Abstract
Animals often face conflicting demands when making movement decisions. To examine the decision process of social animals, we evaluated nest-site preferences of the social spider Stegodyphus dumicola. Colonies engage in collective web building, constructing 3D nests and 2D capture webs on trees and fences. We examined how individuals and groups decide where to construct a nest based on habitat structure and conspecific presence. Individuals had a strong preference for 3D substrates and conspecific presence. Groups were then provided with conflicting options of 3D substrates versus 2D substrates with a conspecific. Groups preferred the 3D structures without presettled conspecifics over a 2D substrate with conspecifics. When a group fragmented and individuals settled on both substrates, the minority group eventually joined the majority. Before rejoining, the collective prey capture behavior of divided groups improved with the size of the majority fragment. The costs of slow responses to prey for split groups and weak conspecific attraction may explain why dispersal is rare in these spiders.
Keywords: collective behavior, conspecific attraction, decision-making, foraging, nest selection
Animals often face conflicting demands when choosing a nest site. Using social spiders, we determined that individuals use conspecific cueing and structural information in their selection process. When presented with conflicting social and structural information regarding nest-site quality, groups chose a preferred structure over another with a pre-established conspecific. These choices influenced collective behavior as groups that reached a consensus had higher prey capture success than groups that were split between nest sites.
INTRODUCTION
Making choices is a challenge for all animals. Often, animals face conflicting information that force them to distinguish between two (or more) options that (all) seem equally good (or bad) (Conradt 2012). Such a situation can arise when animals use more than one cue as an indicator of quality. For example, the quality of all competing choices can be perceived as optimal according to different sets of cues (Czaczkes et al. 2019).
Choosing where to live is an important decision because it can impact an animal’s survival and success (Spencer 2002; Quader 2006; Hatchwell et al. 2008; Misenhelter and Rotenberry 2009). Individuals can rarely assess habitat or nest quality directly because of the complexity of the environment (Johnson et al. 2013). Instead, animals use cues, such as the presence of conspecifics and certain features of the environment, to guide their choices (Miller et al. 2013). Conspecific attraction may play an important role in the decision-making process because the presence of conspecifics can indicate suitable habitat (Stamps 1988; Ramsay et al. 1999; Schuck-Paim and Alonso 2001; Mariette and Griffith 2012; Jeanson and Deneubourg 2017). Furthermore, organisms identify which nest sites will provide the highest pay-off by cueing in on environmental cues, such as light, humidity, and temperature (Franks et al. 2003; Pärt and Doligez 2003; Fletcher 2007; Seeley and Visscher 2007; Mönkkönen et al. 2009). However, animals often face opposing, or incomplete information, and such uncertainty can lead to poor choices (Spencer 2002; Marshall et al. 2006; Platt and Huettel 2008; Johnson et al. 2013; Czaczkes and Heinze 2015; Götmark et al. 2016). When the quality of different habitats seems optimal according to different cues (e.g., one habitat seems good because of light levels but another one seems good because of humidity), it could be challenging to decide where to settle. In such cases, certain nest characteristics may be weighted more heavily than others (Franks et al. 2003; Sasaki et al. 2013) and individuals may differ in their preferences. Conflicting priorities between individuals lead heterogeneous groups to break up (Conradt and Roper 2003; Doering et al. 2020).
Proper assessment and decision-making require comparisons between multiple options. Therefore, choices are prone to mistakes that originate from the available options and can be costly (Mallon et al. 2001; Marshall et al. 2006; Sasaki and Pratt 2011; Sasaki et al. 2013). One of the many benefits of group living is the mitigation of risk in making choices (Franks et al. 2002; Couzin 2009; Sasaki and Pratt 2011; Sasaki et al. 2013; Greening et al. 2015; Sasaki and Pratt 2018). When multiple individuals are choosing a nesting site, collective decision-making emerges, and groups assess nest-site quality more accurately and efficiently than individuals (Mallon et al. 2001; Franks et al. 2002; Pratt et al. 2002; Conradt and Roper 2003; Pratt 2005; Sasaki and Pratt 2011; Sasaki and Pratt 2012; Sasaki and Pratt 2018). However, in a rugged landscape of nest-site options, with multiple choice axes, groups may still be unable to find an optimal solution.
Social invertebrates are an ideal system for studying the links between individual and collective decision-making (Sasaki and Pratt 2018) because they often need to choose between many nest sites when relocating. Furthermore, while information is gathered by individuals, the decision is made at the collective level. While these processes have been well documented in eusocial insects, how individual and collective decision-making occurs in other social invertebrates, such as social spiders, remains largely unknown. Sociality in spiders differs from eusocial insects in that spiders do not have a queen and worker castes. Stegodyphus dumicola (Araneae, Eresidae) is a social spider that lives in colonies of tens to hundreds of females that cooperate in collective foraging, reproduction, and parental care (Wright et al. 2015). Colonies build 3D clusters of webs that form a retreat, or nest, and 2D webs for capturing prey (Kamath et al. 2019). Colonies typically reside in a single retreat; however, groups can build multiple nests connected by capture webs. Stegodyphus dumicola colonies are characterized by low dispersal, serial inbreeding, and low within-colony genetic variation (Lubin et al. 2009; Smith et al. 2009; Avilés and Purcell 2012; Settepani et al. 2017). Colony prey capture success is influenced by the scaffolds on which colonies build their webs. In the field, fence-dwelling colonies capture more prey than tree-dwelling ones (Kamath et al. 2019) and, in the lab, the shape of the structure a colony builds their nest on influences their latency to respond to prey (Modlmeier et al. 2014). Although subsocial spiders have been shown to use natal philopatry (Johannesen and Lubin 1999) and sericophily (Rao and Lubin 2010; Rao and Aceves-Aparicio 2012) in selecting a nest site, the process by which permanently social spiders select a nest site is unknown. Furthermore, uncovering the preferences of both individuals and groups can be important for explaining dispersal patterns in this species (Ward and Lubin 1993; Salomon and Lubin 2007; Řezáč et al. 2018).
To uncover the process by which social spiders choose a nest site, we experimentally tested if individual spiders exhibit conspecific attraction and have a preference regarding the physical properties of a nest site. We hypothesized that individuals will use more than one type of cue to determine where to settle. Specifically, we predicted that the presence of a conspecific on a nest scaffold will be an attractive cue. Furthermore, we predicted that a 3D nest scaffold, which is similar to a natural nest, will be preferable over a 2D nest scaffold. We then tested how groups overcome conflicting cues in choosing a nest site when presented with suboptimal characteristics. We hypothesized that when presented with two preferred cues, one relating to the presence/absence of a conspecific and the other one relating to the structure of the nest scaffold, groups will place a greater weight on one cue over the other. Alternatively, if individuals are highly variable in their preferences, the group will split between the two choices. Finally, we examined if collective foraging is influenced by nest-site decisions. We hypothesized that whether or not a group was able to reach consensus when deciding where to settle would impact their success in other ecological situations. Specifically, we predicted that if groups split between two nest locations, their prey capture success would be negatively impacted because of the importance of social interactions (Hunt et al. 2019; Wright et al. 2019) and nest structure (Modlmeier et al. 2014; Kamath et al. 2019) for this collective behavior.
METHODS
Collection and maintenance
Colonies were collected along roadways in Kalkrand, Namibia, in March 2018 and shipped to UCLA (Permit Number RPIV00632019), where they were kept with their natural webbing in 700-mL containers and fed with crickets weekly. Experiments took place in July 2018. Only adult female spiders were used because females perform the majority of colony maintenance tasks and males are extremely scarce (Pruitt and Keiser 2014).
Individual preference
To test if spiders used conspecific cueing, eight individuals from three different source colonies were each placed in the center of a clear plastic box (29.85 × 12.38 × 6.19 cm) with identical flat 2D wire meshes (5.5 cm2) on each end, 20 cm from each other (Supplementary Figure S1a). These spiders acted as the conspecific cue and were allowed to choose a structure overnight. On the following day, we placed an individually marked spider from the same source colony in the center of the box. The next day we recorded whether the new spider chose the side with the conspecific or without it.
To test if individuals had a preference regarding the structural properties of the nest site, we placed two wire mesh structures: a flat 2D square and a closed 3D cone (Modlmeier et al. 2014) on opposite sides of a plastic box (dimensions as above) 20 cm away from each other (Supplementary Figure S1b). Each spider, from three different source colonies (nindividuals = 6/colony, ntotal = 18), was placed in the center of the box for 24 h. We then recorded the structure on which they settled. Each spider was tested three times to determine the repeatability of structure choice. All other environmental conditions (light, temperature, etc.) were homogenous throughout the arena in all choice tests. One individual failed to build a nest on either structure during the second trial. This was not included in our final analysis. All boxes were cleaned with ethanol at the end of each trial.
Group nest-site preference
To test how opposing information is assessed collectively, we presented groups with conflicting options: an open 2D structure with a conspecific and an empty closed 3D structure (Supplementary Figure S1b). We placed the “cue spider” directly onto the flat mesh and covered it with a cup overnight to ensure that it established and built webbing on the flat mesh. The cup was removed the next day and nine other spiders, from the same source colony, were placed in the center of the box. Thus, each of our experimental colonies consisted of 10 individuals. To determine group preference, we recorded the number of spiders on each structure 1, 2, and 6 days after introducing the groups to the apparatus.
Collective prey capture
To quantify collective prey capture, we assayed the colonies twice on the first day and twice on the second day after groups were added to the apparatus. To assay prey capture, we placed a 1 cm2 piece of paper in the center of the box, on the capture web that connected the two structures, and vibrated it with a wire connected to an Arduino programmed to vibrate at random intervals (Pinter-Wollman et al. 2017; Wright et al. 2019). We recorded the latency until a spider touched the paper (attack latency). Groups with shorter attack latencies are more successful (Pinter-Wollman et al. 2017; Pruitt et al. 2018; Kamath et al. 2019; Pruitt et al. 2019).
Data analysis
To determine individual, conspecific, and group preference, we used chi-squared tests. To determine the repeatability of individual nest structure choice, we calculated the intraclass correlation coefficient (ICC) using the “ICC” R package (Wolak et al. 2012). To determine if attack latency in groups that remained cohesive (9–10 individuals on a single structure) was significantly different from groups that were split between the two structures (6–8 individuals on a single structure), we used a Welch two-sample t-test. All data analysis was conducted in R version 3.5.0 (R Core Team 2018).
RESULTS
Individual preference
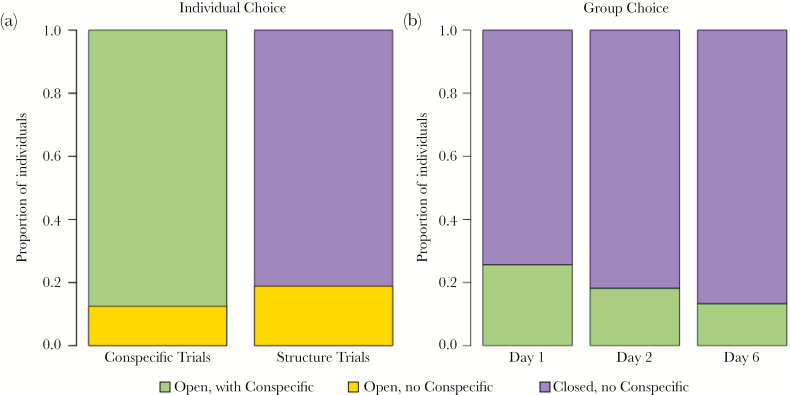
Individuals preferred the structure with a conspecific. We found individuals on the flat mesh with a conspecific significantly more often than expected at random compared with the flat mesh without a conspecific (chi-square: X2 = 7, degrees of freedom [df] = 1, P = 0.008; Figure 1a).
Figure 1.
Conspecific attraction and structural preference by (a) individuals and (b) groups. (a) The proportion of individuals who chose the occupied flat mesh structure is in green, the unoccupied flat structure is in yellow, and the unoccupied closed structure is in purple. (b) Preference for the unoccupied closed structure by groups increased over time.
Individuals preferred the closed cone over the flat mesh structure. Individuals were found on the closed cone more frequently than expected at random compared with the flat mesh (chi-square: X2 = 43, df = 1, P < 0.0001; Figure 1a). Individuals were not repeatable in their preference (ICC = 0.024, 95% confidence interval [CI]: lower CI = −0.21, upper CI = 0.34) because none of the individuals ever chose the open structure 2 days in a row. For each of the three source colonies, individuals chose the closed structure 66%, 78%, and 100% of the time. Half of the individuals always chose the closed structure, seven chose the closed structure twice, and two chose the closed structure once.
Group composition and nest-site preference
Groups preferred the closed structure without a conspecific over the flat mesh with a conspecific. We found individuals in the groups on the closed structure significantly more than expected by random chance, and this preference increased over the course of the experiment (Figure 1b; n = 80, chi-square; Day 1: X2 = 18.513, df = 1, P < 0.0001; Day 2: X2 = 31.182, df = 1, P < 0.0001; Day 6: X2 = 40.333, df = 1, P < 0.0001). In seven of the eight groups, the majority of individuals positioned themselves on the “closed” nest option within the first 24 h.
Collective prey capture
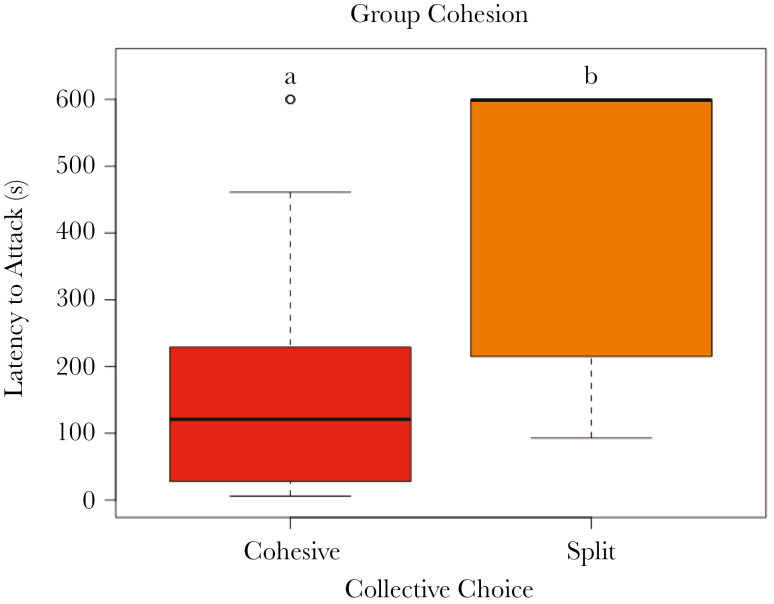
Groups that remained cohesive (9–10 individuals on a single structure; n = 26) had significantly greater foraging aggressiveness compared to groups that were split between the two structures (Figure 2; 6–8 individuals on a single structure; n = 6; t = −2.645; df = 6.652; P-value = 0.035; 95% CI: lower CI = −515.54, upper CI = −26.08). Of the 11 attacks that occurred in split groups, eight occurred from the majority side.
Figure 2.
Latency to collectively attack prey in cohesive versus split groups. Groups that had 9–10 individuals on a single structure and 0–1 individuals on the other were considered cohesive, and groups that had 8-6 individuals on one side and 2–4 individuals on the other side of the arena were considered split.
DISCUSSION
Here, we show that individual spiders use conspecific cueing when selecting a site in which to settle and that they prefer closed 3D substrates over flat 2D ones. When faced with the two options that are preferred by individuals, groups choose closed structures over flat structures with conspecifics. Reaching a consensus decision on where to settle improved the prey capture success of groups.
Individuals strongly preferred to be with conspecifics, suggesting that they use conspecific cueing, which is common in social animals (Stamps 1988; Pärt and Doligez 2003; Fletcher 2007; Jeanson and Deneubourg 2017). Because social spider colonies exhibit many collective behaviors, it is possible that the conspecific acted not only as a cue for nest-site quality but also as a partner with whom to form a group. Such conspecific attraction might reduce the potential costs and risks of building a new web and may explain why dispersal is rare in this species (Lubin et al. 2009; Smith et al. 2009).
Individuals strongly preferred the closed over the flat structure. This preference may be due to the perceived safety of being fully enclosed and protected from predation. Avian and wasp predators often attack web-building spiders, and individuals may benefit from selecting a structure that provides protection on all sides (Henschel 1998). Preference for the closed structure could also be due to the similarity between the interior of this structure and the interior of natural nests, thus providing a familiar structural cue. These explanations are not mutually exclusive. Individuals did not exhibit highly repeatable choices for the closed structure perhaps because they were sampling their environment (Robinson et al. 2009) as none of the individuals chose the open structure two trials in a row, although we were unable to state this with statistical confidence due to our sample size. While we did not observe high activity or movement between the two structures, webbing was always observed connecting the two structures, indicating that the spiders sampled both. Future work should examine the decision process itself and how individuals and groups interact with each potential site before choosing one. In addition, our findings focus on the initial, short-term decisions that individuals make. Future work should also aim to study the long-term nest-site choices made in the laboratory and field. Groups preferred to be on the closed structure over an open mesh with a conspecific on it, and this preference increased over time. Thus, the apparent safety or familiarity provided by a closed structure seems to outweigh the benefits related to conspecific attraction for groups (Franks et al. 2003). Preference for the closed structure could also be due to the similarity between the interior of this structure and the interior of natural nests, or because it gives the spiders a “head start” on building a structure that is similar to naturally occurring structures, thus providing a familiar structural cue (Rao and Poyyamoli 2001). Furthermore, closed structures result in faster prey capture compared with open structures (Modlmeier et al. 2014), providing another potential explanation for the preference of structure over conspecifics, especially for groups of individuals that may already be enjoying the benefits of sociality. Over time, the minority group always joined the majority group, so conspecific attraction and group cohesion may still have an influence on nest-site selection over time. Colonies in nature can be polydomous and can disperse through budding, so the movement of group fragments over time that we observed could explain how budding events can occur (Lubin et al. 2009; Pruitt et al. 2019). The propensity to fragment may also differ among colonies due to a colony’s environment. Kamath et al. (2019) found that tree-dwelling colonies of S. dumicola were more likely to split into multiple retreat nests in the field and were more likely to experience emigration and immigration into nearby colonies when deployed in a greenhouse compared to fence-dwelling colonies.
Although the same number of individuals was exposed to the simulated prey in all trials when the majority fragment was larger, groups responded quicker to prey and, if an attack occurred, it was more likely to come from the majority side. There is strength in numbers, and total group size improves foraging responses in this species (Keiser and Pruitt 2014). However, we show that it is not only group size that is important but that physical proximity to a large number of individuals within a group may be important too (Wright et al. 2019). Hunt et al. (2019) recently showed that S. dumicola groups that exhibit more interactions before prey capture assays attack at faster speeds, supporting our finding that group cohesiveness can influence foraging speed.
Group cohesion may have a greater influence on collective foraging success than nest-site structure. Collective decision-making is more effective than an individual’s choices when negotiating trade-offs (Sasaki and Pratt 2018). While conflicts between individual choice and group choice can occur, we found that democratic decision-making repeatedly emerged in S. dumicola as group fragments always joined the majority over time. This drive toward maintaining group cohesion may contribute to the low dispersal rate and reduced genetic diversity characteristic of most social spiders.
Societies frequently contend with conflicting information, and groups can resolve this conflict in different ways. To form a collective decision in complex environments, individuals can prioritize one informative cue over others and settle on a single choice. However, this may result in a higher frequency of incorrect choices if the error can occur in the assessment of primary cues. Conversely, if there is variation among individuals in the prioritization of information, groups can fragment and remain split in their collective choice. As we found here, when faced with conflicting information, variation in the prioritization of cues may cause groups to initially split. Nonetheless, a strong overall preference for social cohesion resulted in reunification across all groups, allowing for a successful collective decision-making strategy to emerge.
FUNDING
This work was supported by the National Science Foundation IOS grants 1456010 to N.P.W. and 1455895 to J.N.P. and the National Institutes of Health grant GM115509 to N.P.W. and J.N.P.
Supplementary Material
Acknowledgments
We thank Brenden McEwen and Steven Cassidy for their help collecting spiders in the field.
Conflict of interest: The authors declare that they have no competing interests.
Authors’ contributions: G.M.N. and A.P. carried out the experiment; G.M.N. and N.P.W analyzed the results; G.M.N. wrote the first draft of the manuscript; G.M.N., N.P.W., and J.N.P. designed the experiment and prepared the manuscript.
Ethics: All activities were conducted in compliance with all relevant guidelines for the care and use of invertebrate study species.
Data accessibility: Data supporting this work can be found in Najm et al. 2020.
REFERENCES
- Avilés L, Purcell J. 2012. The evolution of inbred social systems in spiders and other organisms. From short-term gains to long-term evolutionary dead ends? Adv Study Behav. 44:99–133. [Google Scholar]
- Conradt L. 2012. Models in animal collective decision-making: information uncertainty and conflicting preferences. Interface Focus. 2:226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature. 421(January):1996–1999. [DOI] [PubMed] [Google Scholar]
- Couzin ID. 2009. Collective cognition in animal groups. Trends Cogn Sci. 13:36–43. [DOI] [PubMed] [Google Scholar]
- Czaczkes TJ, Beckwith JJ, Horsch AL, Hartig F. 2019. The multi-dimensional nature of information drives prioritization of private over social information in ants. Proc Biol Sci. 286:20191136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaczkes TJ, Heinze J. 2015. Ants adjust their pheromone deposition to a changing environment and their probability of making errors. Proc R Soc B Biol Sci. 282(1810):20150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering GN, Sheehy KA, Barnett JB, Pruitt JN. 2020. Colony size and initial conditions combine to shape colony reunification dynamics. Behav Processes. 170:103994. [DOI] [PubMed] [Google Scholar]
- Fletcher RJ., Jr 2007. Species interactions and population density mediate the use of social cues for habitat selection. J Anim Ecol. 76:598–606. [DOI] [PubMed] [Google Scholar]
- Franks NR, Mallon EB, Bray HE, Hamilton MJ, Mischler TC. 2003. Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim Behav. 65:215–223. [Google Scholar]
- Franks NR, Pratt SC, Mallon EB, Britton NF, Sumpter DJ. 2002. Information flow, opinion polling and collective intelligence in house-hunting social insects. Philos Trans R Soc Lond B Biol Sci. 357:1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götmark F, Blomqvist D, Johansson OC, Bergkvist J, Giitmark F, Blomqvist D, Johansson OC, Bergkvist J. 2016. Nordic Society Oikos nest site selection : a trade-off between concealment and view of the surroundings ? Oikos. 26(4):305–312. [Google Scholar]
- Greening BR, Pinter-Wollman N, Fefferman NH. 2015. Higher-order interactions: understanding the knowledge capacity of social groups using simplicial sets. Curr Zool. 61(1):114–127. [Google Scholar]
- Hatchwell BJ, Chamberlain DE, Perrins CM. 2008. The reproductive success of Blackbirds Turdus merula in relation to habitat structure and choice of nest site. Ibis (Lond 1859). 138(2):256–262. [Google Scholar]
- Henschel JR. 1998. Predation on social and solitary individuals of the spider Stegodyphus dumicola (Araneae, Eresidae). J Arachnol. 26(1):61–69. [Google Scholar]
- Hunt ER, Mi B, Geremew R, Fernandez C, Wong BM, Pruitt JN, Pinter-Wollman N. 2019. Resting networks and personality predict attack speed in social spiders. Behav Ecol Sociobiol. 73(7):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanson R, Deneubourg JL. 2017. Conspecific attraction and shelter selection in gregarious insects. Am Nat. 170(1):47–58. [DOI] [PubMed] [Google Scholar]
- Johannesen J, Lubin Y. 1999. Group founding and breeding structure in the subsocial spider Stegodyphus lineatus (Eresidae). Heredity (Edinb). 82 (Pt 6):677–686. [DOI] [PubMed] [Google Scholar]
- Johnson DD, Blumstein DT, Fowler JH, Haselton MG. 2013. The evolution of error: error management, cognitive constraints, and adaptive decision-making biases. Trends Ecol Evol. 28:474–481. [DOI] [PubMed] [Google Scholar]
- Kamath A, Primavera SD, Wright CM, Doering GN, Sheehy KA, Pinter-Wollman N, Pruitt JN. 2019. Collective behavior and colony persistence of social spiders depends on their physical environment. Behav Ecol. 30:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser CN, Pruitt JN. 2014. Personality composition is more important than group size in determining collective foraging behaviour in the wild. Proc Biol Sci. 281:20141424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin Y, Birkhofer K, Berger-Tal R, Bilde T. 2009. Limited male dispersal in a social spider with extreme inbreeding. Biol J Linn Soc. 97(2):227–234. [Google Scholar]
- Mallon EB, Pratt SC, Franks NR. 2001. Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav Ecol Sociobiol. 50(4):352–359. [Google Scholar]
- Mariette MM, Griffith SC. 2012. Conspecific attraction and nest site selection in a nomadic species, the zebra finch. Oikos. 121(6):823–834. [Google Scholar]
- Marshall JA, Dornhaus A, Franks NR, Kovacs T. 2006. Noise, cost and speed-accuracy trade-offs: decision-making in a decentralized system. J R Soc Interface. 3:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Garnier S, Hartnett AT, Couzin ID. 2013. Both information and social cohesion determine collective decisions in animal groups. Proc Natl Acad Sci USA. 110:5263–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misenhelter MD, Rotenberry JT. 2009. Choices and consequences of habitat occupancy and nest site selection in sage sparrows. Ecology. 81(10):2892–2901. [Google Scholar]
- Modlmeier AP, Forrester NJ, Pruitt JN. 2014. Habitat structure helps guide the emergence of colony-level personality in social spiders. Behav Ecol Sociobiol. 68(12):1965–1972. [Google Scholar]
- Mönkkönen M, Forsman JT, Kananoja T, Ylönen H. 2009. Indirect cues of nest predation risk and avian reproductive decisions. Biol Lett. 5:176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm G, Pe A, Pruitt J, Pinter-Wollman N. 2020. Data from: physical and social cues shape nest site preference and prey capture behavior in social spiders, UC Los Angeles. Behav Ecol. doi:10.5068/D1W37T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärt T, Doligez B. 2003. Gathering public information for habitat selection: prospecting birds cue on parental activity. Proc Biol Sci. 270:1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter-Wollman N, Mi B, Pruitt JN. 2017. Replacing bold individuals has a smaller impact on group performance than replacing shy individuals. Behav Ecol. 28(3):883–889. [Google Scholar]
- Platt ML, Huettel SA. 2008. Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci. 11:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SC. 2005. Behavioral mechanisms of collective nest-site choice by the ant Temnothorax curvispinosus. Insectes Soc. 52(4):383–392. [Google Scholar]
- Pratt SC, Mallon EB, Sumpter DJT, Franks NR. 2002. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav Ecol Sociobiol. 52(2):117–127. [Google Scholar]
- Pruitt JN, McEwen BL, Cassidy ST, Najm GM, Pinter-Wollman N. 2019. Experimental evidence of frequency-dependent selection on group behaviour. Nat Ecol Evol. 3:702–707. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Keiser CN. 2014. The personality types of key catalytic individuals shape colonies’ collective behaviour and success. Anim Behav. 93:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN, Wright CM, Lichtenstein JLL, Chism GT, McEwen BL, Kamath A, Pinter-Wollman N. 2018. Selection for collective aggressiveness favors social susceptibility in social spiders. Curr Biol. 28:100–105.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader S. 2006. What makes a good nest? Benefits of nest choice to female baya weavers (Ploceus philippinus). Auk. 123(2):475–486. [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. http://www.R-project.org/. [Google Scholar]
- Ramsay SM, Otter K, Ratcliffe L. 1999. Nest-site selection by female black-capped chickadees: settlement based on conspecific attraction? Auk. 116(3):604–617. [Google Scholar]
- Rao D, Aceves-Aparicio A. 2012. Notes on the ecology and behavior of a subsocial spider Anelosimus baeza (Araneae: Theridiidae) in Mexico. J Arachnol. 40(3):325–331. [Google Scholar]
- Rao D, Lubin Y. 2010. Conditions favoring group living in web-building spiders in an extreme desert environment. Isr J Ecol Evol. 56(1):21–33. [Google Scholar]
- Rao D, Poyyamoli G. 2001. Role of structural requirements in web-site selection in Cyrtophora cicatrosa Stoliczka (Araneae: Araneidae). Curr Sci. 81(6):678–680. [Google Scholar]
- Řezáč M, Tošner J, Heneberg P. 2018. Habitat selection by threatened burrowing spiders (Araneae: Atypidae, Eresidae) of central Europe: evidence base for conservation management. J Insect Conserv. 22(1):135–149. [Google Scholar]
- Robinson EJ, Smith FD, Sullivan KM, Franks NR. 2009. Do ants make direct comparisons? Proc Biol Sci. 276:2635–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Lubin Y. 2007. Cooperative breeding increases reproductive success in the social spider Stegodyphus dumicola (Araneae, Eresidae). Behav Ecol Sociobiol. 61(11):1743–1750. [Google Scholar]
- Sasaki T, Granovskiy B, Mann RP, Sumpter DJ, Pratt SC. 2013. Ant colonies outperform individuals when a sensory discrimination task is difficult but not when it is easy. Proc Natl Acad Sci USA. 110:13769–13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Pratt SC. 2011. Emergence of group rationality from irrational individuals. Behav Ecol. 22(2):276–281. [Google Scholar]
- Sasaki T, Pratt SC. 2012. Groups have a larger cognitive capacity than individuals. Curr Biol. 22:R827–R829. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Pratt SC. 2018. The psychology of superorganisms: collective decision making by insect societies. Annu Rev Entomol. 63:259–275. [DOI] [PubMed] [Google Scholar]
- Schuck-Paim C, Alonso WJ. 2001. Deciding where to settle: conspecific attraction and web site selection in the orb-web spider Nephilengys cruentata. Anim Behav. 62(5):1007–1012. [Google Scholar]
- Seeley TD, Visscher PK. 2007. Group decision making in nest-site selection by honey bees. Apidologie. 38:101–116. [Google Scholar]
- Settepani V, Schou MF, Greve M, Grinsted L, Bechsgaard J, Bilde T. 2017. Evolution of sociality in spiders leads to depleted genomic diversity at both population and species levels. Mol Ecol. 26:4197–4210. [DOI] [PubMed] [Google Scholar]
- Smith D, Van Rijn S, Henschel JR, Bilde T, Lubin Y. 2009. Amplified fragment length polymorphism fingerprints support limited gene flow among social spider populations. Biol J Linn Soc. 97(2):235–246. [Google Scholar]
- Spencer RJ. 2002. Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology. 83(8):2136–2144. [Google Scholar]
- Stamps JA. 1988. Conspecific attraction and aggregation in territorial species. Am Nat. 131(3):329–347. [Google Scholar]
- Ward D, Lubin Y. 1993. Habitat selection and the life history of a desert spider, Stegodyphus lineatus (Eresidae). J Anim Ecol. 62:353–363. [Google Scholar]
- Wolak ME, Fairbairn DJ, Paulsen YR. 2012. Guidelines for estimating repeatability. Methods Ecol Evol. 3(1):129–137. [Google Scholar]
- Wright CM, Keiser CN, Pruitt JN. 2015. Personality and morphology shape task participation, collective foraging and escape behaviour in the social spider Stegodyphus dumicola. Anim Behav. 105:47–54. [Google Scholar]
- Wright CM, Lichtenstein JL, Luscuskie LP, Montgomery GA, Geary S, Pruitt JN, Pinter-Wollman N, Keiser CN. 2019. Spatial proximity and prey vibratory cues influence collective hunting in social spiders. Isr J Ecol Evol. 1(aop):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.