Abstract
Extracranial metastasis from glioblastoma multiforme (GBM) is rare, especially multi-site metastases without intracranial recurrence. However, the metastatic mechanism of GBM remains unknown and there is currently no consensus regarding the best therapeutic regimen. We report the case of a 46-year-old man with primary GBM who developed scalp metastases and subsequent multiple pulmonary metastases. He was treated with the Stupp regimen after surgery for the intracranial tumor. However, a series of soft masses in the scalp were subsequently identified, and new nodules were found in his left eyebrow arch during chemoradiotherapy. Despite salvage chemotherapy and targeted therapy, the patient eventually died of respiratory failure with multiple pulmonary metastases. This case highlights the need for rigorous follow-up, including brain magnetic resonance imaging, in patients with GBM. The occurrence of extra-central nervous system symptoms indicates the possibility of metastasis, and the relevant examinations should be conducted promptly. Positive therapies may help to relieve symptoms and prolong survival in patients with metastatic GBM.
Keywords: Glioblastoma multiforme, multiple extracranial metastases, lung metastasis, scalp metastasis, follow-up, case report
Introduction
Glioblastoma multiforme (GBM) is the most common malignant central nervous system (CNS) tumor in adults, accounting for 47.7% of all primary malignant brain tumors.1 Surgery is the first-line treatment for GBM, and gross total resection improves survival. Postoperative radiotherapy plus concomitant and adjuvant chemotherapy with temozolomide (TMZ) has also been the standard treatment for patients with GBM.2 However, GBM is the most aggressive type of brain tumor with a poor prognosis and a median survival time of only about 14 months.3
Extracranial metastasis of GBM is rare, with a reported incidence of about 0.4% to 2.0%.3 Extra-CNS metastases mainly occur in adults, particularly men.4 The prognosis of metastatic GBM is poor, with a median overall survival (OS) from diagnosis of metastasis of 6.0 ± 0.8 months.5
Case Report
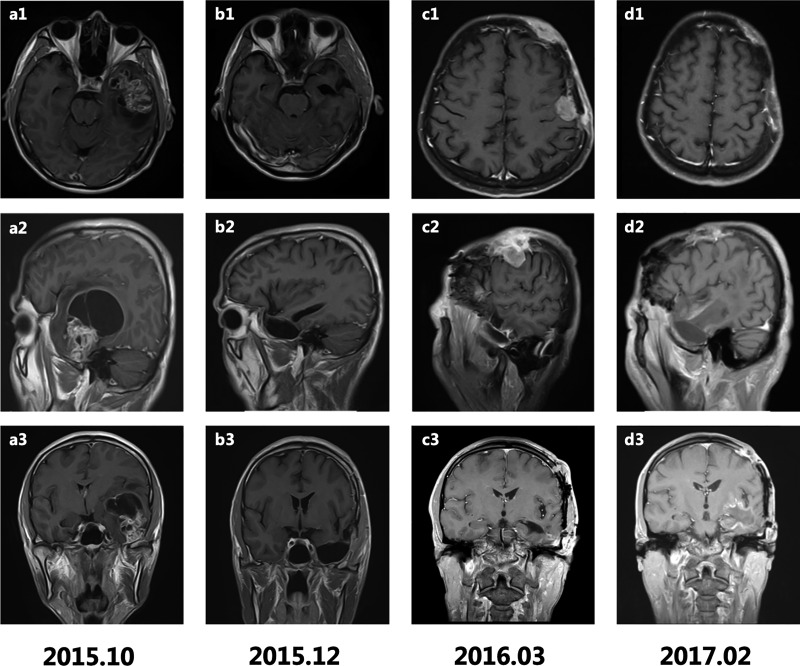
We report an otherwise healthy 46-year-old man with primary GBM who developed widespread extracranial progression and distant metastasis. He was admitted to hospital in October 2015 with history of a headache for >10 days, aggravated for 2 days, accompanied by a decline in language function and memory. Magnetic resonance imaging (MRI) scans (Figure 1) revealed a contrast-enhanced mass (60 × 60 × 54 mm) with partial cystic degeneration in his left temporal lobe (Figure 1a1–3). He underwent craniotomy with complete lesion resection, and primary GBM (World Health Organization grade IV) was diagnosed by postoperative pathological examination (Figure 2). Immunohistochemistry (IHC) of the brain tumor demonstrated positive staining for glial fibrillary acidic protein (GFAP) and Ki67 (+30%) (Figure 2d, g), but negative staining for O6-methylguanine-DNA methyltransferase (MGMT) and isocitrate dehydrogenase 1 (IDH1). The MGMT promoter was confirmed to be unmethylated by methylation-specific polymerase chain reaction, and Sanger sequencing revealed wild-type IDH1/2. The operative Karnofsky performance scale score was high (≥90), and he therefore underwent a postoperative Stupp regimen including concurrent chemoradiotherapy (CRT) followed by adjuvant TMZ chemotherapy. The primary tumor disappeared on MRI scans after concurrent CRT (Figure 1b1–3). However, the patient experienced disease progression after only four cycles of adjuvant chemotherapy.
Figure 1.
T1-weighted gadolinium-enhanced axial, sagittal, and coronal magnetic resonance imaging scans. (a1–3) Contrast-enhanced mass (60 × 60 × 54 mm) with partial cystic degeneration in the left temporal lobe before craniotomy; (b1–3) postoperative changes without residual tumor after concurrent chemoradiotherapy (CRT); (c1–3) multiple scalp masses in the frontal and temporal regions; (d1–3) relief of scalp masses after re-operation and CRT.
Figure 2.
Histopathology of primary tumor and metastases. Hematoxylin and eosin staining (a–c), immunostaining for glial fibrillary acidic protein (d–f) and Ki67 (g–i) in primary glioblastoma multiforme, scalp metastases, and lung metastases. HE, hematoxylin and eosin; GFAP, glial fibrillary acidic protein.
In March 2016, the patient presented with a series of soft neoplasms in his scalp, and MRI scans showed multiple scalp masses in the frontal and temporal regions (Figure 1c1–3). The left frontal scalp lesion was resected in May 2016 and postoperative pathological examination showed malignant tumors of the left frontotemporal scalp and epidural space (Figure 2b), confirmed as GBM by positive IHC for GFAP and Ki-67(+60%) (Figure 2e, h). After the second surgery, the patient received further radiotherapy (60 Gy/2 Gy, 30 fractions planned) and synchronous systemic irinotecan chemotherapy. However, he was unable to tolerate irinotecan chemotherapy because of grade 4 bone marrow suppression, high fever, and diarrhea. In addition, he only received 30 Gy irradiation because of the appearance of new nodules in the left eyebrow arch after the 15th fraction of radiotherapy. We then redesigned the target area and started the second phase of radiotherapy (32.1 Gy/2.14 Gy for 15 fractions) with concurrent dose-dense TMZ chemotherapy. At the end of CRT, MRI surveillance showed that the eyebrow nodules were slightly reduced (about 25 × 20 mm) and had almost disappeared after 2 months of CRT. Lesion resection and CRT therefore helped to achieve local control of the scalp metastases in this patient.
The patient continued to take oral dose-dense TMZ after discharge. However, he presented with chest pain 6 months later, in December 2016. Physical examination revealed low breath sounds in his bilateral lower lungs. Chest radiography demonstrated multiple pulmonary nodules of different sizes (Figure 3), although no obvious abnormalities had been detected during the previous few months (Figure 3a, b). Repeat MRI scans in February 2017 showed reduced scalp masses (Figure 1d1–3). Whole-body 18F-fluorodeoxyglucose-positron emission tomography conducted 1 month later revealed hypermetabolism in the lungs, the bilateral pleural area, left hilar lymph nodes, left internal mammary region, and para-thoracic aorta (Figure 3d). Biopsy of nodules in the left lung in April 2017 led to a pathological diagnosis of poorly differentiated malignant tumor (Figure 2c), considered to be metastases from the GBM (WHO grade IV). IHC was strongly positive for GFAP(+++) and Ki67(+10%) (Figure 2f, i). Dose-dense TMZ chemotherapy combined with bevacizumab targeted therapy was administered. However, the patient eventually died of respiratory failure with multiple pulmonary metastases in June 2017. The time interval between the diagnosis of primary GBM and the diagnosis of scalp metastases was > 6 months, and the interval between the diagnosis of GBM and the biopsy-based identification of multiple pulmonary metastases was about 18 months. The patient’s survival was thus 20 months from resection of the primary GBM to the time of death.
Figure 3.
Chest X-ray image and 18F-fluorodeoxyglucose positron emission tomography (PET) scan. (a) Negative chest X-ray image before craniotomy of primary glioblastoma multiforme; (b) negative chest X-ray image before scalp metastases; (c) chest X-ray image showed multiple lung nodules after 9 months of scalp metastases; (d) PET-computed tomography scan revealed multiple areas of hypermetabolism in the lungs.
The patient and his family provided signed informed consent for publication of this report.
Discussion
GBM is a highly aggressive brain tumor with high heterogeneity leading to therapeutic resistance, thus posing a serious threat to patient survival. Only about one third of patients survive for 1 year and the 5-year survival rate is about 4% to 5%.6 A meta-analysis by Binabaj et al.7 revealed that MGMT methylation was associated with longer OS in patients with GBM, but no similar relationship with progression-free survival (PFS). The median OS rates of adult GBM patients with methylated and unmethylated MGMT gene promoter were 21.2 and 14.0 months, respectively.8 Moreover, survival of patients with wild-type IDH remains poor compared with patients with IDH mutation.9
The rarity of extracranial metastasis is attributed to the short survival period, and the presence of the blood–brain barrier (BBB) and lack of a classic lymphatic drainage system.10 In a recent meta-analysis by Cunha et al.11 of 114 cases of metastatic GBM, most cases involved metastases in a single organ or site, and, to the best of our knowledge, only 12 cases of GBM multi-site metastases (including the current case) have been described with or without intracranial recurrence (Table 1). The most common primary site of GBM with extracranial metastases is the temporal lobe, and its metastatic sites include the lungs, pleura, lymph nodes, liver, skin, scalp, parotid gland, spleen, pancreas, bowel mesentery, peritoneum, epidural space, and bones, while spread to the meninges or spinal cord via the cerebrospinal fluid (CSF) is also frequent. Piccirilli et al.12 reported that the common metastatic sites included the lungs and pleura (60%), lymph nodes (51%), and bones (31%). Extracranial metastases of GBM to soft tissues are extremely unusual, and the first well-documented case was reported by Davis in 1928.13 Patients with pulmonary metastasis from GBM have the worst prognosis.14 As seen in the current study, the patient developed a primary GBM with uncommon local scalp metastases and diffuse pulmonary metastases, and survived for approximately 6 months from the onset of chest pain to death.
Table 1.
Summary of previous and present cases of patients with multiple extracranial metastases from GBM.
| Reference | Sex | Age (years) | Primary site | Metastatic sites | Treatments after GBM operation | Time interval from operation/biopsy to metastasis | Treatments after metastasis | Time interval from metastasis to death | Intracranial recurrence |
|---|---|---|---|---|---|---|---|---|---|
| Simonetti et al.29 | M | 38 | L parietal lobe | Lung, lymph nodes, bones (right iliac crest, thoracic vertebrae) | RT, CT (TMZ); BV after recurrence | 4 years | Local RT of the iliac crest, CT (etoposide, oncocarbide) | 8 weeks | Yes |
| Karatas et al.30 | M | 55 | R temporal lobe | Thoracic spine (T4-T7); cerebellar, cervical spine (C5-C6) | RT, CT | 2 years; 5 years | S, RT, CT; S | 5 years; 2 years | No; no |
| Anghileri et al.31 | M | 30 | L central sulcus | R lateral cervical and occipital areas, lungs | RT, CT (TMZ); re-S after first recurrence; BV after second recurrence | 86 months | S of the cervical lesion, CT refused | 4 months | Yes (second) |
| Romero-Rojas et al.32 | M | 26 | L frontal lobe | Parotid gland, cervical lymph nodes (levels IIB, III and IV), vertebral bones | RT, CT (TMZ) | 6 months | RT, TMZ CT | 18 months | No |
| Zhen et al.33 | M | 25 | R parietofrontal lobe | R cervical lymph nodes, bones (mainly pelvic bone) | RT | About 2 months | S of lymph nodes, CT | NS | NS |
| Saad et al.34 | M | 13.5 | L frontal lobe | Leptomeninges, cranial skin, subcutaneous tissue, L temporalis muscle; liver, L lung after autopsy | RT, CT (TMZ); antiangiogenic therapy after recurrence | About 6 months | CT (procarbazine, CCNU) | About 4 months | Yes |
| Toledano Delgado et al.35 | M | 65 | R temporal lobe | Cerebellum, spine | RT, CT | 10 months | NS | NS | NS |
| Mujic et al.36 | M | 39 | L frontal lobe | Soft tissue mass within the abdomen, small bowel mesentery, pancreas; L lung, pleura, L hilar lymph nodes | RT, re-S of intracranial recurrence | 25 months | Treatments refused | 1 month | Yes |
| Taha et al.37 | M | 33 | L frontal lobe | L parotid gland, cervical lymph nodes | RT, CT and re-S of intracranial recurrence | About 6 months | Local RT of L parotid gland, CT (PCV) | 3 months | Yes |
| Ogungbo et al.38 | F | 49 | L occipital lobe | L parotid gland, cervical lymph nodes, lungs | RT, CT (CCNU, procarbazine) | About 12 months | Palliative treatment | 2 months | Yes |
| Beauchesne et al.39 | M | 54 | R temporal lobe | Dorsolumbar vertebrae and R iliac bone; L medulla, L lung and heart after autopsy | RT, CT (etoposide) | 8 months | No aggressive treatment | <1 month | No |
| Present case | M | 46 | L temporal lobe | Scalp masses (in frontal and temporal regions, L eyebrow arch); lungs | RT, CT (TMZ) | >6 months; 18 months | S of the frontal scalp mass, local RT, CT; CT (TMZ), BV | 13 months; 2 months | No |
GBM, glioblastoma multiforme; M, male; F, female; L, left; R, right; NS, not stated; S, surgery; re-S, re-operation; RT, radiotherapy; TMZ, temozolomide; CT, chemotherapy; BV, bevacizumab.
The possible mechanisms, including the genetic and molecular factors, leading to extracranial metastases remain unclear and require further investigation. Extracranial GBMs are most commonly found in patients with prior invasive surgery or biopsy, which create iatrogenic access to extracranial structures.3 Metastatic GBM may thus occur by migration of GBM cells in the CSF through shunts to the peritoneum or peritoneal cavity,15 or direct seeding to soft tissues through craniotomy defects.16 However, surgery is not a prerequisite for extracranial metastasis of GBM, and extra-CNS metastases have been reported in both adults and children without craniotomy.17–20 Endogenous factors may also result in the extracranial spread of GBM. Current studies21,22 propose that GBM metastasis occurs as a result of breakdown of the BBB. This barrier is breached in GBM, as visualized on T1-weighted MRI. Breakdown of the BBB in patients with metastases and high-grade gliomas is associated with disruption of both endothelial tight junctions and astrocyte–endothelial cell interactions. BBB disruption also affects peritumoral edema, and tumor development and progression.21,22 In addition to the above factors, other mechanisms of cancer cell escape include vascular invasion, lymphatic spread, cranial nerve perineural spread, and direct invasion. However, transmission of GBM cells to distant organs via a hematogenous or lymphatic route is infrequent because of the existence of the BBB or lack of lymphatic drainage system. Further studies are needed to investigate potential molecular biomarkers and genetic factors that may help to predict these complex mechanisms of metastatic potential.3
Regarding diagnostic techniques, MRI, especially T1-weighted MRI, is the first-choice method and should be repeated periodically to assess the primary lesion in patients with GBM. Furthermore, extensive high signals in FLAIR and T2-weighted images may reflect a combination of peritumoral edema and tumor extension. Diffusion-weighted and perfusion-weighted MRI, as well as X-ray computed tomography (CT), single-photon emission CT (SPECT), and positron emission tomography (PET)-CT also contribute to the detection of primary brain lesions. SPECT is used to detect bone destruction, while PET-CT is a systemic examination that helps to detect the presence of systemic metastases. Pathological biopsy is also necessary, when feasible, for making a precise diagnosis of GBM, and liquid biopsy of circulating tumor cells or peripheral blood biomarkers could be considered if invasive tumor biopsy or surgery is restricted and the effectiveness of imaging is limited. A blood test using GFAP as a marker for GBM cells has been established, and the presence of circulating GFAP-positive cells opens up the possibility of a liquid biopsy for primary brain tumors.23 Detection of extracellular vesicles and circulating cell-free nucleic acids may also be an alternative option to avoid high-risk neurosurgical interventions in patients with GBM.24 However, the sensitivity and specificity of liquid biopsy need to be improved before its clinical application. In the current case, the patient presented with chest pain and chest X-ray examination showed diffuse scattered dot-like changes, which did not preclude fungal infection. However, PET-CT 3 months later detected multiple pulmonary metastases. The possibility of lung metastases should thus be considered when symptoms first appear, especially in patients with scalp metastases, and CT examination should thus be carried out as soon as possible to confirm the patient’s pulmonary condition and allow active systemic treatment to be carried out.
Multidisciplinary therapies are required for patients with GBM, and the standard regimen includes surgery and postoperative concurrent CRT. Resection of intracranial GBM remains the first step in the treatment after diagnosis, and the extent of resection is crucial in terms of increased survival.2,6 The Stupp regimen is widely used during clinical practice, and other treatments, including targeted agents such as bevacizumab, result in no significant improvement in OS but longer PFS.2 Prophylactic craniospinal irradiation may be considered in patients at high risk of CSF seeding, if the ventricles were opened during surgery or if the tumor was in close contact with the CSF.16,25 Previous studies have also suggested that local chemotherapy combined with anti-programmed cell death protein 1 agents can facilitate an antitumor immune response and improve survival in GBM patients.26 Moreover, tumor-treating fields (TTFields) was confirmed to improve OS and PFS in the phase 3 EF-14 trial in patients with GBM.27 TTFields has been approved by the United States Food and Drug Administration for use in patients with newly diagnosed and recurrent GBM, with no obvious toxicity.28 In the current patient, good local control of scalp metastases was achieved, indicating that re-irradiation combined with chemotherapy was effective for local metastases. However, the patient’s memory declined, suggesting possible radiation encephalopathy caused by re-irradiation; however, we unfortunately did not perform a functional MRI examination. Furthermore, the patient’s condition progressed rapidly after the development of lung metastases, and we therefore did not observe any efficacy of bevacizumab and had no time to try other medicines. In short, this case suggests that patients with GBM and extra-CNS metastases should be treated actively to relieve symptoms and prolong survival.
Although extracranial metastasis of GBM is not uncommon, multiple metastases are rare, especially in patients without intracranial recurrence. Long-term survivors may be at increased risk of developing extracranial metastases. The current patient represents the first reported case of GBM with scalp metastases followed by multiple lung metastases. Rigorous follow-up should be carried out in patients with GBM, especially using advanced MRI techniques, and aggressive locoregional and systemic treatments are necessary to reduce recurrence and metastasis and prolong survival. Further studies of the complex mechanism of metastatic GBM and its therapeutic regimen are needed to improve its currently poor prognosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China [No. 81974466] and the Science Foundation of Xiangya Hospital for Young Scholars [No. 2015Q09].
ORCID iD
References
- 1.Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol 2018; 20: iv1–iv86. DOI: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reni M, Mazza E, Zanon S, et al. Central nervous system gliomas. Crit Rev Oncol Hematol 2017; 113: 213–234. DOI: 10.1016/j.critrevonc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JD, Rapp M, Schneiderhan T, et al. Glioblastoma multiforme metastasis outside the CNS: three case reports and possible mechanisms of escape. J Clin Oncol 2014; 32: e80–e84. DOI: 10.1200/JCO.2013.48.7546. [DOI] [PubMed] [Google Scholar]
- 4.Pasquier B, Pasquier D, N’Golet A, et al. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer 1980; 45: 112–125. DOI: 10.1002/1097-0142(19800101)45: 1<112: : aid-cncr2820450121>3.0.co; 2-9. [DOI] [PubMed] [Google Scholar]
- 5.Pietschmann S, Von Bueren AO, Kerber MJ, et al. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/gliosarcoma patients with metastases outside of the central nervous system. PLoS One 2015; 10: e0121592. DOI: 10.1371/journal.pone.0121592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batash R, Asna N, Schaffer P, et al. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem 2017; 24: 3002–3009. DOI: 10.2174/0929867324666170516123206. [DOI] [PubMed] [Google Scholar]
- 7.Binabaj MM, Bahrami A, ShahidSales S, et al. The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J Cell Physiol 2018; 233: 378–386. DOI: 10.1002/jcp.25896. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 2013; 31: 4085–4091. DOI: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong D, Yip S. Finding a four-leaf clover-identifying long-term survivors in IDH-wildtype glioblastoma. Neuro Oncol 2019; 21: 1352–1353. DOI: 10.1093/neuonc/noz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Wang Y, Xu J, et al. Extensive therapies for extraneural metastases from glioblastoma, as confirmed with the OncoScan assay. World Neurosurg 2016; 90: 698.e7–698.e11. [DOI] [PubMed] [Google Scholar]
- 11.Cunha MLVD, Maldaun MVC. Metastasis from glioblastoma multiforme: a meta-analysis. Rev Assoc Med Bras (1992) 2019; 65: 424–433. DOI: 10.1590/1806-9282.65.3.424. [DOI] [PubMed] [Google Scholar]
- 12.Piccirilli M, Brunetto GM, Rocchi G, et al. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori 2008; 94: 40–51. [DOI] [PubMed] [Google Scholar]
- 13.Davis L. Spongioblastoma multiforme of the brain. Ann Surg 1928; 87: 8–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Lun M, Lok E, Gautam S, et al. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol 2011; 105: 261–273. DOI: 10.1007/s11060-011-0575-8. [DOI] [PubMed] [Google Scholar]
- 15.Newton HB, Rosenblum MK, Walker RW. Extraneural metastases of infratentorial glioblastoma multiforme to the peritoneal cavity. Cancer 1992; 69: 2149–2153. [DOI] [PubMed] [Google Scholar]
- 16.Forsyth TM, Bi WL, Abedalthagafi M, et al. Extracranial growth of glioblastoma multiforme. J Clin Neurosci 2015; 22: 1521–1523. DOI: 10.1016/j.jocn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Anzil AP. Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case report. J Neurosurg 1970; 33: 88–94. [DOI] [PubMed] [Google Scholar]
- 18.Dolman CL. Lymph node metastasis as first manifestation of glioblastoma. Case report. J Neurosurg 1974; 41: 607–609. [DOI] [PubMed] [Google Scholar]
- 19.Hulbanni S, Goodman PA. Glioblastoma multiforme with extraneural metastases in the absence of previous surgery. Cancer 1976; 37: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 20.Gamis AS, Egelhoff J, Roloson G, et al. Diffuse bony metastases at presentation in a child with glioblastoma multiforme. A case report. Cancer 1990; 66: 180–184. [DOI] [PubMed] [Google Scholar]
- 21.Nduom EK, Yang C, Merrill MJ, et al. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J Neurosurg 2013; 119: 427–433. DOI: 10.3171/2013.3.JNS122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong Y, Durden DL, Van Meir EG, et al. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol 2006; 65: 529–539. [DOI] [PubMed] [Google Scholar]
- 23.Burki TK. Blood-borne glioblastoma cells discovered. Lancet Oncol 2014; 15: e422. [DOI] [PubMed] [Google Scholar]
- 24.Klekner Á, Szivos L, Virga J, et al. Significance of liquid biopsy in glioblastoma - A review. J Biotechnol 2019; 298: 82–87. [DOI] [PubMed] [Google Scholar]
- 25.Kuo LT, Tsai SY, Yang CY, et al. Meningeal seeding from glioblastoma multiforme treated with radiotherapy and temozolomide. Asian J Surg 2017; 40: 61–65. DOI: 10.1016/j.asjsur.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med 2016; 8: 370ra180. DOI: 10.1126/scitranslmed.aag2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 2015; 314: 2535–2543. DOI: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 28.Mun EJ, Babiker HM, Weinberg U, et al. Tumor-treating fields: a fourth modality in cancer treatment. Clin Cancer Res 2018; 24: 266–275. DOI: 10.1158/1078-0432.Ccr-17-1117. [DOI] [PubMed] [Google Scholar]
- 29.Simonetti G, Silvani A, Fariselli L, et al. Extra central nervous system metastases from glioblastoma: a new possible trigger event? Neurol Sci 2017; 38: 1873–1875. DOI: 10.1007/s10072-017-3036-0. [DOI] [PubMed] [Google Scholar]
- 30.Karatas Y, Cengiz SL, Ustun ME. A case of symptomatic synchronous cervical and cerebellar metastasis after resection of thoracal metastasis from temporal glioblastoma multiforme without any local recurrence. Asian J Neurosurg 2016; 11: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anghileri E, Elena A, Castiglione M, et al. Extraneural metastases in glioblastoma patients: two cases with YKL-40-positive glioblastomas and a meta-analysis of the literature. Neurosurg Rev 2016; 39: 37–45; discussion 45–36. DOI: 10.1007/s10143-015-0656-9. [DOI] [PubMed] [Google Scholar]
- 32.Romero-Rojas AE, Diaz-Perez JA, Amaro D, et al. Glioblastoma metastasis to parotid gland and neck lymph nodes: fine-needle aspiration cytology with histopathologic correlation. Head Neck Pathol 2013; 7: 409–415. DOI: 10.1007/s12105-013-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhen L, Yufeng C, Zhenyu S, et al. Multiple extracranial metastases from secondary glioblastoma multiforme: a case report and review of the literature. J Neurooncol 2010; 97: 451–457. DOI: 10.1007/s11060-009-0044-9. [DOI] [PubMed] [Google Scholar]
- 34.Saad AG, Sachs J, Turner CD, et al. Extracranial metastases of glioblastoma in a child: case report and review of the literature. J Pediatr Hematol Oncol 2007; 29: 190–194. [DOI] [PubMed] [Google Scholar]
- 35.Toledano Delgado R, Garcia N, Riva-Amarante E, et al. [Spinal leptomeningeal metastasis from cerebral glioblastoma: case report]. Neurologia 2006; 21: 378–381. [PubMed] [Google Scholar]
- 36.Mujic A, Hunn A, Taylor AB, et al. Extracranial metastases of a glioblastoma multiforme to the pleura, small bowel and pancreas. J Clin Neurosci 2006; 13: 677–681. [DOI] [PubMed] [Google Scholar]
- 37.Taha M, Ahmad A, Wharton S, et al. Extra-cranial metastasis of glioblastoma multiforme presenting as acute parotitis. Br Journal Neurosurg 2005; 19: 348–351. [DOI] [PubMed] [Google Scholar]
- 38.Ogungbo BI, Perry RH, Bozzino J, et al. Report of GBM metastasis to the parotid gland. J Neurooncol 2005; 74: 337–338. [DOI] [PubMed] [Google Scholar]
- 39.Beauchesne P, Soler C, Mosnier JF. Diffuse vertebral body metastasis from a glioblastoma multiforme: a technetium-99m Sestamibi single-photon emission computerized tomography study. J Neurosurg 2000; 93: 887–890. [DOI] [PubMed] [Google Scholar]