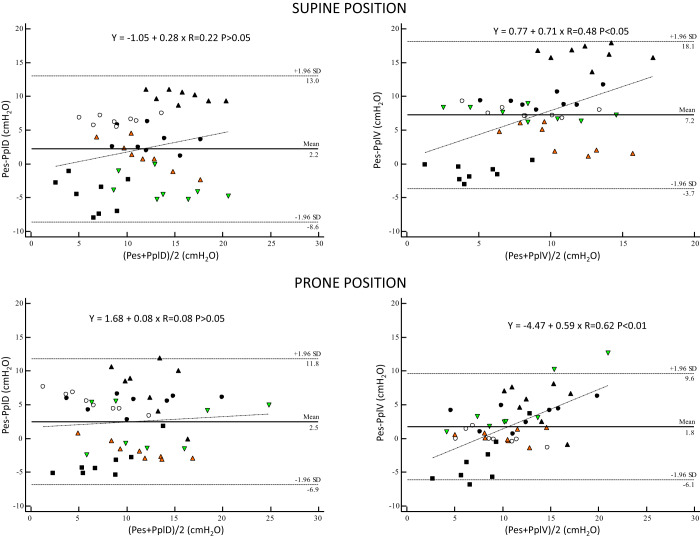
The linear regression analysis of Pes to PplD and PplV at end-expiration and end-inspiration across PEEP in each pig in supine and prone positions is displayed in Table 1. Bias and limits of agreement between static chest wall pressures are shown in Fig. 1 over all pigs. There was an overestimation of PplV by Pes at higher values of PplV in supine and prone positions, with greater bias in the latter (Fig. 1).
Table 1.
Linear regression between esophageal and pleural pressures at end-expiration and end-inspiration over 4 positive end-expiratory pressure levels in supine and prone positions in each pig
| Pig | Pes vs. | Supine Position |
Prone Position |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | Spearman | P value | Slope | Intercept | Spearman | P value | ||
| 1 | PplD | 1.04 | 6.30 | 0.95 | 0.0003 | 0.66 | 6.70 | 1.00 | <0.0001 |
| 1 | PplV | 0.85 | 8.30 | 1.00 | <0.0001 | 0.77 | 2.24 | 1.00 | <0.0001 |
| 2 | PplD | 0.73 | 0.70 | 0.91 | 0.0020 | 0.99 | 1.90 | 0.79 | 0.0020 |
| 2 | PplV | 0.84 | 8.20 | 0.98 | <0.0001 | 1.89 | −3.30 | 0.98 | <0.0001 |
| 3 | PplD | 0.75 | 6.10 | 0.91 | 0.0020 | 1.03 | 5.20 | 0.93 | 0.0020 |
| 3 | PplV | 1.10 | 8.90 | 0.98 | <0.0001 | 1.14 | 2.10 | 0.98 | <0.0001 |
| 4 | PplD | 0.82 | 11.90 | 0.93 | 0.0009 | 0.85 | 17.00 | 0.69 | 0.0500 |
| 4 | PplV | 0.21 | 14.30 | 0.74 | 0.0400 | 0.37 | 12.10 | 0.81 | 0.0100 |
| 5 | PplD | 0.47 | 0.10 | 0.65 | 0.0800 | 1.57 | −9.00 | 0.95 | 0.0003 |
| 5 | PplV | 0.99 | −1.20 | 0.74 | 0.0100 | 1.65 | −9.40 | 0.60 | 0.1200 |
| 6 | PplD | 0.50 | 6.90 | 0.86 | 0.0070 | 0.69 | 1.90 | 0.97 | 0.0001 |
| 6 | PplV | 0.52 | 7.80 | 0.91 | 0.0020 | 0.96 | 0.80 | 0.95 | 0.0004 |
Pes, esophageal pressure, PplD and PplV, dorsal and ventral pleural pressure.
Fig. 1.
Bias (horizontal solid line) and 95% upper and lower limits of agreement (horizontal dashed lines) between static end-expiratory and end-inspiratory chest wall pressures in supine and prone positions. The values of bias (horizontal solid black lines) and limits of agreement (horizontal black dashed lines) and linear regression lines with regression equations were computed over the 6 pigs, but individual pig values are highlighted. Open circles, pig 1; green triangles, pig 2; black circles, pig 3; black triangles, pig 4; black squares, pig 5; orange triangles, pig 6. Pes, esophageal pressure; PplD and PplV, dorsal and ventral pleural pressure.
Static end-expiratory chest wall pressures.
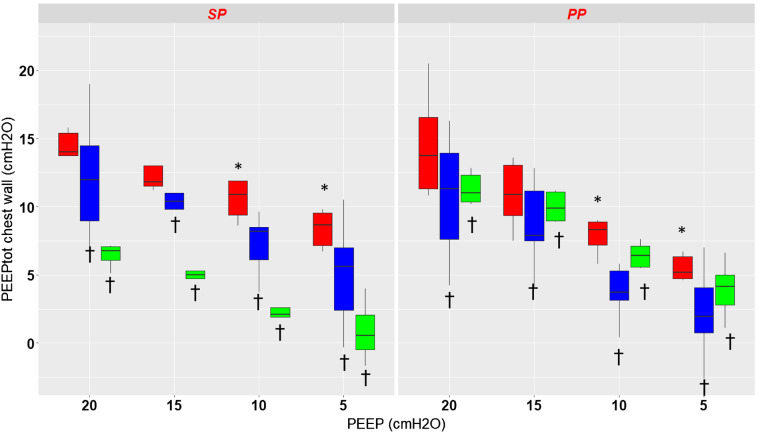
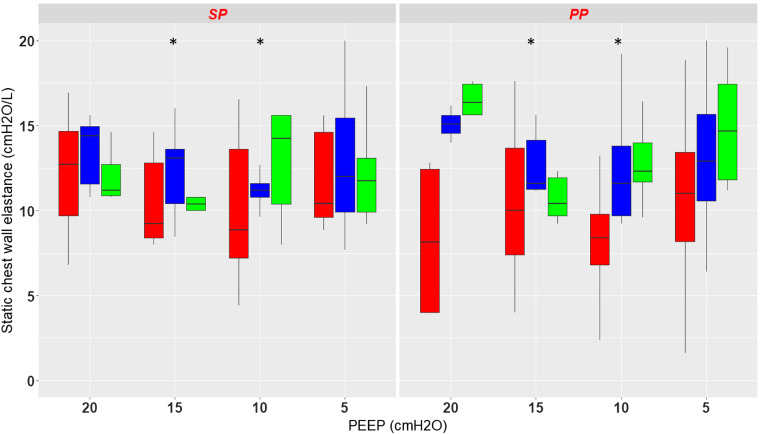
Figure 2 shows the effect of PEEP and position on static end-expiratory Pes, PplD, and PplV. Overall, there was a significant effect of PEEP (P < 0.0001) and pressure measurement site (P < 0.0001) and no effect of position (P = 0.95) on the site of chest wall pressures measurement. As there was an interaction between position and PEEP (P < 0.0001), a separate analysis was done for each position. In the supine position both PEEP and pressure measurement site had a significant effect on static end-expiratory chest wall pressure. In the prone position the results are similar, with, however, a smaller difference between PplV and Pes. The complete results are displayed in Table 2.
Fig. 2.
Box-and-whisker plots of total end-expiratory static chest wall pressure (PEEPtot) measured with the esophageal probe (red) and the dorsal (blue) and ventral (green) pleural sensors in supine (SP) and prone (PP) positions at different levels of positive end-expiratory pressure (PEEP) from 20 to 5 by steps of 5 cmH2O. *P < 0.05 vs. PEEP 20 cmH2O, †P < 0.05 vs. esophageal pressure. Vertical lines indicate , where IQR is interquartile range.
Table 2.
Mixed-effects models for static end-expiratory and end-inspiratory chest wall pressures in supine and prone positions
| Pressure | End-Expiratory Pressures |
End-Inspiratory Pressures |
||||||
|---|---|---|---|---|---|---|---|---|
| Supine |
Prone |
Supine |
Prone |
|||||
| Coefficient | P value | Coefficient | P value | Coefficient | P value | Coefficient | P value | |
| Pes at PEEP 20 (reference)* | 15 (13;18) | <0.0001* | 14 (12;16) | <0.0001* | 18 (16;21) | <0.0001* | 18 (16;20) | <0.0001* |
| Change with PplD | −4 (−5;−2) | <0.0001* | −4 (−5;−2) | <0.0001* | −4 (−6;−2) | <0.0001* | −3 (−5;−2) | <0.0001* |
| Change with PplV | −8 (−10;−7) | <0.0001* | −3 (−4;−2) | <0.0001* | −9 (−10;-7) | <0.0001* | −3 (−4;−1) | <0.0001* |
| Change with PEEP 15 | −2 (−4;0) | 0.06* | −2 (−4;0) | 0.02* | −2 (−4;0) | 0.07* | −3 (−5;−2) | <0.0001* |
| Change with PEEP 10 | −5 (−7;−3) | <0.0001* | −5 (−7;−4) | <0.0001* | −5 (−7;−3) | <0.0001* | −7 (−8;−5) | <0.0001* |
| Change with PEEP 5 | −7 (−8;−5) | <0.0001* | −8 (−9;−6) | <0.0001* | −6 (−8;−4) | <0.0001* | −9 (−10;−7) | <0.0001* |
| Fixed-effect PEEP** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| PEEP 15 vs. 20 | 0.69† | 0.26† | 0.74† | 0.02† | ||||
| PEEP 10 vs. 20 | 0.03† | <0.0001† | 0.0475† | <0.00001† | ||||
| PEEP 5 vs. 20 | 0.0004† | <0.0001† | 0.003† | <0.00001† | ||||
| Fixed-effect pressure measurement site** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| PplD vs. Pes | 0.011† | 0.009† | 0.006† | 0.045† | ||||
| PplV vs. Pes | <0.0001† | 0.04† | 0.0001† | 0.11† | ||||
PEEP, positive end-expiratory pressure; Pes, esophageal pressure; PplD, dorsal pleural pressure; PplV, ventral pleural pressure.
P values for the test of the coefficients vs. 0;
P values for the fixed effects with ANOVA;
comparisons to the reference (PEEP 20 for the fixed-effect PEEP and Pes for the fixed-effect site of measurement) with Dunnett’s test.
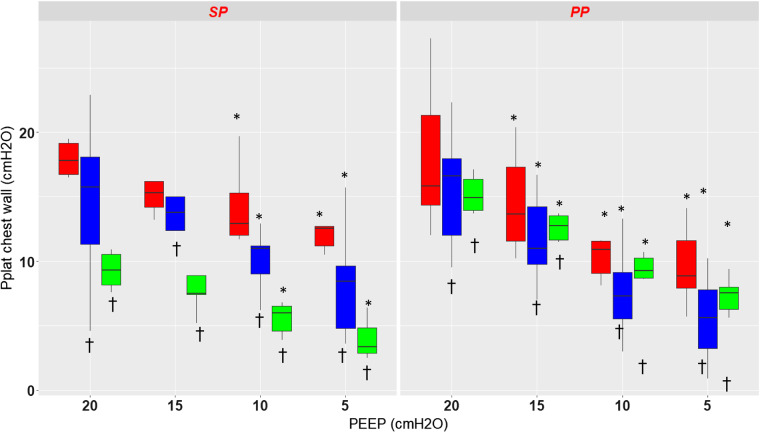
Static end-inspiratory chest wall pressures.
As for static end-expiratory Pes, there was a significant effect of PEEP (P < 0.0001) and pressure measurement site (P < 0.0001) but no effect of position (P = 0.47) on the static end-inspiratory Pes (Fig. 3). Because of a significant interaction between position and pressure measurement site (P < 0.0001), a separate analysis was performed for each position. In the supine position PplD and PplV were significantly lower than Pes, the values at PEEP 15 not being different from PEEP 20 (Table 2 and Fig. 3). The result is the same in the prone position except that the values at any PEEP were different from PEEP 20.
Fig. 3.
Box-and-whisker plots of end-inspiratory static pressure chest wall (Pplat) measured with the esophageal probe (red) and the dorsal (blue) and the ventral (green) pleural sensors in supine (SP) and prone (PP) positions at different levels of positive end-expiratory pressure (PEEP) from 20 to 5 by steps of 5 cmH2O. *P < 0.05 vs. PEEP 20 cmH2O, †P < 0.05 vs. esophageal pressure. Vertical lines indicate , where IQR is interquartile range.
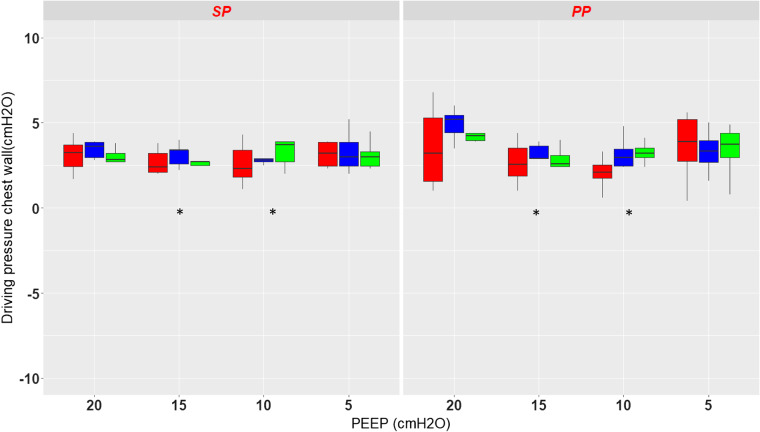
Static driving chest wall pressures.
There was a significant effect of PEEP (P = 0.0038) but not of position (P = 0.16) or pressure measurement site (P = 0.39) on static driving pressure of the chest wall (Fig. 4). There was no interaction between any combinations of these factors. Therefore, DP,es, DP,PplD, and DP,PplV did not significantly differ between supine and prone positions. As can be seen from Fig. 4, DPes and DPpl exhibited a U-shaped profile in the prone position, but not in the supine position, between PEEP 20 and PEEP 5. Finally, the variability in DP,es was of notice at PEEP 20 and PEEP 5 in the prone position. In the supine position, the mean difference between DPplD was small, i.e., −0.2 cmH2O, with narrow limits of agreement (−2.3 to 0.9 cmH2O) without any bias. The same was observed with DPplV, i.e., 0 (−1.7 to 1.8) cmH2O (Supplemental Fig. S6). In the prone position the mean difference between DPes and DPplD and DPplV was −0.4 and 0.3 cmH2O, respectively, with limits of agreement −3.9 to 3.1 and −3.7 to 4.2 cmH2O, respectively, with an overestimation of DPpl by DPes at the higher DPpl values (Supplemental Fig. S6).
Fig. 4.
Box-and-whisker plots of driving pressure chest wall measured with the esophageal probe (red) and the dorsal (blue) and ventral (green) pleural sensors in supine (SP) and prone (PP) positions at different levels of positive end-expiratory pressure (PEEP) from 20 to 5 by steps of 5 cmH2O. *P < 0.05 vs. PEEP 20 cmH2O. Vertical lines indicate , where IQR is interquartile range.
Static chest wall elastance.
The results pertaining to Est,cw are in line with these of chest wall driving pressure. There was a significant effect of PEEP (P = 0.0042) with no effect of position (P = 0.16) and pressure measurement site (P = 0.39) without any significant interaction between the above factors (Fig. 5). That means that Est,cw was not significantly different whether it was computed from Pes, PplD, or PplV in either position. The U-shape profile exhibited by Est,cw across PEEP is even clearer than for the driving pressure, in particular in the prone position, except for Est,cw from Pes at PEEP 20 (Fig. 4).
Fig. 5.
Box-and-whisker plots of the static elastance of chest wall measured with the esophageal probe (red) and the dorsal (blue) and ventral (green) pleural sensors in supine (SP) and prone (PP) positions at different levels of positive end-expiratory pressure (PEEP) from 20 to 5 by steps of 5 cmH2O. *P < 0.05 vs. PEEP 20 cmH2O. Vertical lines indicate , where IQR is interquartile range.
Pleural pressure dorsal-to-ventral gradient.
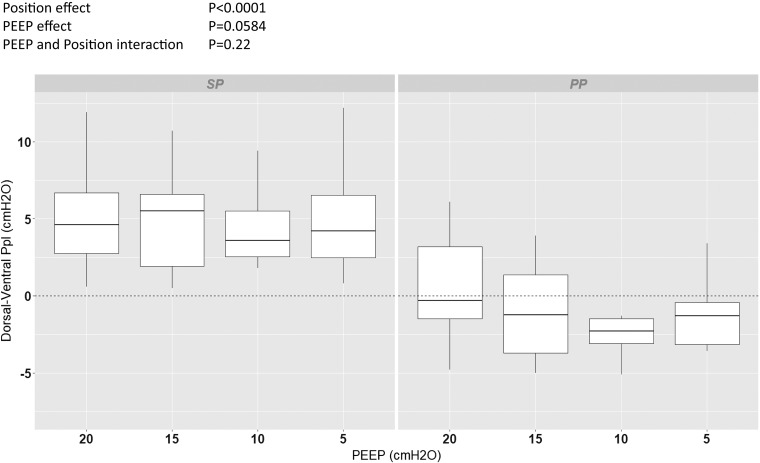
The dorsal-to-ventral Ppl gradient at end-expiration was 4.4 (2.4–6.8) cmH2O in the supine position and −1.5 (−3.5 to +1.1) cmH2O in the prone position (P < 0.0001), over all PEEP levels, and was not significantly influenced by PEEP (P = 0.0584), without interaction between PEEP and position (P = 0.22) (Fig. 6).
Fig. 6.
Box-and-whisker plots of the dorsal-ventral pleural pressure (Ppl) gradient at end-expiration in supine (SP) and prone (PP) positions at different levels of positive end-expiratory pressure (PEEP) from 20 to 5 by steps of 5 cmH2O. The results of ANOVA for the fixed effects in the mixed-effect model are shown at top left. Vertical lines indicate , where IQR is interquartile range.
DISCUSSION
The main findings of the present study were that 1) PEEPtot,es was higher than PEEPtot,PplD and PEEPtot,PplV in both supine and prone positions; 2) similar results were obtained for end-inspiratory chest wall static pressures; 3) as a result, the chest wall driving pressure and the static elastance were not different between supine and prone positions; 4) the dorsal-to-ventral Ppl gradient was partly reverted and of smaller absolute magnitude when animals were prone.
Ppl measurement in supine position.
In this study we measured pleural surface pressure, which is the chest wall recoil pressure (1). To do this, we used custom-made sensors inserted surgically into the pleural space, as other investigators did in previous experiments (19, 20, 24). We expected that Ppl measurement would hardly compare between studies given the differences in animal species, type of sensors, the nature of ARDS, ventilator settings, and in particular PEEP. However, the end-expiratory dorsal-to-ventral Ppl gradient measured in the supine position in the present study was close to that found in previous experimental studies performed by others (Supplemental Table S1). This can be seen as an external validation, which is reassuring for our findings.
Comparison between Ppl and Pes measurement in supine position.
Many studies have compared Ppl and Pes in various clinical or experimental settings, and the majority of them have reported discrepancies between measurements within and across studies. This can be explained by differences in the type of sensors used to measure Ppl and Pes, such as air- or liquid-filled catheters, the accurate placement of Pes, and the distance between the Pes probe and Ppl sensors. Using CT scan imaging, Yoshida et al. (24) showed that the Pes probe actually measures the regional Ppl close to it. When PEEP is increased, the vertical lung height measured with the CT increases and so does the distance between Pes and Ppl in the dorsal lung regions, resulting in an increased difference between them (24).
Compared with Pelosi et al. (20), the mean difference between Pes and Ppl,D was 2.2 cmH2O in our study versus −4 cmH2O, and it was 7.2 cmH2O vs. −4 cmH2O between Pes and PplV, respectively, at PEEP 10. This means that in our study Pes overestimates PplV at higher pressures, whereas the opposite is true in the study by Pelosi et al. (20). We have no explanation for this discrepant finding. In contrast to the results pertaining to the absolute values of Pes, we found a good agreement, however, between the change of Ppl and that of Pes, i.e., the DP (Fig. 4 and Supplemental Fig. S5). This concordance between relative change in Pes and that in Ppl has been demonstrated in previous studies (6, 9).
Effects of prone position on Ppl and Pes measurements.
We explored two factors that may affect the concordance between Ppl and Pes: one is gravity (change in body position) and the other the level of Paw (different PEEP levels).
The factors that govern the gravitational gradient of Ppl include the shape and the mechanical properties of the chest wall (21), the inherent stress-free shape of the lung (10), the weight of the lung (3), and possible friction between the two pleural surfaces. In the absence of gravity, the lung is more uniformly inflated (15), suggesting that Ppl is more uniform over the entire pleural surface. Although gravity still influences Ppl in the prone position, its effect is offset by positional differences in the forces generated in the thoracic cavity; for example, the heart and upper abdomen rest on the sternum when prone and therefore have a much smaller effect on Ppl and regional lung expansion (15).
As a matter of fact, a few studies have assessed the effect of prone position on the regional distribution of Ppl in experimental ARDS. Mutoh et al. (18) compared the effect of volume infusion in supine and prone positions on regional Ppl in mechanically ventilated pigs. They demonstrated that Ppl was lower in the dorsal lung regions (nondependent with respect to gravity) in the prone position at baseline, and this effect was even larger after volume infusion. Consequently, the Ppl gradient, which was enhanced by volume infusion in the supine position, was reduced in the prone position. Other studies have also demonstrated that the gravitational gradient of Ppl is decreased in the prone position compared with the supine position in dogs (15, 23). We found similar findings, since PplV increased in the prone position and exceeded PplD, resulting in at least a partial reversal of the gravity-dependent vertical Ppl gradient. This explains why our data analysis, which aimed at assessing the effect of measurement site, position, and PEEP together, concluded that even though position had no significant effect on chest wall pressures, there was a significant interaction between position and measurement site of chest wall pressures, meaning that the effect of the latter was not the same in each position. In the prone position, end-expiratory and end-inspiratory Pes still exhibited higher values than PplD or PplV.
In our study we found that Paw influenced the relationship of Pes to Ppl. The effect of PEEP was indeed significant, without interaction with either the measurement site of chest wall pressure or position on end-expiratory and end-inspiratory chest wall static pressures, their difference, and Est,cw. This was particularly true between PEEP 10 and PEEP 5 compared with PEEP 20 that was used as a control. Conversely, the Ppl vertical gradient was marginally affected by the PEEP level in our analysis. The Ppl vertical gradient is important because it governs the vertical distribution of transpulmonary pressure, and hence that of lung stress throughout the lung. In our study, the vertical gradient of Ppl tended to increase with increasing PEEP, a result in line with that found by Pelosi et al. (20).
Clinical implications.
The most important clinical implication of measuring Pes is deriving transpulmonary pressure. In pigs investigated in the supine position, Yoshida et al. (24) found that transpulmonary pressure measured from Pes closely reflected dependent, i.e., dorsal, Ppl, whereas this was less true with the nondependent, i.e., ventral, Ppl. We confirm this result in the supine position (Figs. 2 and 3). In the prone position, our results suggest that this relationship may be less clear, as the difference between Pes, PplD, and PplV is smaller. This consideration has two implications. On one hand, in the prone position Pes would estimate a more homogeneous Ppl and therefore be more relevant than in the supine position. On the other hand, the regional character of transpulmonary pressure based on Pes would be lost in the prone position, which can limit its use in that position.
Following the above consideration, another clinical implication is PEEP selection. The Pes-guided strategy proposed to set PEEP in order to obtain a positive end-expiratory transpulmonary pressure by using absolute PEEPtot,es (22). Our Fig. 1 clearly shows that this strategy would have resulted in 1) a higher PEEP than setting PEEP based on Ppl; 2) an inhomogeneous distribution of PEEP across the vertical lung dimension in the supine position; and 3) a more homogeneous distribution of PEEP in the prone position. The Pes-guided strategy of PEEP titration has failed to demonstrate clinical benefit as yet. Indeed, the large multicenter EPVent-2 trial found no difference in outcome between the Pes-guided strategy and the control strategy to set PEEP (5), maybe because in that trial PEEP was similar between the two groups, unlike in the pilot study (22). In the present study, PEEPtot,es was not different between supine and prone positions at any given PEEP, in line with our previous human study (16). Even though the Pes-guided strategy can individualize PEEP level, the average PEEP could be very close between prone and supine position.
Study limitations and strengths.
Our study had some limitations. The present findings should be applied to humans with caution because of interspecies differences in chest morphology and the quadruped nature of pigs. To the best of our knowledge, a systematic comparison of chest morphology in supine and prone positions across species has not been performed. The normalization required for such a comparison would use variables not easy to define. The larger dorso-ventral diameter of the chest in pigs implies a greater change in the hydrostatic forces from supine to prone than in humans, suggesting that larger effects on variables subjected to such forces may be expected in pigs. On the other hand, in humans with a larger lateral than dorso-ventral chest dimension, rib displacement from its center of attachment to the spine from supine to prone would be greater than in swine because of a larger lever effect; therefore, more effects could be expected in humans. The weight of the thoracic and abdominal viscera, which is different between species, should also be taken into account. We did not perform a comprehensive lung CT study to assess regional lung volume or superimposed pressure as in previous studies (20, 24). Finally, despite the relatively small sample size, which limits the statistical power, our study has strengths, however, as this is the first study to compare Pes and Ppl in supine and prone positions at different PEEP levels in a porcine model of ARDS. Mutoh et al. investigated pigs with pulmonary edema induced by volume infusion and measured Ppl and Pes in supine and prone positions at functional residual capacity.
Conclusions.
In a porcine model of ARDS, we found that in prone compared with supine position Pes overestimated PplV and reduced the end-expiratory dorsal-to-ventral Ppl vertical gradient, likely due to a more even distribution of mechanical forces over the chest wall.
GRANTS
This work is supported by the French National Research Agency in the framework of the “Investissements d’avenir” program (ANR-15-IDEX-02).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.T., S.B., N.N., W.H., and C.G. conceived and designed research; N.T., S.B., N.N., E.T., W.H., and C.G. performed experiments; N.T., S.B., N.N., W.H., L.A., M.C., B.L., and C.G. interpreted results of experiments; S.B., B.L., and C.G. analyzed data; B.L. and C.G. prepared figures; N.T., S.B., and C.G. drafted manuscript; N.T., S.B., N.N., E.T., W.H., L.A., M.C., B.L., and C.G. edited and revised manuscript; N.T., S.B., N.N., E.T., W.H., L.A., M.C., B.L., and C.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jean-Pierre Giliberto and Sylvie Roulet for technical assistance in animal handling and surgical preparations.
REFERENCES
- 1.Agostoni E. Mechanics of the pleural space. In: Handbook of Physiology, The Respiratory System, Mechanics of Breathing, edited by Fenn WO, Rahn H. Bethesda, MD: American Physiological Society, 1986, p. 531–559. doi: 10.1002/cphy.cp030330 [DOI] [Google Scholar]
- 2.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guérin C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L; PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine) . The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 189: 520–531, 2014. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 3.Albert RK, Leasa D, Sanderson M, Robertson HT, Hlastala MP. The prone position improves arterial oxygenation and reduces shunt in oleic-acid-induced acute lung injury. Am Rev Respir Dis 135: 628–633, 1987. doi: 10.1164/arrd.1987.135.3.628. [DOI] [PubMed] [Google Scholar]
- 4.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 5.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D; EPVent-2 Study Group . Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs. an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 321: 846–857, 2019. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chartrand DA, Ye TH, Maarek JM, Chang HK. Measurement of pleural pressure at low and high frequencies in normal rabbits. J Appl Physiol (1985) 63: 1142–1146, 1987. doi: 10.1152/jappl.1987.63.3.1142. [DOI] [PubMed] [Google Scholar]
- 7.Chiumello D, Cressoni M, Carlesso E, Caspani ML, Marino A, Gallazzi E, Caironi P, Lazzerini M, Moerer O, Quintel M, Gattinoni L. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med 42: 252–264, 2014. doi: 10.1097/CCM.0b013e3182a6384f. [DOI] [PubMed] [Google Scholar]
- 8.Cressoni M, Chiumello D, Carlesso E, Chiurazzi C, Amini M, Brioni M, Cadringher P, Quintel M, Gattinoni L. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology 121: 572–581, 2014. doi: 10.1097/ALN.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 9.Dechman G, Sato J, Bates JH. Factors affecting the accuracy of esophageal balloon measurement of pleural pressure in dogs. J Appl Physiol (1985) 72: 383–388, 1992. doi: 10.1152/jappl.1992.72.1.383. [DOI] [PubMed] [Google Scholar]
- 10.Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis 115: 559–566, 1977. doi: 10.1164/arrd.1977.115.4.559. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 158: 3–11, 1998. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 12.Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L, Ranieri VM. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 38: 395–403, 2012. doi: 10.1007/s00134-012-2490-7. [DOI] [PubMed] [Google Scholar]
- 13.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group . Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368: 2159–2168, 2013. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 14.Hedenstierna G. Esophageal pressure: benefit and limitations. Minerva Anestesiol 78: 959–966, 2012. [PubMed] [Google Scholar]
- 15.Liu S, Margulies SS, Wilson TA. Deformation of the dog lung in the chest wall. J Appl Physiol (1985) 68: 1979–1987, 1990. doi: 10.1152/jappl.1990.68.5.1979. [DOI] [PubMed] [Google Scholar]
- 16.Mezidi M, Parrilla FJ, Yonis H, Riad Z, Böhm SH, Waldmann AD, Richard JC, Lissonde F, Tapponnier R, Baboi L, Mancebo J, Guérin C. Effects of positive end-expiratory pressure strategy in supine and prone position on lung and chest wall mechanics in acute respiratory distress syndrome. Ann Intensive Care 8: 86, 2018. doi: 10.1186/s13613-018-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mojoli F, Iotti GA, Torriglia F, Pozzi M, Volta CA, Bianzina S, Braschi A, Brochard L. In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care 20: 98, 2016. doi: 10.1186/s13054-016-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutoh T, Guest RJ, Lamm WJ, Albert RK. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis 146: 300–306, 1992. doi: 10.1164/ajrccm/146.2.300. [DOI] [PubMed] [Google Scholar]
- 19.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol (1985) 72: 575–582, 1992. doi: 10.1152/jappl.1992.72.2.575. [DOI] [PubMed] [Google Scholar]
- 20.Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164: 122–130, 2001. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- 21.Piehl MA, Brown RS. Use of extreme position changes in acute respiratory failure. Crit Care Med 4: 13–14, 1976. doi: 10.1097/00003246-197601000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359: 2095–2104, 2008. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiener CM, Kirk W, Albert RK. Prone position reverses gravitational distribution of perfusion in dog lungs with oleic acid-induced injury. J Appl Physiol (1985) 68: 1386–1392, 1990. doi: 10.1152/jappl.1990.68.4.1386. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida T, Amato MB, Grieco DL, Chen L, Lima CAS, Roldan R, Morais CC, Gomes S, Costa EL, Cardoso PF, Charbonney E, Richard JM, Brochard L, Kavanagh BP. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med 197: 1018–1026, 2018. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]