Abstract
Background
Cefiderocol is a novel siderophore cephalosporin, developed for activity against MDR Gram-negative bacilli (MDR-GNB).
Objectives
To assess the in vitro antibacterial activity of cefiderocol against a collection of MDR-GNB clinical isolates from hospitals in southern Spain.
Methods
Two hundred and thirty-one isolates of successful clones were tested: 125 Enterobacterales (121 ESBL- and/or carbapenemase-producing Klebsiella pneumoniae and 4 carbapenemase-producing Enterobacter cloacae), 80 Acinetobacter baumannii, 6 Pseudomonas aeruginosa and 20 Stenotrophomonas maltophilia. Ceftolozane/tazobactam, ceftazidime, ceftazidime/avibactam, cefepime, aztreonam, meropenem, amikacin, ciprofloxacin, colistin and tigecycline were used as comparators against Enterobacterales, P. aeruginosa and A. baumannii. Minocycline, levofloxacin and trimethoprim/sulfamethoxazole were studied against S. maltophilia instead of aztreonam, ciprofloxacin and cefepime. MICs were determined by broth microdilution according to CLSI guidelines. MIC determination was performed in CAMHB for all antimicrobials except cefiderocol, where iron-depleted CAMHB was used.
Results
Cefiderocol showed potent in vitro activity against the isolates analysed. MIC50 and MIC90 values were in the ranges 0.125–8 mg/L and 0.5–8 mg/L, respectively, and 98% of isolates were inhibited at ≤4 mg/L. Only five isolates showed cefiderocol MICs of >4 mg/L: three ST2/OXA-24/40-producing A. baumannii, one ST114/VIM-1-producing E. cloacae and one ST114/VIM-1 + OXA-48-producing E. cloacae. All KPC-3-producing K. pneumoniae were susceptible to cefiderocol, even those resistant to ceftazidime/avibactam. P. aeruginosa isolates showed cefiderocol MICs of <4 mg/L, including those resistant to ceftolozane/tazobactam. S. maltophilia isolates displayed cefiderocol MICs of <4 mg/L, including those resistant to levofloxacin and/or trimethoprim/sulfamethoxazole.
Conclusions
Cefiderocol showed excellent activity against MDR-GNB, including carbapenem-resistant isolates, and was the most active antimicrobial tested against this collection.
Introduction
MDR Gram-negative bacilli (MDR-GNB) represent an important public health problem, which was recently described by the WHO as a global crisis.1 Nosocomial and healthcare-associated infections caused by MDR organisms significantly increase morbidity, mortality and medical costs.2 Infections caused by these bacteria are very difficult to treat since strains are resistant to all first-line anti-Gram-negative antibiotics, such as β-lactams, and to fluoroquinolones. A few new antimicrobials such as ceftazidime/avibactam and ceftolozane/tazobactam are available in many countries, but these compounds have very limited activity against bacteria producing MBLs.3 Alternative drugs, such as colistin, tigecycline, fosfomycin and aminoglycosides, are frequently the only options.3
The WHO has published a priority list of pathogens for which the development of new antibiotics is urgently required. Among these microorganisms are carbapenem-resistant Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumannii.4
The spread and persistence of these pathogens is favoured by selection for successful, high-risk (HR) clones. These clones are characterized by their enhanced ability to cause nosocomial outbreaks and to develop or acquire resistance to multiple antimicrobials, including extended-spectrum agents such as carbapenems. Horizontal transfer of plasmids carrying genes that encode carbapenemases is probably one of the most important factors in the success of these epidemic clones.5 MBL-producing GNB are also increasing, including in Spain.6
Cefiderocol is a new parenteral catechol-substituted siderophore cephalosporin. This antimicrobial has a unique mechanism of penetration into bacterial cells. Experiments with P. aeruginosa have shown that cefiderocol can easily enter the periplasmic space using the iron transport system, via the binding of the catechol moiety of the drug to extracellular trivalent iron.7 The high activity of cefiderocol is also due to its increased stability against various types of β-lactamases, including serine-based and metallo-type carbapenemases.8 In contrast, cefiderocol shows weak or no activity against Gram-positive and anaerobic bacteria.9
Cefiderocol has been reported to have potent in vitro activity against MDR-GNB, including carbapenem-resistant strains of Enterobacterales, P. aeruginosa and A. baumannii, as well as against Stenotrophomonas maltophilia.10–12
In vivo human pharmacokinetic and pharmacokinetics/pharmacodynamics data from an animal infection model have been published.13,14 Cefiderocol has demonstrated efficacy against GNB based on human pharmacokinetic properties.14
A recent report demonstrated the efficacy and safety of cefiderocol as monotherapy for the treatment of invasive infections caused by MDR A. baumannii and Klebsiella pneumoniae.15
The purpose of this study was to provide data on the comparative in vitro antimicrobial activity of cefiderocol against a collection of cephalosporin- and/or carbapenem-resistant Gram-negative HR clones with characterized antibiotic resistance mechanisms from clinical sources, isolated in hospitals in southern Spain.
Methods
Bacteria
The isolates of MDR-GNB tested in this study (n = 231) were selected from a well-characterized collection held in the Reference Laboratory of the Andalusian programme for the surveillance and control of healthcare-associated infections and antibiotic stewardship (PIRASOA programme), based in the Hospital Universitario Virgen Macarena, Seville, Spain.16,17 The selected isolates came from 27 hospitals in the eight provinces of Andalusia. Sixteen isolates from 2014 were selected, 37 from 2015, 92 from 2016, 84 from 2017 and 2 from 2018. The inclusion criteria for selection of isolates were those belonging to HR clones, except for S. maltophilia, and MDR isolates. Isolates of Enterobacterales (n = 125; 121 K. pneumoniae and 4 Enterobacter cloacae) were chosen if they were ESBL and/or carbapenemase producers. Isolates of A. baumannii (n = 80) were selected if they demonstrated oxacillinase production and P. aeruginosa (n = 6) if they were resistant to carbapenems. Twenty isolates of S. maltophilia were included, based solely on identification and MDR phenotype. The characteristics and resistance determinants of selected isolates are shown in Table 1.
Table 1.
Isolates of MDR GNB tested (n = 231)
| Species | Clone/resistance determinant | No. of isolates (no. of pulsotypes) |
|---|---|---|
| A. baumannii | ST2/OXA-23 | 25 (17) |
| ST2/OXA-58 | 25 (14) | |
| ST2/OXA-24/40 | 25 (15) | |
| ST745/OXA-23 | 5 (4) | |
| P. aeruginosa | ST175 | 5 (NA) |
| ST253/IMP-16 | 1 (NA) | |
| K. pneumoniae | ST11/CTX-M-15 | 5 (5) |
| ST11/CTX-M-15 + OXA-48 | 25 (9) | |
| ST15/CTX-M-15 | 5 (3) | |
| ST15/CTX-M-15 + OXA-48 | 25 (6) | |
| ST512/KPC-3 | 25 (9) | |
| ST258/KPC-3 | 25 (9) | |
| ST147/KPC-3 | 1 (NA) | |
| ST147/CTX-M-15 + OXA-48 | 2 (NA) | |
| ST147/OXA-48 | 1 (NA) | |
| ST392/CTX-M-15 | 3 (3) | |
| ST392/CTX-M-15 + OXA-48 | 4 (3) | |
| E. cloacae | ST114/CTX-M-15 | 2 (NA) |
| ST114/VIM-1 | 1 (NA) | |
| ST114/OXA-48 + VIM-1 | 1 (NA) | |
| S. maltophilia | – | 20 (NA) |
NA, not applicable; a dash indicates data not available.
Bacterial identification, identification of resistance genes and molecular epidemiology
The identification of isolates was confirmed at the reference laboratory using MALDI-TOF MS (MALDI-TOF Biotyper 3.1; Microflex Bruker, Madrid, Spain).
The presence of ESBL (CTX-M group) and carbapenemases (NDM/VIM/KPC/IMP/OXA-48 groups for Enterobacterales and P. aeruginosa, and OXA for A. baumannii) was evaluated by PCR and DNA sequencing.18–20
PFGE analysis of XbaI- (Enterobacterales), SpeI- (P. aeruginosa) and ApaI-digested (A. baumannii) DNA (http://www.cdc.gov/pulsenet) was used to determine the degree of genetic relatedness between isolates. Isolates differing by two or more bands in PFGE assays were assigned to different pulsotypes, except for K. pneumoniae ST512 and ST258, for which different pulsotypes were assigned when they differed in one band. A dendrogram was created with Fingerprinting 3.0 software (Bio-Rad), using the Dice coefficient with position tolerance settings of 1% optimization and 1.2% band position tolerance. PFGE was not performed for S. maltophilia. The MLST scheme developed by the Institut Pasteur was used to characterize a subset of isolates representing different PFGE clusters and all S. maltophilia were included (https://bigsdb.pasteur.fr/index.html). All pulsotypes assigned to the same MLST were considered to belong to the same clone. Whenever possible, isolates from the same clone with different pulsotypes were selected.
Drug susceptibility testing
Susceptibility testing was performed using frozen broth microdilution plates prepared by International Health Management Associates (IHMA; Schaumburg, IL, USA) and Shionogi & Co., Ltd. (Osaka, Japan) to determine the MICs of cefiderocol and comparators. Broth microdilution panels included the following ranges of antimicrobial agents (mg/L) for Enterobacterales, P. aeruginosa and A. baumannii: cefiderocol (doubling dilution range tested: 0.03–64), ceftolozane/tazobactam (0.03–64), meropenem (0.03–64), ceftazidime (0.03–64), ceftazidime/avibactam (0.03–64), colistin (0.5–8), aztreonam (0.5–32), amikacin (4–64), ciprofloxacin (0.25–4), cefepime (0.5–16) and tigecycline (0.25–4). For S. maltophilia, the activities of aztreonam, ciprofloxacin and cefepime were not tested and the in vitro activity of minocycline (2–64), levofloxacin (1–16) and trimethoprim/sulfamethoxazole (0.25/4.75–16/304) were studied instead.
MICs were determined by broth microdilution in CAMHB, according to CLSI guidelines.21,22 To evaluate the antimicrobial activity of cefiderocol, iron-depleted (ID) CAMHB was used.23 The microdilution panels included growth control wells for both CAMHB and ID-CAMHB. Panels were incubated in ambient air at 35°C for 16–20 h before reading MIC endpoints. In parallel, Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as control strains on each day of testing, checking that all quality control results were within the specified CLSI ranges for ceftolozane/tazobactam, meropenem, ceftazidime, ceftazidime/avibactam, colistin, aztreonam, amikacin, ciprofloxacin, cefepime, tigecycline, minocycline, levofloxacin and trimethoprim/sulfamethoxazole.24 The CLSI-approved range for cefiderocol (E. coli ATCC 25922, 0.06 to 0.5 mg/L; P. aeruginosa ATCC 27853, 0.06 to 0.5 mg/L) was also included.25
Cefiderocol MICs were read as the first drug well in which growth was significantly reduced (i.e. a button of <1 mm or light/faint turbidity) relative to the growth observed in the ID-CAMHB growth control well. The method used for reading MIC endpoints for cefiderocol, described above, was approved by the CLSI Subcommittee on Antimicrobial Susceptibility Testing in January 2016.25
CLSI investigational breakpoints for cefiderocol (≤4mg/L for susceptible; 8 for intermediate; and ≥16 mg/L for resistant)26 were used for assignment to clinical category.
CLSI interpretive criteria, when available,26 were used to interpret the MIC values of comparators. Colistin and tigecycline lack CLSI breakpoints for Enterobacterales and the EUCAST MIC breakpoints for Enterobacterales were applied for these antimicrobials.27
Results
Antimicrobial activity against MDR-GNB
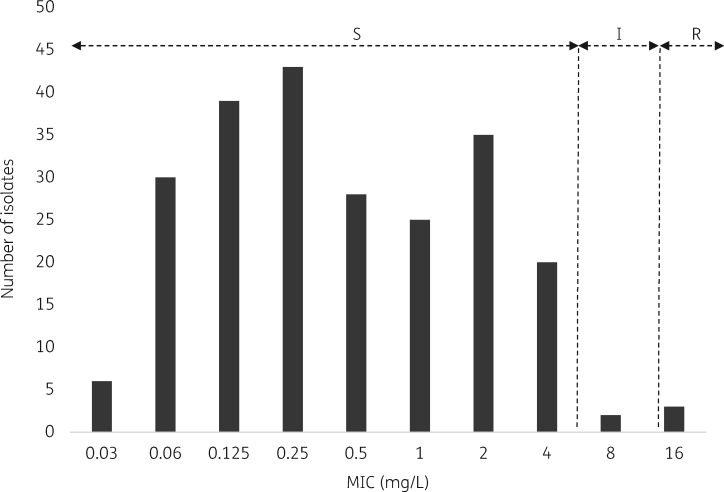
MIC distributions of cefiderocol for the clinical isolates included in this study are shown in Figure 1. After applying the CLSI clinical breakpoints,26 98% of isolates were susceptible, 0.8% intermediate and 1.2% resistant. Non-susceptible isolates were: three A. baumannii ST2 OXA-24/40 producers, one E. cloacae ST114 VIM-1 producer and one E. cloacae ST114 co-producing VIM-1 plus OXA-48 (Figure 1).
Figure 1.
Distribution of cefiderocol MICs for all isolates and clinical category according to investigational CLSI breakpoints. I, intermediate; R, resistant; S, susceptible.
Table 2 shows summary data of MIC ranges and MIC50 and MIC90 values of all antibiotics against the bacterial isolates tested and their respective resistance percentages. Against K. pneumoniae, A. baumannii and S. maltophilia, the concentrations of cefiderocol inhibiting 90% of isolates tested (MIC90) were 2, 4 and 0.5 mg/L, respectively. Cefiderocol MICs ranged from ≤0.03 to 16 mg/L for all isolates, ≤0.03 to 8 mg/L for Enterobacterales (≤0.03 to 4 mg/L for K. pneumoniae) and 0.06 to 16 mg/L for non-fermenting GNB (0.06 to 16 mg/L for A. baumannii, 0.125 to 0.5 for P. aeruginosa and ≤0.03 to 2 mg/L for S. maltophilia).
Table 2.
MIC ranges, MIC50s and MIC90s (mg/L) of cefiderocol and comparators and distribution by clinical category (according to CLSI, when available, and EUCAST when not established) against the MDR GNB tested (n = 231)
| Species (no. of isolates) | Antimicrobial agent | MIC (mg/L) |
Clinical category (%)a |
||||
|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | S | I | R | ||
| K. pneumoniae (n = 121) | |||||||
| cefiderocol | ≤0.03–4 | 0.5 | 2 | 100 | 0 | 0 | |
| ceftolozane/tazobactam | 0.25 to >64 | >64 | >64 | 7.4 | 1.6 | 90.9 | |
| meropenem | ≤0.03 to >64 | 8 | >64 | 23.9 | 12.4 | 63.6 | |
| ceftazidime | 0.5 to >64 | >64 | >64 | 3.3 | 0.8 | 95.9 | |
| ceftazidime/avibactam | ≤0.03–32 | 1 | 4 | 98.3 | NA | 1.7 | |
| colistinb | ≤0.5–8 | ≤0.5 | >8 | 85.9 | NA | 15.1 | |
| aztreonam | ≤0.5 to >32 | >32 | >32 | 0 | 0.8 | 99.1 | |
| amikacin | ≤4 to >64 | 8 | 64 | 60.3 | 28.1 | 11.6 | |
| ciprofloxacin | 1 to >4 | >4 | >4 | 0.8 | 0.8 | 98.3 | |
| cefepime | ≤0.5 to >16 | >16 | >16 | 1.7 | 6.6 | 91.7 | |
| tigecyclineb | ≤0.25 to >4 | 1 | 2 | 85.9 | 9.1 | 4.9 | |
| E. cloacae (n = 4) | |||||||
| cefiderocol | 0.5–8 | NC | NC | 50.0 | 50.0 | 0 | |
| ceftolozane/tazobactam | 0.25 to >64 | NC | NC | 25.0 | NA | 75.0 | |
| meropenem | 0.06–8 | NC | NC | 75.0 | 0 | 25.0 | |
| ceftazidime | 8 to >64 | NC | NC | 0 | 25.0 | 75.0 | |
| ceftazidime/avibactam | 0.25 to >64 | NC | NC | 50.0 | NA | 50.0 | |
| colistinb | ≤0.5 | NC | NC | 100 | NA | 0 | |
| aztreonam | 8 to >32 | NC | NC | 0 | 25.0 | 75.0 | |
| amikacin | ≤4–16 | NC | NC | 100 | 0 | 0 | |
| ciprofloxacin | 2 to >4 | NC | NC | 0 | 25.0 | 75.0 | |
| cefepime | 4 to >16 | NC | NC | 0 | 25.0 | 75.0 | |
| tigecyclineb | ≤0.25–2 | NC | NC | 50.0 | 50.0 | 0 | |
| A. baumannii (n = 80) | |||||||
| cefiderocol | 0.06–16 | 0.25 | 4 | 95 | 0 | 5 | |
| ceftolozane/tazobactam | 4 to >64 | 16 | >64 | NA | NA | NA | |
| meropenem | 4 to >64 | 64 | >64 | 0 | 3.8 | 96.2 | |
| ceftazidime | 4 to >64 | 64 | >64 | 6.3 | 2.5 | 91.2 | |
| ceftazidime/avibactam | 8 to >64 | 32 | 64 | NA | NA | NA | |
| colistin | ≤0.5 to >8 | 1 | 4 | 83.7 | NA | 16.3 | |
| aztreonam | 32 to >32 | >32 | >32 | NA | NA | NA | |
| amikacin | ≤4 to >64 | 64 | >64 | 37.5 | 11.3 | 51.2 | |
| ciprofloxacin | >4 | >4 | >4 | 0 | 0 | 100 | |
| cefepime | 8 to >16 | >16 | >16 | 1.2 | 11.3 | 87.5 | |
| tigecycline | 1 to >4 | 2 | 4 | NA | NA | NA | |
| P. aeruginosa (n = 6) | |||||||
| cefiderocol | 0.125–0.5 | NC | NC | 100 | 0 | 0 | |
| ceftolozane/tazobactam | 2 to >64 | NC | NC | 83.3 | 0 | 16.7 | |
| meropenem | 8–64 | NC | NC | 0 | 0 | 100 | |
| ceftazidime | 16 to >64 | NC | NC | 0 | 16.7 | 83.3 | |
| ceftazidime/avibactam | 4 to >64 | NC | NC | 83.3 | NA | 16.7 | |
| colistin | ≤0.5–1 | NC | NC | 100 | NA | 0 | |
| aztreonam | 32 to >32 | NC | NC | 0 | 0 | 100 | |
| amikacin | ≤4–16 | NC | NC | 100 | 0 | 0 | |
| ciprofloxacin | >4 | NC | NC | 0 | 0 | 100 | |
| cefepime | 16 to >16 | NC | NC | 0 | 83.3 | 16.7 | |
| tigecycline | >4 | NC | NC | NA | NA | NA | |
| S. maltophilia (n = 20) | |||||||
| cefiderocol | ≤0.03–2 | 0.25 | 0.5 | 100 | 0 | 0 | |
| ceftolozane/tazobactam | 0.5 to >64 | 32 | >64 | NA | NA | NA | |
| meropenem | 16 to >64 | >64 | >64 | NA | NA | NA | |
| ceftazidime | 4 to >64 | >64 | >64 | 15.0 | 0 | 85.0 | |
| ceftazidime/avibactam | 2 to >64 | 32 | >64 | NA | NA | NA | |
| colistin | ≤0.5 to >8 | >8 | >8 | NA | NA | NA | |
| amikacin | ≤4 to >64 | >64 | >64 | NA | NA | NA | |
| tigecycline | ≤0.25–4 | 0.5 | 2 | NA | NA | NA | |
| levofloxacin | ≤1–8 | ≤1 | 4 | 80.0 | 15.0 | 5.0 | |
| minocycline | ≤2–4 | ≤2 | 4 | 100 | 0 | 0 | |
| trimethoprim/sulfamethoxazole | ≤0.25 to >16 | ≤0.25 | 2 | 95.0 | NA | 5.0 | |
NA, not applicable; NC, not calculated because the number of test strains was <10; I, intermediate; R, resistant; S, susceptible.
CLSI breakpoints.
EUCAST breakpoints.
In Enterobacterales, the overall rates of resistance to ceftazidime/avibactam, ceftolozane/tazobactam, meropenem, amikacin, colistin and tigecycline were 3.2%, 90.4%, 62.4%, 43.2%, 19.2% and 62.4%, respectively. In A. baumannii and P. aeruginosa, the overall rates of resistance to ceftazidime, cefepime, meropenem, amikacin and colistin were 90.7%, 82.6%, 96.5%, 47.7% and 15.11%, respectively. All isolates tested were resistant to ciprofloxacin.
Antimicrobial activity against MDR Enterobacterales
One hundred and ten of the Enterobacterales included in the study were carbapenemase producers (108 K. pneumoniae and 2 E. cloacae), one isolate co-produced two carbapenemases (E. cloacae ST114; VIM-1 plus OXA-48) and 15 produced only ESBLs (13 K. pneumoniae and 2 E. cloacae) (Table 1).
The MIC ranges and MIC50 and MIC90 values of cefiderocol and comparators against this group of isolates are shown in Table 3. Applying CLSI breakpoints for cefiderocol, 100% of isolates, including KPC-3 producers, were susceptible to this cephalosporin. All isolates were ciprofloxacin resistant, 90% (46/51) were amikacin resistant and one (1.9%) isolate (ST512) was resistant to ceftazidime/avibactam; 13 (12 ST512 and 1 ST258) (25.5%) and 3 (one ST512, one ST258 and one ST147) (5.9%) isolates showed MICs of ≥4 mg/L for colistin and tigecycline, respectively. Among OXA-48 producers, 43.9% (25/57) were resistant to meropenem, 3.5% (2/57) to amikacin and 11 (19.3%) and 4 (7.0%) isolates, respectively, showed colistin and tigecycline MICs of ≥4 mg/L. After cefiderocol, the second most active agent against carbapenemase-producing K. pneumoniae was the ceftazidime/avibactam combination (1.8% of isolates were resistant; 2/108), regardless of the type of carbapenemase or clone.
Table 3.
MIC ranges, MIC50s and MIC90s (mg/L) of cefiderocol and comparators for 107 carbapenemase-producing K. pneumoniae, grouped by clone and type of carbapenemase produced
| Isolates | Antimicrobial agent | MIC range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) |
|---|---|---|---|---|
| ST11/OXA-48 + CTX-M-15 (n = 25) | ||||
| cefiderocol | ≤0.03–4 | 0.25 | 2 | |
| ceftolozane/tazobactam | 16 to >64 | >64 | >64 | |
| meropenem | 0.25–64 | 2 | 8 | |
| ceftazidime | 8 to >64 | 64 | >64 | |
| ceftazidime/avibactam | 0.5–2 | 1 | 2 | |
| colistin | ≤0.5 to >8 | ≤0.5 | 4 | |
| aztreonam | 32 to >32 | >32 | >32 | |
| amikacin | ≤4–64 | 8 | 16 | |
| ciprofloxacin | >4 | >4 | >4 | |
| cefepime | 8 to >16 | >16 | >16 | |
| tigecycline | 0.5–2 | 1 | 2 | |
| ST15/OXA-48 + CTX-M-15 (n = 25) | ||||
| cefiderocol | ≤0.03–4 | 0.25 | 4 | |
| ceftolozane/tazobactam | 0.5 to >64 | 64 | >64 | |
| meropenem | 1–64 | 2 | 64 | |
| ceftazidime | 0.5 to >64 | 64 | >64 | |
| ceftazidime/avibactam | ≤0.03–4 | 0.5 | 1 | |
| colistin | ≤0.5 to >8 | ≤0.5 | 2 | |
| aztreonam | ≤0.5 to >32 | >32 | >32 | |
| amikacin | ≤4–8 | ≤4 | ≤4 | |
| ciprofloxacin | 1 to >4 | >4 | >4 | |
| cefepime | ≤0.5 to >16 | >16 | >16 | |
| tigecycline | ≤0.25–2 | 0.5 | 2 | |
| ST512/KPC-3 (n = 25) | ||||
| cefiderocol | 0.25–4 | 2 | 4 | |
| ceftolozane/tazobactam | >64 | >64 | >64 | |
| meropenem | >64 | >64 | >64 | |
| ceftazidime | >64 | >64 | >64 | |
| ceftazidime/avibactam | 2–16 | 4 | 8 | |
| colistin | ≤0.5 to >8 | 2 | >8 | |
| aztreonam | >32 | >32 | >32 | |
| amikacin | 32 to >64 | 32 | 64 | |
| ciprofloxacin | >4 | >4 | >4 | |
| cefepime | >16 | >16 | >16 | |
| tigecycline | 1–4 | 1 | 2 | |
| ST258/KPC-3 (n = 25) | ||||
| cefiderocol | 0.06–4 | 2 | 2 | |
| ceftolozane/tazobactam | 64 to >64 | >64 | >64 | |
| meropenem | 8 to >64 | 16 | 64 | |
| ceftazidime | >64 | >64 | >64 | |
| ceftazidime/avibactam | 2–4 | 2 | 2 | |
| colistin | ≤0.5 to >8 | ≤0.5 | 1 | |
| aztreonam | >32 | >32 | >32 | |
| amikacin | ≤4–32 | 32 | 32 | |
| ciprofloxacin | >4 | >4 | >4 | |
| cefepime | 16 to >16 | >16 | >16 | |
| tigecycline | 0.5–4 | 0.5 | 1 | |
| ST147/OXA-48 (n = 3) | ||||
| cefiderocol | 0.06–0.5 | 0.25 | 0.5 | |
| ceftolozane/tazobactam | 8 to >64 | 64 | >64 | |
| meropenem | 0.5 to >64 | 1 | >64 | |
| ceftazidime | 32 to >64 | 64 | >64 | |
| ceftazidime/avibactam | 0.125–32 | 2 | 32 | |
| colistin | ≤0.5 | ≤0.5 | ≤0.5 | |
| aztreonam | 32 to >32 | >32 | >32 | |
| amikacin | ≤4–64 | ≤4 | 64 | |
| ciprofloxacin | >4 | >4 | >4 | |
| cefepime | 8 to >16 | >16 | >16 | |
| tigecycline | ≤0.25–4 | 0.5 | 4 | |
| ST392/OXA-48 + CTX-M-15 (n = 4) | ||||
| cefiderocol | 0.06–1 | 0.25 | 1 | |
| ceftolozane/tazobactam | 8–64 | 32 | 64 | |
| meropenem | 1–16 | 8 | 16 | |
| ceftazidime | 16–64 | 32 | 64 | |
| ceftazidime/avibactam | 0.25–0.5 | 0.25 | 0.5 | |
| colistin | ≤0.5–1 | ≤0.5 | 1 | |
| aztreonam | 32 to >32 | >32 | >32 | |
| amikacin | ≤4 | ≤4 | ≤4 | |
| ciprofloxacin | >4 | >4 | >4 | |
| cefepime | 4 to >16 | >16 | >16 | |
| tigecycline | 1 to >4 | >4 | >4 | |
I, intermediate; R, resistant; S, susceptible.
All isolates producing only ESBLs, with no carbapenemases, were CTX-M-15 producers (Table 1). The cefiderocol MIC for all of this group of isolates was ≤2 mg/L and rates of resistance to ciprofloxacin and meropenem were 100% and 7%, respectively. No resistance to ceftazidime/avibactam was observed, nor any differences in activity between different clones.
All E. cloacae isolates belonged to the ST114 clone (Table 1). Two of the four were CTX-M-15 producers and the other two produced VIM-1; one of them co-produced VIM-1 and OXA-48. MIC range, MIC50 and MIC90 values are shown in Table 2. According to CLSI breakpoints, two isolates were intermediate to cefiderocol and both were VIM-1 producers. The other two produced only CTX-M-15 and displayed cefiderocol MICs of ≤0.5 mg/L.
Activity of cefiderocol against non-fermenting GNB
The distribution of non-fermenting GNB included in the study is shown in Table 1.
The MIC ranges and MIC50 and MIC90 values of cefiderocol and comparators for oxacillinase-producing A. baumannii, grouped by clone and type of carbapenemase, are shown in Table 4. Three out of 80 isolates presented MIC values of 16 mg/L, all of which were ST2/OXA-24/40 producers and presented different pulsotypes. Applying CLSI breakpoints, the global resistance rate was 3.8% for all A. baumannii and 12.0% for the ST2/OXA-24/40 group.
Table 4.
MIC ranges, MIC50s and MIC90s (mg/L) of cefiderocol and commercial comparators for 80 oxacillinase-producing A. baumannii, grouped by clone and type of carbapenemase produced
| Species | Antimicrobial agent | MIC (mg/L) |
Clinical category (%) |
||||
|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | S | I | R | ||
| ST2/OXA-23 (n = 25) | |||||||
| cefiderocol | 0.06–1 | 0.25 | 0.5 | 100 | 0 | 0 | |
| meropenem | 16 to >64 | 64 | 64 | 0 | 0 | 100 | |
| ceftazidime | 4 to >64 | 64 | >64 | 20 | 8 | 72 | |
| colistin | ≤0.5 to >8 | 1 | >8 | 84 | NA | 16 | |
| amikacin | ≤4 to >64 | >64 | >64 | 44 | 0 | 56 | |
| ciprofloxacin | >4 | >4 | >4 | 0 | 0 | 100 | |
| cefepime | >16 | >16 | >16 | 0 | 0 | 100 | |
| ST2/OXA-24/40 (n = 25) | |||||||
| cefiderocol | 0.5–16 | 2 | 16 | 88 | 0 | 12 | |
| meropenem | >64 | >64 | >64 | 0 | 0 | 100 | |
| ceftazidime | 32 to >64 | >64 | >64 | 0 | 0 | 100 | |
| colistin | ≤0.5–1 | ≤0.5 | 2 | 100 | NA | 0 | |
| amikacin | ≤4 to >64 | 64 | >64 | 16 | 4 | 80 | |
| ciprofloxacin | >4 | >4 | >4 | 0 | 0 | 100 | |
| cefepime | >16 | >16 | >16 | 0 | 0 | 100 | |
| ST2/OXA-58 (n = 25) | |||||||
| cefiderocol | 0.06–0.5 | 0.125 | 0.5 | 100 | 0 | 0 | |
| meropenem | 4–64 | 8 | 64 | 0 | 12 | 88 | |
| ceftazidime | 32 to >64 | >64 | >64 | 0 | 0 | 100 | |
| colistin | ≤0.5 to >8 | 1 | 8 | 64 | NA | 36 | |
| amikacin | ≤4 to >64 | 32 | >64 | 44 | 32 | 24 | |
| ciprofloxacin | >4 | >4 | >4 | 0 | 0 | 100 | |
| cefepime | 8 to >16 | >16 | >16 | 4 | 28 | 68 | |
| ST745/OXA-58 (n = 5) | |||||||
| cefiderocol | 0.06–0.25 | NC | NC | 100 | 0 | 0 | |
| meropenem | 8–16 | NC | NC | 0 | 0 | 100 | |
| ceftazidime | >64 | NC | NC | 0 | 0 | 100 | |
| colistin | ≤0.5–2 | NC | NC | 100 | NA | 0 | |
| amikacin | ≤4 to >64 | NC | NC | 80 | 0 | 20 | |
| ciprofloxacin | >4 | NC | NC | 0 | 0 | 100 | |
| cefepime | 16 to >16 | NC | NC | 0 | 40 | 60 | |
NA, not applicable; NC, not calculated because the number of test strains was <10; I, intermediate; R, resistant; S, susceptible.
Colistin resistance was observed in 13 isolates (16.3%: 5 ST2/OXA-23 and 8 ST2/OXA-58) and all were susceptible to cefiderocol (MIC range: 0.06–0.5 mg/L). Forty-one (51.3%) isolates were resistant to amikacin (14 ST2/OXA-23, 20 ST2/OXA-24/40, 6 ST2/OXA-58 and 1 ST745/OXA-23). The MIC50 and MIC90 values of cefiderocol for isolates resistant or intermediate to amikacin were one dilution higher than for those that were amikacin susceptible (MIC50: 0.5 and 0.25 mg/L, respectively; MIC90: 4 and 2 mg/L, respectively). The two isolates with cefiderocol MIC >4 mg/L were also amikacin resistant.
Five of the carbapenem-resistant P. aeruginosa isolates evaluated were ST175 non-carbapenemase producers and one was an ST253/IMP-16 producer (Table 1). MIC ranges and MIC50 and MIC90 values of cefiderocol and comparators for these isolates are shown in Table 2. The MICs of cefiderocol ranged from 0.125 to 0.5 mg/L. The carbapenemase isolate displayed the highest MIC values for this species. All isolates were susceptible to cefiderocol, including one IMP producer that was resistant to ceftolozane/tazobactam and ceftazidime/avibactam.
Finally, 20 S. maltophilia isolates were included (Table 1) and 15 different MLST profiles were identified among these isolates (data not shown). MIC ranges and MIC50 and MIC90 values of cefiderocol and comparators for these isolates, as well as resistance rates, are shown in Table 2. All of these isolates were susceptible to cefiderocol (MIC ≤2mg/L). Resistance rates to ceftazidime, levofloxacin and trimethoprim/sulfamethoxazole were 85%, 5% and 5%, respectively; three (15%) isolates were intermediate to levofloxacin. Apart from cefiderocol, minocycline was the most active agent, with 100% of isolates being susceptible.
Discussion
To our knowledge, this is the first study to evaluate the in vitro activity of cefiderocol against a well-characterized collection of HR isolates of MDR-GNB. This collection includes the most representative MDR bacteria causing healthcare-associated infections in southern Spain (Andalusia has a population of more than 8 million people) and very similar to those that cause infections in neighbouring countries.
The results obtained from the current study showed that the in vitro activity of cefiderocol was superior to that of comparators against recent clinical HR clone isolates of ESBL- and/or carbapenemase-producing Enterobacterales, carbapenem-non-susceptible P. aeruginosa, oxacillinase-producing A. baumannii and MDR S. maltophilia.
Cefiderocol showed potent antimicrobial activity, with MIC90 values of ≤4 mg/L for all groups of organisms evaluated. The MIC90 of cefiderocol was lower for Enterobacterales than for non-fermenting GNB, except for S. maltophilia where the MIC90 was 0.5 mg/L.
Against K. pneumoniae, cefiderocol exhibited MIC90 values that were 2 to 16 times lower than those of comparator agents. Against A. baumannii, cefiderocol (MIC90, 4 mg/L) was up to four times more potent than the comparator agents tested, with the exception of colistin and tigecycline, which also had MIC90 values of 4 mg/L. S. maltophilia was highly susceptible to cefiderocol (MIC90 0.5 mg/L) and cefiderocol was at least as active as comparators, including trimethoprim/sulfamethoxazole (MIC90 2 mg/L). These data demonstrate that cefiderocol has markedly high activity against the isolates of K. pneumoniae, A. baumannii, P. aeruginosa and S. maltophilia that were tested, including carbapenemase-producing strains.
Our results are consistent with previous studies.8,28 Ito-Horiyama et al.8 evaluated the activity of cefiderocol, meropenem, levofloxacin, cefepime, ceftazidime and piperacillin/tazobactam against a collection of carbapenem-resistant non-fermenting GNB, including A. baumannii, P. aeruginosa and S. maltophilia. In their study, cefiderocol demonstrated significantly lower MIC90 values than all comparators for all groups of bacteria. Kohira et al.28 analysed the activity of cefiderocol against Enterobacterales. MIC90 values of cefiderocol against K. pneumoniae and E. cloacae were 0.125 and 1 mg/L, respectively, and comparators had higher values in all cases. In both studies, medium containing apo-transferrine was used instead of ID-CAMHB.
In our study, all carbapenemase-producing K. pneumoniae were susceptible to cefiderocol, whereas a previous study found 12 isolates of carbapenem-non-susceptible K. pneumoniae with MIC values of >4 mg/L.29 The latter study did not specify which underlying mechanism of action was present in their isolates that were not susceptible to carbapenems. As far as we know, few KPC-3-producing K. pneumoniae have been evaluated. Other studies have analysed cefiderocol activity against KPC-producing K. pneumoniae isolates,8,12,28 although the proportion of KPC-3 is unknown, whereas in our study, all isolates belonging to the HR ST512 and ST258 clones were KPC-3 producers. Dobias et al.12 demonstrated that cefiderocol MICs for KPC-type carbapenemase-producing K. pneumoniae ranged between 0.03 and 64 mg/L. Nevertheless, cefiderocol MICs of ≤4 mg/L were obtained for all KPC-type producers analysed by other authors, as was the case in our study.
Several studies have analysed the activity of cefiderocol against OXA-48-type-producing K. pneumoniae isolates. According to previous data, the cefiderocol MIC range for 88 OXA-48-like-producing K. pneumoniae was 0.03–64 mg/L, with an MIC90 of 1 mg/L, and just one resistant isolate.12 Kohira et al.28 reported that 3 out of 81 carbapenemase-producing K. pneumoniae were OXA-48 producers, all with MICs of ≤0.5 mg/L; the medium used for testing cefiderocol MIC in this study was supplemented with apo-transferrine, while in our previously cited study the medium used was ID-CAMHB. In our study, all OXA-48-producing isolates demonstrated cefiderocol MICs of ≤4 mg/L. In addition, the results of our study showed that isolates co-producing OXA-48 and CTX-M-15 had MICs of ≤4 mg/L, whereas isolates that only produced OXA-48 displayed MICs ≤1 mg/L, independently of the clone. A possible explanation for this finding is that the combination of β-lactamases could decrease cefiderocol activity against these isolates. Nevertheless, they continued to demonstrate MICs in the susceptible range.
Two isolates of E. cloacae ST114/VIM-1 (one of them co-producing OXA-48) showed MICs of 8 mg/L. Previously, Enterobacter spp. demonstrated higher MIC90 (8 mg/L) values than other genera/species of Enterobacterales.29 Hackel et al.29 showed that cefiderocol inhibited 97.8% (222/227) of carbapenem-non-susceptible Enterobacterales, 15 of which were E. cloacae. VIM-1-producing E. cloacae strains have previously been reported as resistant to cefiderocol.12,28 However, cefiderocol showed 100% susceptibility in 53 VIM producers from the multinational SIDERO-WT-2014 study, including 7 E. cloacae.30
Three of our A. baumannii isolates (12.0%) were resistant to cefiderocol (16 mg/L), all of them belonging to the HR ST2 clone and producers of OXA-24/40. Previous studies have reported MICs of >8 mg/L for A. baumannii,12,29,31 although other studies have reported all A. baumannii isolates as susceptible, with MIC90 values of 0.5 mg/L.11 Ito et al.31 found five strains showing cefiderocol MICs of 8 mg/L and two strains with MICs of 32 mg/L. Dobias et al.12 reported seven OXA-23-producing A. baumannii, representing 30% of all isolates with cefiderocol MICs >8 mg/L. In that study, the number of isolates that produced OXA-23 and OXA-58, respectively and the clone to which they belonged was not specified. None of the isolates produced OXA-24/40. With the available data, it is not possible to establish whether one type of oxacillinase has more activity than another against cefiderocol. Our results suggest that the lower activity is not related to the clone, since most isolates in our collection belonged to the same clone. Even so, all resistant isolates produced a single type of oxacillinase.
With respect to P. aeruginosa, the number of isolates of HR clones included in the present study was small and only one produced a carbapenemase (VIM-1), although all were resistant to carbapenems. All data available show that cefiderocol has excellent activity against MDR P. aeruginosa.8,9,11,12 The numbers of cefiderocol-resistant P. aeruginosa isolates reported are small: Hackel et al.29 described two isolates of MDR P. aeruginosa with cefiderocol MICs of >8 mg/L and Ito et al.9 found two isolates of VIM-type-producing P. aeruginosa with cefiderocol MICs of 8 mg/L.
In our study, cefiderocol displayed more potent in vitro activity against S. maltophilia compared with previously published data, in which the MIC values obtained for isolates of this species were ≤4 mg/L.32 The isolates in our collection showed cefiderocol MICs of ≤2 mg/L.
Our study has some strengths and limitations. The main strength of this study is that it is the first to include a well-characterized collection of MDR-GNB isolates belonging to HR clones. Second, the collection reflects the current local epidemiology of a large and specific geographical area. The first limitation is the low number of isolates of enterobacteria other than K. pneumoniae included, as well as of P. aeruginosa isolates. The second limitation is the low number of MBL-producing isolates. Most of the isolates reported as non-susceptible to cefiderocol in the literature correspond to isolates producing MBLs, so it would be necessary to conduct a larger-scale study focusing on isolates producing these enzymes.
In conclusion, cefiderocol demonstrated excellent activity against MDR-GNB isolates, including carbapenemase-producing isolates, regardless of the type of carbapenemase or clone. Isolates of A. baumannii ST2/OXA-24/40 and E. cloacae ST114/VIM-1 showed resistance to this antimicrobial, so it will be necessary to conduct further studies to establish the spectrum of cefiderocol activity. This new antimicrobial has great potential for the treatment of infections caused by MDR-GNB.
Acknowledgements
We thank all hospitals that took part in the PIRASOA programme.
Funding
This study was partially supported by Shionogi B. V.
Transparency declarations
All authors: none to declare.
References
- 1.WHO. Antimicrobial resistance. Fact sheet. 2018. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
- 2. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P. et al. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis 2017; 65: 644–52. [DOI] [PubMed] [Google Scholar]
- 3. Van Duin D, Bonomo RA.. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 2016; 63: 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
- 5. Pitout JDD, Nordmann P, Poirel L.. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 2015; 59: 5873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. David S, Reuter S, Harris SR. et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol 2019; 4: 1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito A, Nishikawa T, Matsumoto S. et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60: 7396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito-Horiyama T, Ishii Y, Ito A. et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60: 4384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito A, Sato T, Ota M. et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother 2018; 62: e01454-17. doi:10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karlowsky JA, Hackel MA, Tsuji M. et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int J Antimicrob Agents 2019; 53: 456–66. [DOI] [PubMed] [Google Scholar]
- 11. Falagas M, Skalidis T, Vardakas K. et al. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 2017; 72: 1704–8. [DOI] [PubMed] [Google Scholar]
- 12. Dobias J, Dénervaud-Tendon V, Poirel L. et al. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 2017; 36: 2319–27. [DOI] [PubMed] [Google Scholar]
- 13. Monogue ML, Tsuji M, Yamano Y. et al. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61: doi:10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katsube T, Wajima T, Ishibashi T. et al. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother 2017; 61: doi:10.1128/AAC.01381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trecarichi EM, Quirino A, Scaglione V. et al. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: a case report. J Antimicrob Chemother 2019; 74: 3399–401. [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez-Baño J, Pérez-Moreno MA, Peñalva G. et al. Outcomes of the PIRASOA programme, an antimicrobial stewardship programme implemented in hospitals of the Public Health System of Andalusia, Spain: an ecologic study of time-trend analysis. Clin Microbiol Infect 2020; 26: 358–65. [DOI] [PubMed] [Google Scholar]
- 17.PIRASOA. PIRASOA programme, an antimicrobial stewardship programme implemented in hospitals of the public Health system of Andalusia, Spain. Junta Andalucía. 2014. http://pirasoa.iavante.es/. [DOI] [PubMed]
- 18. Oteo J, Navarro C, Cercenado E. et al. Spread of Escherichia coli strains with high-level cefotaxime and ceftazidime resistance between the community, long-term care facilities, and hospital institutions. J Clin Microbiol 2006; 44: 2359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doyle D, Peirano G, Lascols C. et al. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol 2012; 50: 3877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woodford N, Ellington MJ, Coelho JM. et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 2006; 27: 351–3. [DOI] [PubMed] [Google Scholar]
- 21.International Organization for Standardization. Clinical laboratory testing and in vitro diagnostic test systems—Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices—Part 1: Reference method for testing the in vitro activity of antimicrobials. Geneva, Switzerland; 2006. https://www.iso.org/standard/41630.html.
- 22.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically–Eleventh Edition: M07 2018.
- 23. Hackel MA, Tsuji M, Yamano Y. et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant gram-negative Bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 study). Antimicrob Agents Chemother 2017; 61: e00093–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Eighth Edition: M100 2018.
- 25.CLSI. Minutes of Methods Development and Standardization Working Group. June 2015. https://clsi.org/meetings/ast/ast-meeting-files-resources/.
- 26.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Ninth Edition: M100 2019.
- 27.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0. 2019.
- 28. Kohira N, West J, Ito A. et al. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 2016; 60: 729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hackel MA, Tsuji M, Yamano Y. et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62: doi:10.1128/AAC.01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kazmierczak KM, Tsuji M, Wise MG. et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53: 177–84. [DOI] [PubMed] [Google Scholar]
- 31. Ito A, Kohira N, Bouchillon SK. et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother 2016; 71: 670–7. [DOI] [PubMed] [Google Scholar]
- 32. Zhanel GG, Golden AR, Zelenitsky S. et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs 2019; 79: 271–89. [DOI] [PubMed] [Google Scholar]