Abstract
In recent months, the coronavirus disease 2019 (COVID-19) pandemic has sent many countries into crisis. Studies have shown that this virus causes worse outcomes and a higher mortality in men than in women. It has been recognized that sex can affect the immune response to a pathogenic agent, as well as the susceptibility for some respiratory diseases. These different responses in males and females may be related to the actions of sex hormones. Angiotensin-converting enzyme 2 (ACE2) acts as the receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19. The expression of ACE2 is influenced by sex hormones; therefore, we discuss in this article that this could be one of the reasons why COVID-19 is more prevalent in men than in women.
Keywords: ACE2, hormones, pandemic, SARS-CoV, sex difference
Recently, we wrote a review about sex differences in breathing control (13). More than ever, we recognize that there is a higher prevalence in men of respiratory diseases than in women. In the last months, coronavirus disease 2019 (COVID-19) showed us this fact very clearly. As of April 30th, 2020, only 35 countries have reported official data regarding sex prevalence in confirmed cases of COVID-19. Of the 35 countries reporting, 14 reported having a higher number of cases in men, 2 reported having a similar incidence in both men and women, and 19 reported having a higher number of cases in women. This shows that for infection, it seems that both sexes can be affected (14) (see Supplementary Table S1, available at https://doi.org/10.6084/m9.figshare.12240707.v1). That sex difference in mortality can involve many factors, such as sociocultural gender factors and behaviors (3). In fact, a recent study also pointed out that ethnic/racial minority groups are being disproportionately affected by COVID-19 (3). Therefore, in addition to sex, other factors such as age, gender, and socioeconomic status need to be examined and disclosed to have a clearer picture of this pandemic.
However, 33 of those countries reported that the number of deaths was higher in men compared with women, which might indicate a clear sex difference. The highest proportion of men/women was seen in the Dominican Republic (men represented 78% of all deaths) (14) (Fig. 1). In China, where the disease is thought to have begun, the COVID-19 mortality in men was reported to be 4.7%, whereas mortality in women was 2.8% (number of deaths/number of cases), showing that men died 68% more often than women (14).
Fig. 1.
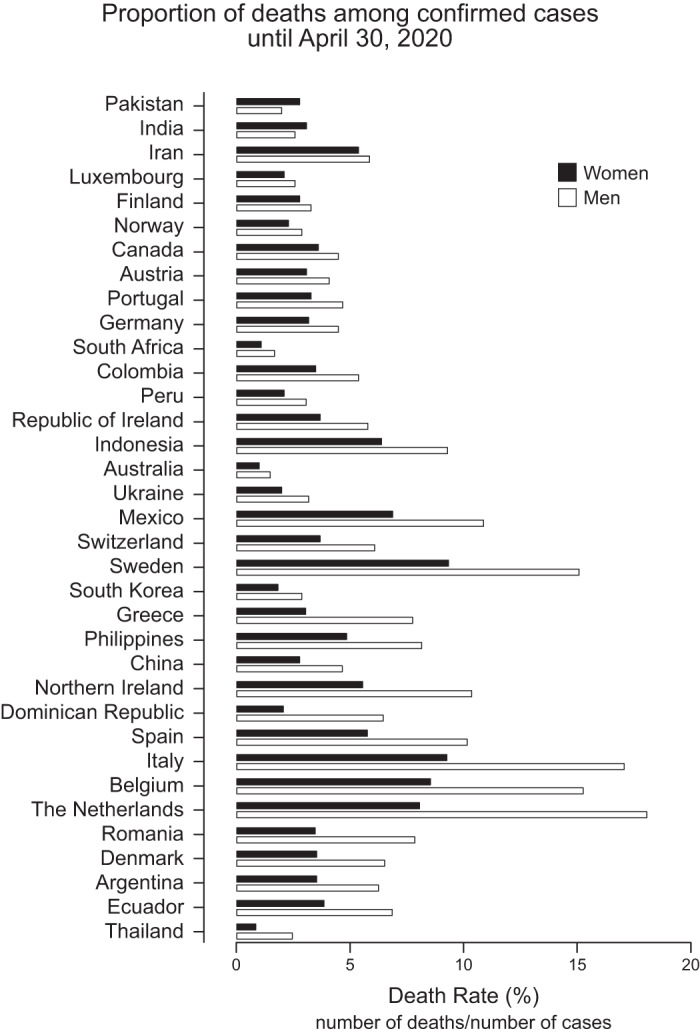
Graphs of data from 35 countries who provided information on the number of COVID-19-related deaths by sex. Death rate expressed in % of data using only number of confirmed deaths. Represented are the data acquired on or before April 30, 2020.
But why this “preference” in males?
One putative answer to this question is sex hormones. The main sex hormones are testosterone (T), progesterone (P), and estrogen (E2), produced by men and women during their adult lives in different concentrations. Men produce more T compared with women, who, in turn, produce E2 and P in higher concentrations (13). Sex hormones exert their actions via nuclear and membrane receptors (36) and are capable of changing membrane fluidity and the expression of mitochondrial proteins and genes that mediate many cellular mechanisms (22).
Sex hormones are very important for the development and activity of the immune system (36), contributing to the sexual dimorphism observed in immunological responses to viral infections (see Ref. 35 for review). In general, E2 has immunostimulatory roles, while P and T are immunosuppressive and counteract the pathways affected by E2 (36). Therefore, men produce less robust immune responses and are more susceptible to a variety of infectious agents (21). Specifically, regarding the severe acute respiratory syndrome coronavirus (SARS-CoV), a mouse model was developed to mimic SARS-CoV in humans (41). Roberts et al. (41) performed 15 passages of the virus in the respiratory tract of young BALB/c mice, which resulted in a lethal mouse-adapted SARS-CoV virus (MA15). These mice died from a very strong viral infection, with extensive destruction of pneumocytes and ciliated epithelial cells of the respiratory tract (41). Using this MA15 mouse model, Channappanavar et al. (7) evaluated whether SARS-CoV infection is sex or age dependent. First, the authors demonstrated that sexually immature, 2-mo-old male and female mice were completely resistant to developing SARS. They then used different doses of MA15 and evaluated the survival rate in sexually mature 8- to 10-mo-old male and female C57BL/6 mice. When the mice were infected with 5,000 PFU (plaque-forming units, which indicates the number of viral particles capable of lysing host cells and forming a plaque), the mortality rate was ~90% in male mice and ~20% in female mice. Upon increasing the MA15 dose to 10,000 PFU, all male mice died during the 5th day, while 50% of female mice died. Interestingly, when they gonadectomized the animals, the mortality rate increased in females, but did not change in males, suggesting a protective effect of E2 in female mice infected with SARS-CoV. The authors also observed that the degree of sex bias to viral infections increased with advancing age such that middle-aged mice (8–9 mo old) showed much more pronounced differences compared with young mice (2 mo old). All of these sex and age biases of SARS-CoV were also observed in humans (20).
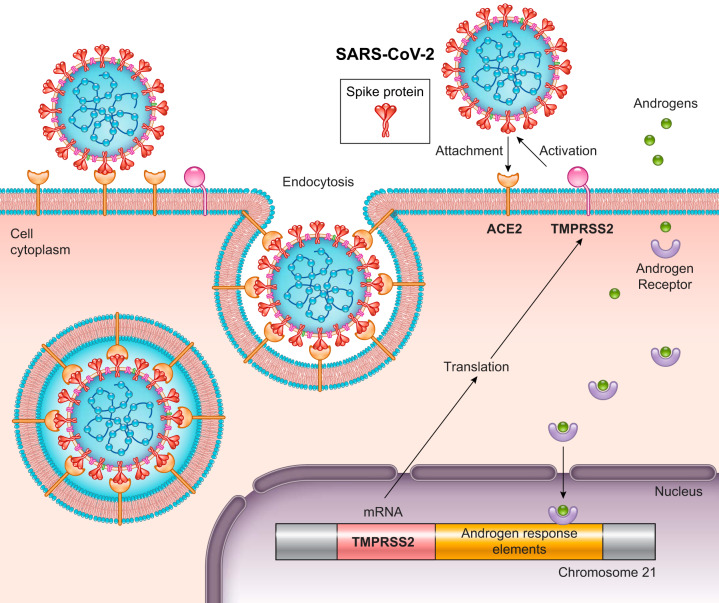
Coronavirus is formed by type 1 transmembrane spike (S) glycoproteins, which contain two functional domains (S1 and S2) that associate with cellular receptors to promote infection of their host cells (17). The angiotensin-converting enzyme 2 (ACE2) is considered the functional receptor for SARS-CoV. The receptor binding domain of the S1 spike protein enters cells by binding to ACE2 (28). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus identified as the cause of coronavirus disease (COVID-19), uses ACE2 as its receptor for cellular entry, similarly to the SARS-CoV (17). Additionally, viral S glycoproteins are activated by the transmembrane serine protease 2 (TMPRSS2), which facilitates virus-cell membrane fusion (17) (Fig. 2). In fact, an in vivo study using ACE2-knockout mice demonstrated that ACE2 is essential for the viral infection (24). These data provide the first genetic proof that ACE2 is indeed a crucial in vivo SARS receptor required for effective replication of infectious SARS-CoV. For instance, lung injury was reduced in ACE2-knockout mice compared with wild-type mice.
Fig. 2.
Schematic illustration showing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binding of the spike protein with angiotensin-converting enzyme 2 (ACE2), which acts as a receptor for the virus. Viral S glycoproteins are activated by transmembrane serine protease 2 (TMPRSS2), which facilitates virus-cell membrane fusion. TMPRSS2 transcription is upregulated by the androgen receptor. This leads to viral entry into the cell by endocytosis.
ACE2 mRNA and protein have been identified in several tissues (27). Recently, mRNA expression patterns of ACE2 were analyzed in 31 normal human tissues using two databases (27). The highest ACE2 expression was observed in the small intestine, testis, kidneys, heart, thyroid, and adipose tissue. The lungs, colon, liver, bladder, and adrenal gland showed moderate levels of ACE2 expression, while blood, spleen, bone marrow, brain, blood vessels, and muscle presented the lowest expression. Despite other tissues having higher ACE2 expression than the lungs, pneumonia is one of the main symptoms of patients infected with SARS-CoV-2. This might be attributed to the fact that the respiratory tract is the most readily available route of transmission for the virus (27). Besides ACE2, TMPRSS2 is also widely expressed in multiple tissues in the gastrointestinal system, lung, and kidney (46). Accordingly, multiorgan expression of these enzymes may explain the multiorgan dysfunction observed in patients with COVID-19 (48).
Recently, Sommerstein and Gräni (44) suggested that the use of angiotensin-converting enzyme inhibitors (ACE-Is) by patients with cardiovascular diseases could represent a lethal danger for COVID-19 by upregulating ACE2 and increasing the infection. However, this is not a consensus. Some authors believe the use of ACE-Is might be protective against COVID-19, since there is overwhelming evidence of mortality reduction in cardiovascular disease with this drug (25).
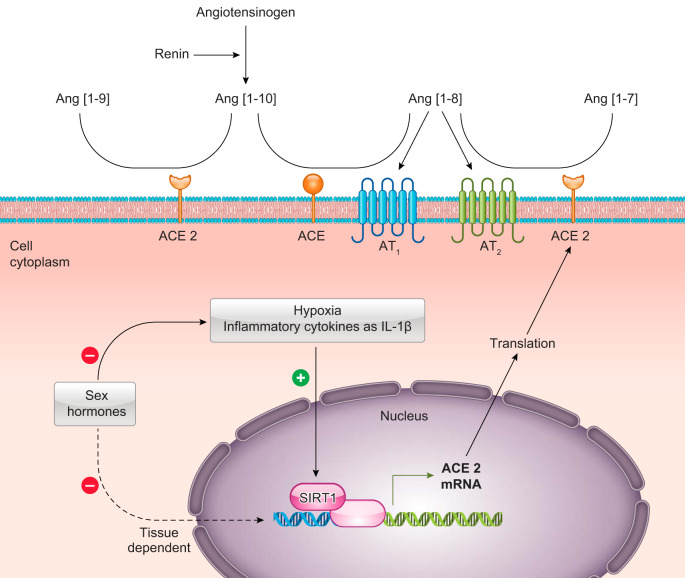
ACE converts angiotensin (Ang)-[1–10] into Ang-[1–8], which can promote vasoconstriction by acting on the angiotensin type 1 receptor (AT1) or vasodilation via the angiotensin type 2 receptor (AT2). ACE2 is a monocarboxypeptidase that catabolizes the conversion of Ang-[1–8] to the heptapeptide Ang-[1–7], which is described to have vasodilatory effects by binding MAS or MAS-related G protein-coupled receptors. ACE2 also converts Ang-[1–10] into the nonopeptide Ang-[1–9], also described to have vasodilatory effects by binding to AT2 (15). ACE2 has been identified as an important renin-angiotensin system (RAS) regulator, which is a peptidergic system that regulates extracellular fluid volume and controls homeostasis of the cardiovascular and renal systems (10, 15, 31). The gene that codes for ACE2 is located on the X chromosome (23), which raises the possibility that females, having two X chromosomes, could have differences in ACE2 expression compared with males with only one X chromosome. However, the expression of ACE2 appears to be more influenced by sex hormones, such as E2, than the presence of a second X chromosome, as demonstrated by Liu et al. (30). Using the four-core genotype mouse model which enables separation of sex-chromosome effects from gonadal hormone effects, this study showed that E2-mediated downregulation of ACE2 expression in the kidney is sex-chromosome independent.
Major sex differences have been identified in the expression levels of components of the RAS (45). For instance, ACE2 protein and mRNA expression, as well as ACE2 activity, are higher in the kidney in male mice than in female mice under basal conditions (30), which would imply that SARS-CoV would find ACE2 to be more readily available in males. Remarkably, this sex difference in ACE2 activity was not observed in the heart or lung in the same animals. Likewise, supplementation with E2 caused a downregulation of ACE2 mRNA expression in the mouse kidney, but not in the lung, showing that sex hormones can also regulate ACE2 in a tissue-specific manner (30). However, under pathological conditions, several studies have found that Ang-[1–7] levels are higher in women than in men, particularly in those with hypertension (45), indicating a higher ACE2 activity. Thus, increased levels of this vasodilator could also contribute to female protection from Ang-[1–8]-induced hypertension (45). In this regard, a recent study in mice showed that ACE2 plays a larger role in protecting females than males from Ang-[1–8]-induced hypertension by increasing catabolism of Ang-[1–8] and by downregulating AT1 (19). Hypertension is a recognized major risk factor for COVID-19 morbidity and mortality (26). Women are more protected from hypertension due to higher Ang-[1–7] expression (45), which may play a role in their protection from COVID mortality, despite potential elevations in ACE2 levels. Regarding COVID-19, there are no clear data about sex hormone levels and their association with ACE2 activity in different organs, including the lungs. As previously mentioned, ACE2 is widely distributed in many tissues, and its activity appears to be differentially regulated in different organs. Thus, further studies are urgent to provide new insights into the role of ACE2 in the SARS-CoV-2 pandemic and for understanding the associations of SARS-CoV-2 symptoms and sex differences.
To date, there are no data in the literature regarding the effects of T on the concentration of ACE2 receptors in the lungs. Nevertheless, TMPRSS2 is highly expressed in epithelial cells in adult human lungs, small intestine, heart, liver, thymus, and prostate (46), and its transcription is upregulated by the androgen receptor (Fig. 2) (32). This protease may also cleave ACE2 for augmented viral entry (16). This could also explain the higher susceptibility of males to SARS-CoV-2 infection compared with females. However, Channappanavar and Perlman (8) observed that SARS-CoV infection significantly reduced serum T levels in mice, which was also recently demonstrated in humans infected with COVID-19 (47). Interestingly, low levels of T appear to be linked to increased susceptibility of respiratory diseases, such as asthma and COPD (37), as well as cardiovascular disease, diabetes, and others (1) that represent risk factors for the severity of COVID-19 (33, 49). Additionally, animal and human studies showed that T deficiency is associated with an increase in proinflammatory cytokines (4, 37), independent of other risk factors (2, 38). In illness with COVID-19, an important mediating factor in mortality appears to be excessive release of proinflammatory cytokines (“cytokine storm”). As mentioned before, deaths from COVID-19 are higher in men, affecting mostly elderly men (49), who, in turn, have naturally low levels of T compared with young men. In some cases, the hypogonadism promoted by COVID-19 can cause severe complications in the infected. Therefore, measuring T levels in these patients would be helpful, since androgen suppression would reduce host vulnerability when infection risk is high, but can worsen the systemic inflammatory response during the course of the disease.
The transcriptional regulation of ACE2 is not completely elucidated; however, Clarke et al. (9) demonstrated that ACE2 is upregulated by interleukin (IL)-1β treatment and in response to hypoxic conditions by activation of transcriptional mediators, such as the silent information regulator T1 (SIRT1), which binds to the promoter region and facilitates ACE2 mRNA expression. Although hypoxia, IL-1β, and angiotensin peptides regulate ACE2 expression, steroid hormones appear to be a modulator of its expression (4, 6, 11, 42). Moreover, sex hormones also regulate the hypoxic ventilatory response (13) and the concentrations of proinflammatory cytokines, such as IL-1β (34, 40, 43). Therefore, sex hormones can act directly or indirectly to control ACE2 (Fig. 3).
Fig. 3.
Schematic illustration showing the classic renin-angiotensin system (RAS) and the sequential cleavage of protein substrates by specific proteases. The primary substrate for the RAS is angiotensinogen, which is converted by renin into angiotensin (Ang)-[1–10]. In a sequential reaction, the dicarboxyl-peptidase angiotensin-converting enzyme (ACE) cleaves Ang-[1–10] to form Ang-[1–8]. Angiotensin-[1–8] could bind to angiotensin AT1 and AT2 receptors, each with distinct functions, or could be degraded by angiotensin-converting enzyme 2 (ACE2) into a heptapeptide, Ang-[1–7]. ACE2 also can degrade Ang-[1–10] into a nonapeptide, Ang-[1–9]. ACE2 expression is regulated by hypoxia, IL-1β, and estradiol (E2) via histone deacetylase sirtuin 1 (SIRT1), which binds to the promoter region and facilitates ACE2 mRNA expression. Hypoxia and IL-1β can also be modulated by E2.
Indeed, Brosnihan et al. (4) investigated the role of E2 in the regulation of ACE/ACE2, and in the angiotensin receptor subtypes AT1/AT2, using mRNA measurements in the lung and kidney of female mice. They found that nonpregnant mice treated with E2 without alpha E2 receptors presented a reduction in gene expression of ACE2 in the lungs, suggesting that E2 might be involved in the regulation of the ACE2 gene in the lung. Notably, renal ACE2 activity has been shown to be upregulated during pregnancy in Sprague Dawley rats (5). A recent study suggested that during pregnancy, women may be more susceptible to COVID-19, since pregnant women, in general, are vulnerable to respiratory infection (29). However, a systematic recent review suggests that COVID-19 during pregnancy has a similar clinical presentation and infection severity to nonpregnant adults and may not be associated with poor maternal or perinatal outcomes (12). In fact, COVID-19 is less severe in pregnancy than the two SARS-CoV and Middle East Respiratory Syndrome-related coronavirus (MERS) (12). According to the authors, the concept that during pregnancy occurs an immune suppression increasing the risk for infection is incorrect. During pregnancy, human chorionic gonadotropin inhibits the proinflammatory cytokines, which could be protective for the cytokine storm (12).
At the moment, no vaccine is available to control the spread of COVID-19, and many potential therapeutic drugs have been suggested for treatment of this disease. Here, we point out the potential of sex hormones as therapeutics, which have not yet been explored. We believe that consideration of sex as a factor can increase the efficacy and safety of medicine use and can also help to design effective translational interventions for sexually dimorphic disorders. One good example of how sex can be a determinant in disease treatments is the study of Huded et al. (18) where consideration of sex in the treatment of patients with ST-segment elevation myocardial infarction (STEMI) resulted in a 50% reduction in mortality of women (following 30 days of STEMI, mortality rate was initially 6.1%, and after considering sex, in treatment, was reduced to 3.1%).
Notably, there is an on-going study, led by Dr. Sharon Nachman from Stony Brook University, which aims to evaluate whether E2 delivery to COVID-19-positive or presumed-positive patients via a transdermal patch for 7 days can reduce the severity of COVID-19 symptoms compared with standard care (39). They hypothesized that E2 would reduce symptom severity in adult men and older women if given before intubation.
In summary, from our understanding, patients who are currently receiving oral contraceptives or hormone replacement therapies need to be studied for their potential disparities related to COVID-19 outcomes.
GRANTS
The laboratory of L. H. Gargaglioni is funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.H.G. and D.A.M. drafted manuscript; L.H.G. and D.A.M. edited and revised manuscript; L.H.G. and D.A.M. approved final version of manuscript.
REFERENCES
- 1.AACE Hypogonadism Task Force AACE Medical Guidelines for Clinical Practice for the Evaluation and Treatment of Hypogonadism in Adult Male Patient — 2002 Update. Endocr Pract 8: 439–456, 2002. [Erratum in Endocr Pract 14: 802–803, 2008]. doi: 10.4158/EP.8.6.439. 152600 [DOI] [Google Scholar]
- 2.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 96: 3007–3019, 2011. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhala N, Curry G, Martineau AR, Agyemang C, Bhopal R. Sharpening the global focus on ethnicity and race in the time of COVID-19. Lancet S0140-6736(20)31102-8, 2020. doi: 10.1016/S0140-6736(20)31102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-α knock-out mice. Exp Physiol 93: 658–664, 2008. doi: 10.1113/expphysiol.2007.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosnihan KB, Neves LAA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension 42: 749–753, 2003. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- 6.Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, Bornfleth P, Kutschka I, Gardemann A, Isermann B, Goette A. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med (Maywood) 242: 1412–1423, 2017. doi: 10.1177/1535370217718808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based differences in susceptibility to Severe Acute Respiratory Syndrome Coronavirus infection. J Immunol 198: 4046–4053, 2017. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39: 529–539, 2017. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke NE, Belyaev ND, Lambert DW, Turner AJ. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond) 126: 507–516, 2014. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 10.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res 98: 463–471, 2006. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 11.Elangovan S, Ramachandran S, Venkatesan N, Ananth S, Gnana-Prakasam JP, Martin PM, Browning DD, Schoenlein PV, Prasad PD, Ganapathy V, Thangaraju M. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor α in breast cancer. Cancer Res 71: 6654–6664, 2011. doi: 10.1158/0008-5472.CAN-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshafeey F, Magdi R, Hindi N, Elshebiny M, Farrag N, Mahdy S, Sabbour M, Gebril S, Nasser M, Kamel M, Amir A, Emara MM, Nabhan A. A systematic scoping review of COVID‐19 during pregnancy and childbirth. Int J Gynecol Obstet. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargaglioni LH, Marques DA, Patrone LGA. Sex differences in breathing. Comp Biochem Physiol A Mol Integr Physiol 238: 110543, 2019. doi: 10.1016/j.cbpa.2019.110543. [DOI] [PubMed] [Google Scholar]
- 14.Global Health 50/50 COVID-19 – Global Health 50/50. COVID-19 Sex-Disaggregated Data Tracker (Online). https://globalhealth5050.org/covid19/sex-disaggregated-data-tracker/ [ 4 Apr 2020].
- 15.Guignabert C, de Man F, Lombès M. ACE2 as therapy for pulmonary arterial hypertension: the good outweighs the bad. Eur Respir J 51: 1800848, 2018. doi: 10.1183/13993003.00848-2018. [DOI] [PubMed] [Google Scholar]
- 16.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88: 1293–1307, 2014. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020.01.31.929042, 2020. doi: 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- 18.Huded CP, Johnson M, Kravitz K, Menon V, Abdallah M, Gullett TC, Hantz S, Ellis SG, Podolsky SR, Meldon SW, Kralovic DM, Brosovich D, Smith E, Kapadia SR, Khot UN. 4-Step protocol for disparities in STEMI care and outcomes in women. J Am Coll Cardiol 71: 2122–2132, 2018. doi: 10.1016/j.jacc.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 19.Ji H, de Souza AMA, Bajaj B, Zheng W, Wu X, Speth RC, Sandberg K. Sex-specific modulation of blood pressure and the renin angiotensin system by angiotensin converting enzyme 2. Hypertension. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 159: 229–231, 2004. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 16: 626–638, 2016. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 22.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem 105: 1342–1351, 2008. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsu T, Suzuki Y, Imai J, Sugano S, Hida M, Tanigami A, Muroi S, Yamada Y, Hanaoka K. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2). DNA Seq 13: 217–220, 2002. doi: 10.1080/1042517021000021608. [DOI] [PubMed] [Google Scholar]
- 24.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 41: 1801–1803, 2020. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci 21: 2948, 2020. doi: 10.3390/ijms21082948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9: 45, 2020. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Wang LL, Zhao SJ, Kwak-Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol 139: 103122, 2020. doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 1: 6, 2010. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo J, Patel VB, Wang Z, Levasseur J, Kaufman S, Penninger JM, Oudit GY. Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar-Kyoto rats and spontaneously hypertensive rats. Exp Physiol 98: 109–122, 2013. doi: 10.1113/expphysiol.2012.067165. [DOI] [PubMed] [Google Scholar]
- 32.Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, Morrissey C, Corey E, Montgomery B, Mostaghel E, Clegg N, Coleman I, Brown CM, Schneider EL, Craik C, Simon JA, Bedalov A, Nelson PS. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov 4: 1310–1325, 2014. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 34.Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22: 129–140, 2019. doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 35.Morgan R, Klein SL. The intersection of sex and gender in the treatment of influenza. Curr Opin Virol 35: 35–41, 2019. doi: 10.1016/j.coviro.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol 9: 2279, 2018. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60: 762–769, 2006. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 169: 725–733, 2013. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 39.Nachman S. Estrogen Patch for COVID-19 Symptoms (Online). https://clinicaltrials.gov/ct2/show/NCT04359329 [ 1 May 2020].
- 40.Okabe H, Makino S, Kato K, Matsuoka K, Seki H, Takeda S. The effect of progesterone on genes involved in preterm labor. J Reprod Immunol 104-105: 80–91, 2014. doi: 10.1016/j.jri.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Roberts A, Deming D, Paddock CD, Cheng A, Yount B, Vogel L, Herman BD, Sheahan T, Heise M, Genrich GL, Zaki SR, Baric R, Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog 3: e5, 2007. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santolla MF, Avino S, Pellegrino M, De Francesco EM, De Marco P, Lappano R, Vivacqua A, Cirillo F, Rigiracciolo DC, Scarpelli A, Abonante S, Maggiolini M. SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. Cell Death Dis 6: e1834, 2015. doi: 10.1038/cddis.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 72: 121–129, 2015. doi: 10.1016/j.cyto.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommerstein R, Gräni C. Rapid Response: Re: Preventing a covid-19 pandemic: ACE inhibitors as a potential risk factor for fatal. BMJ 368: m810, 2020. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan JC, Rodriguez-Miguelez P, Zimmerman MA, Harris RA. Differences in angiotensin (1-7) between men and women. Am J Physiol Heart Circ Physiol 308: H1171–H1176, 2015. doi: 10.1152/ajpheart.00897.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaarala MH, Porvari KS, Kellokumpu S, Kyllönen AP, Vihko PT. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J Pathol 193: 134–140, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 47.Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol S0190-9622(20)30608-3, 2020. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]