Abnormal sleep is a prominent feature of Axis I neuropsychiatric disorders and is often included in their DSM-V diagnostic criteria. While often viewed as secondary, as these disorders may themselves diminish sleep quality, there is growing evidence that sleep disorders can aggravate, trigger, and even cause a range of neuropsychiatric conditions. Moreover, as has been shown in major depression and attention deficit hyperactivity disorder, treating sleep can improve symptoms, suggesting that disrupted sleep contributes to the clinical syndrome and is a target for treatment. In addition to its effects on symptoms, sleep disturbance, which is known to impair emotional regulation and cognition in otherwise healthy individuals, may contribute to or cause disabling cognitive deficits. For sleep to be a target for treatment of symptoms and cognitive deficits in neuropsychiatric disorders, its role in initiating and maintaining these disorders needs to be understood.
Posttraumatic stress disorder (PTSD) exemplifies this relationship with sleep. DSM-V criteria for PTSD include “difficulty falling or staying asleep” and recurrent “nightmares related to the traumatic event.” Here the associated sleep disorder is not only insomnia but also a specific disorder of dreaming. Importantly, the nightmares of PTSD are distinctly different from nightmares experienced more generally in the population in that they appear to be near veridical replays of traumatic events. Such replay of episodic memories is unusual in normal dreams, occurring in less than 1–2% of dream reports (1). This replay of traumatic events during dreams in PTSD may be a reflection of defective sleep-dependent memory processing. This is a tenet of the theory that PTSD is a memory disorder that develops when the mechanisms underlying the normal post-encoding evolution of memory fail to convert an emotionally charged episodic memory of a traumatic event into an integrated and emotionally modulated narrative story of the trauma. It further proposes that the mechanisms that normally process traumatic memories depend largely on sleep, and that it is a failure of these sleep-dependent memory processes, perhaps due to sleep disruption, that leads to and perpetuates PTSD (2) (Figure 1A). This theory is supported by epidemiological and prospective studies showing that sleep disturbances that predate trauma exposure, or begin soon after, increase the risk of developing a neuropsychiatric disorder, including PTSD. This theory, that abnormal sleep contributes to the development and perpetuation of PTSD, also has important implications for treatment.
Figure 1:
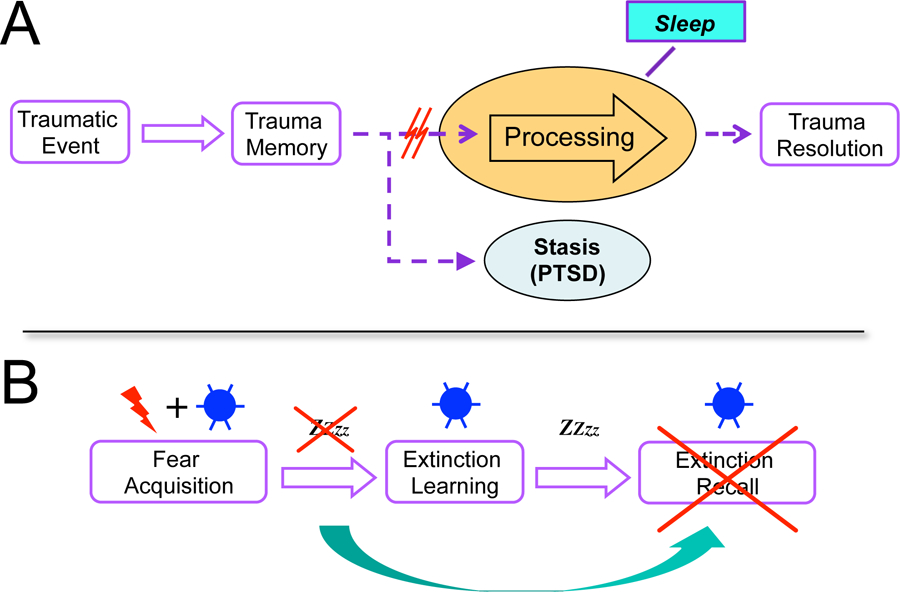
Traumatic events are encoded into trauma memories that are normally processed during sleep, leading to resolution of the trauma. Processing includes (1) reduction in the associated fear response, (2) forgetting of irrelevant details, and (3) integration into pre-existing memory networks. All three of these have been shown to occur preferentially during sleep.
The standard treatment for PTSD is exposure-based therapy, which involves repeatedly re-exposing the individual to elements of the fearful experience in a safe setting until their fear and distress are extinguished. Based on a compelling body of work from human and rodent studies, fear extinction reflects not the erasure of the fear memory but the development of a new safety or “extinction memory” that inhibits the fear memory and its associated emotional response.
In this issue, Straus and colleagues (3) report that total sleep deprivation can impair the retention of such extinction memories. In their study, healthy human participants in three groups successfully learned to associate a blue circle (conditioned stimulus, CS) with the occurrence of an electric shock (unconditioned stimulus) during a fear acquisition session. The following day, during extinction learning, the blue circle was repeatedly presented without the shock. The day after that, extinction recall was tested by again repeatedly presenting the blue circle without the shock. In all three sessions, fearfulness in response to the appearance of the blue circle was measured as startle reactivity (EMG-measured eyeblinks) to an auditory stimulus that occurred after the appearance of the circle, but before the shock was expected. The sleep manipulation was as follows: Controls were allowed to sleep normally throughout the experiment; the Pre-Extinction group was deprived of sleep the night after the fear acquisition session but before the extinction learning session (Figure 1B); and the Post-Extinction group was deprived of sleep the night after the extinction learning session, but before the extinction recall testing session.
As expected, all three groups showed increased startle responses to the blue circle over the course of the fear acquisition session. On the following day, all three groups, including the one that had been deprived of sleep the night before, retained the conditioned startle response (CR), which decreased to below baseline levels over the course of the extinction learning session. During the extinction recall session the next day, the extinguished startle response remained extinguished in Controls. Surprisingly, the Post-Extinction group also showed intact extinction recall, despite having no sleep the previous night. But the most striking result is that the Pre-Extinction group, despite having slept the night before and having shown intact extinction learning, showed impaired recall of this learning – their fear response was greater than that of Controls.
These results are especially surprising given additional findings of this study suggesting that rapid eye movement sleep (REM) contributes to the consolidation of extinction learning. Using a complex composite measure of REM consolidation calculated on the night of the extinction learning session, Straus et al. reported that decreased REM consolidation correlated with increased fear responses during extinction recall in the combined Control and Pre-Extinction groups. This suggests that REM following extinction enhances the retention of extinction learning. Why then did Post-Extinction participants, who had no sleep at all after extinction learning, show intact extinction recall? Further confusing matters, our re-analysis of the data presented in Figure 4 of Straus et al. shows that the correlation was only present for the Pre-Extinction group (r = 0.49, p = 0.013), which was sleep-deprived the night prior to extinction learning, and not for Controls who slept normally throughout (r = 0.11, p = 0.64).
If this all sounds confusing, it is, and reflects the general state of the field. REM deprivation immediately following extinction learning has been shown to impair extinction recall in rats (4), but in the present study total sleep deprivation after extinction learning left extinction recall intact. In addition, while previous work in humans reported that sleep deprivation after fear acquisition reduced subsequent fear responses (5), in the present study sleep-deprivation after fear acquisition did not diminish the fearful response, and in a study of rats, REM deprivation after fear acquisition enhanced fear responses (6). These rats also showed impaired extinction learning, unlike the Pre-Extinction group here, who showed intact extinction learning.
What can we take from this contradictory literature? One point is that the measurement of autonomic responses is difficult. In the current study, an entire second arm of the protocol could not be analyzed because of an unexpected failure of subjects to condition to a second stimulus (red circles). In addition, to reduce noise, the startle responses to the baseline noise-alone trials (noise without the prior presentation of the CS) were averaged across entire sessions, even though these responses clearly decreased across each session (Figure S1, 3) and even though they served as a baseline measures of fear acquisition, extinction learning and extinction recall, which were calculated based only on the initial or final trials of the session. Differences in measurements across studies (e.g., in the present study, the use of an idiosyncratic composite measure of “REM consolidation” instead of more typical REM measures) makes it difficult to compare findings and may lead to the appearance of discrepancies. The authors also raise the question of whether animal models translate directly to humans.
As a result of these inconsistencies in results and methods, convergent findings from multiple laboratories will be needed before clear patterns emerge. In the interim, studies of deficits in sleep-dependent cognitive processes in PTSD sufferers may provide more information about the altered brain processes that lead to PTSD.
What we can conclude from this study and those preceding it is that sleep, and possibly REM in particular, plays an important role in the normal learning and maintenance of fear extinction. Fear extinction is a critical component of the evolution of trauma memories that normally prevents the development of PTSD. And in PTSD, as in other Axis I neuropsychiatric disorders (e.g., 7), rather than viewing abnormal sleep as consequence of the disorder, it may be more valid and productive to study the mechanisms by which it may be causal and target them for treatment.
Acknowledgments:
Support from: R01MH048832 (RS); MH092638 (DSM, RS); MH099421 (DSM).
Footnotes
Financial disclosures: Neither author has any biomedical financial interests or potential conflicts of interest to report.
Cited Literature
- 1.Fosse MJ, Fosse R, Hobson JA, Stickgold RJ (2003): Dreaming and episodic memory: a functional dissociation? J Cogn Neurosci 15:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Stickgold R (2007): Of sleep, memories and trauma. Nat Neurosci 10:540–542. [DOI] [PubMed] [Google Scholar]
- 3.Straus LD, Acheson DT, Risbrough VB, Drummond SPA (2017): Sleep deprivation disrupts recall of conditioned fear extinction. Sleep deprivation disrupts recall of conditioned fear extinction. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, et al. (2007): Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 144:1186–1192. [DOI] [PubMed] [Google Scholar]
- 5.Kuriyama K, Soshi T, Kim Y (2010): Sleep deprivation facilitates extinction of implicit fear generalization and physiological response to fear. Biol Psychiatry. 68:991–998. [DOI] [PubMed] [Google Scholar]
- 6.Silvestri AJ (2005): REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav 84:343–349. [DOI] [PubMed] [Google Scholar]
- 7.Manoach DS, Pan JQ, Purcell SM, Stickgold R (2016): Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry. 80:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]


