Abstract
Epstein‐Barr virus‐related mucocutaneous ulcer lymphoma is a rare entity promoted by immunosuppression. It is less described in inflammatory bowel diseases, and mostly these are refractory diseases. CD30 acts to Epstein‐Barr virus (EBV) local proliferation and thus could be an interesting target. Brentuximab vedotin could become a new helpful tool.
Keywords: brentuximab vedotin, CD30, epstein‐barr virus, immunosuppression, lymphoma
Epstein‐Barr virus‐related mucocutaneous ulcer lymphoma is a rare entity promoted by immunosuppression. It is less described in inflammatory bowel diseases, and mostly these are refractory diseases. CD30 acts to Epstein‐Barr virus (EBV) local proliferation and thus could be an interesting target. Brentuximab vedotin could become a new helpful tool.
1. INTRODUCTION
Epstein‐Barr virus‐positive mucocutaneous ulcer (EBVMCU) is a new entity in the 2016 review of the World Health Organization (WHO) classification of lymphoid neoplasms. It is a rare condition promoted by immunosuppression, well described in patients exposed to long‐term immunosuppressive therapies.1, 2 EBVMCU is characterized by sharply circumscribed ulcers, localized in mucosa (gastrointestinal tract and oropharynx mainly). Cytologic analysis describes polymorphous infiltration of lymphocytes and immunoblasts with Reed‐Sternberg like morphology, and a strong positivity of CD30+ lymphocytes and Epstein‐Barr virus (EBV) presence into cells.
Epstein‐Barr virus reactivation in state of immunosuppression often occurs and can evolve to lymphoma diseases, like in Burkitt lymphoma or post‐transplant lymphoproliferative disease.3, 4 Immune system depression allowed viral replication and amplification into lymphoid cells. CD 30, present in many inflammatory conditions, could be an activator of EBV replication and helps local proliferation.5 CD 30 could be an interesting target in EBVMCU, as we described it herein.
2. CASE PRESENTATION
We present here the case of a 34 years old man suffered from a Crohn's disease for 14 years old, without any other medical and hematological histories. He was first treated for 4 years by azathioprine monotherapy, and then in combination with infliximab. Patient relapsed in 2006, and left colectomy was then performed. Azathioprine with adalimumab was initiated until his third relapse in 2014. Afterward, golimumab, another anti‐TNF drug, was initiated with a limited response until 2016, when Ustekimab was tested in monotherapy. No macroscopic or microscopic improvement were observed at each colonoscopy and biopsy samples under these treatments (Figure S1).
In May 2017, the patient was hospitalized for fatigue, fever, and rectal pain. Colonoscopy highlighted rectum's deep ulcerations, covered by adherent membranes (Figure 1). Rectal biopsy showed a completely ulcerated mucosa, capillary neoangiogenesis, and inflammatory fibrinous exudate. There were many polymorphic inflammatory elements with many large lymphoid cells, with a hyperchromatic nucleus, expressing CD30 demonstrated by the immunohistochemical study (Figure 2). These lymphoid cells also expressed MUM1 (Multiple Myeloma 1) and PAX5 (Paired Box 5) and not CD15, rejecting a Hodgkin lymphoma diagnosis. Finally, a significant number of these cells were marked by the EBER probe in in situ hybridization (Figure 2). These lymphoid elements CD30+ and EBV+ were present in varied proportions on all biopsy samples retrospectively analyzed since 2015. EBV polymerase chain reaction (PCR) done during this hospitalization was >4log in serum.
Figure 1.
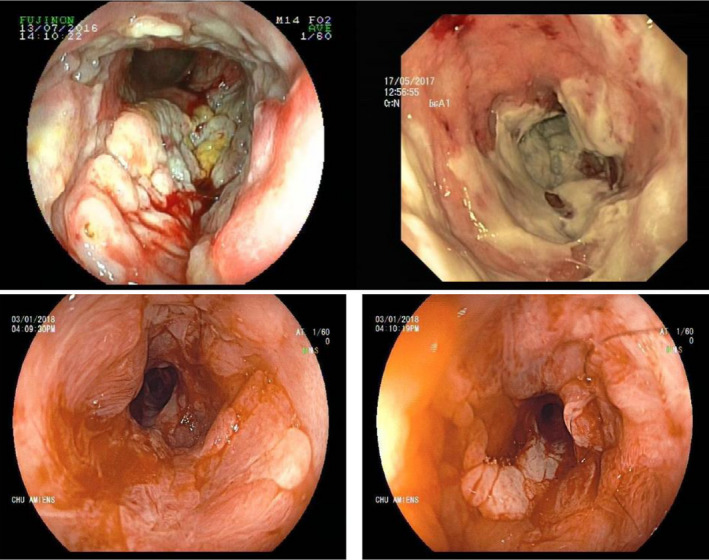
Colonoscopy images: large and deep ulcerations and pseudo‐membranes before treatment (images above). macroscopical improvement after treatment with Brentuximab vedotin (images below)
Figure 2.
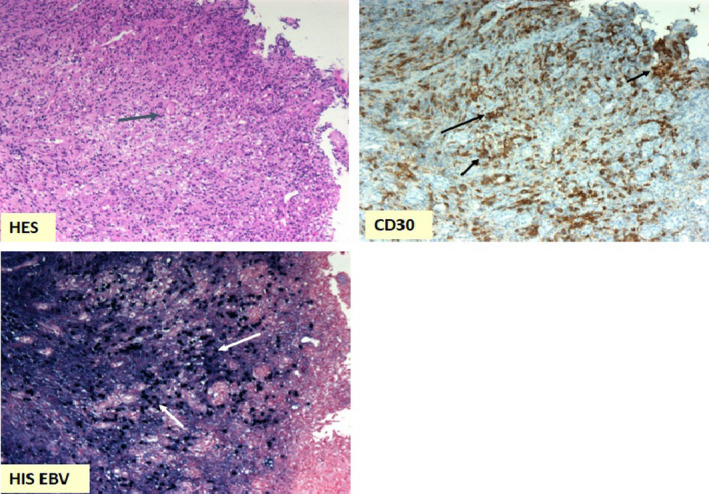
Rectal biopsies at diagnosis: large lymphoid cells seen in HES coloration (blue arrow) (×20 magnification); large lymphoid cells marked by CD30 antibody (black arrows); lymphoid cells nucleus marked by EBER probe in in situ hybridation, highlighting EBV presence (white arrows) (×10 magnification)
A first‐line therapy by rituximab was administered 4 times, once weekly, and was associated with local steroids. Unfortunately, it did not reduce symptoms and did not decrease the blood EBV PCR. A second‐line therapy with brentuximab vedotin, a composed monoclonal antibody anti–CD‐30, was then initiated monthly. After 4 infusions, we observed dramatically clinical and biological improvements, with reduced amount of stool by day, general status improvement, blood EBV PCR, and systemic inflammation markers negativation. Colonoscopy brought to light a reduction of both the pseudomembranous aspect and ulcerations (Figure 1). Biopsies showed cells CD30+ and EBV+ clearance (Figure 3).
Figure 3.
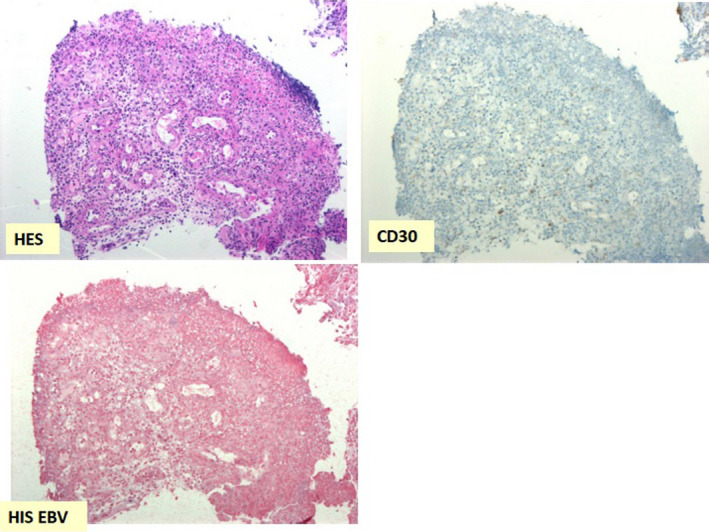
Rectal biopsies after treatment with Brentuximab vedotin: disappearance of large lymphoid cells in HES coloration (×20 magnification); clearance of lymphoid cells marked by CD30 antibody (×20 magnification); disappearance of lymphoid cells nucleus marked by EBER probe in in situ hybridation (×10 magnification)
Sadly, two months after the first course end, the patient had relapse. Colonoscopy showed resurgence of macroscopic ulcerations and anal stenosis. Blood EBV PCR was once again positive. Lymphoid cells, CD30+, and EBV+ were again highlighted on rectal biopsies. Therefore, further management consisted on maintenance therapy with brentuximab vedotin, one infusion every two months. At this time, the patient still going through maintenance therapy. Clinical improvement is already back with macroscopical remission on colonoscopy.
3. DISCUSSION AND CONCLUSION
Epstein‐Barr virus is an endemic virus infecting 90%‐95% of the whole population, mainly in childhood. Virus infection first occurs in the oropharyngeal cells and rejoins B‐cells circulating nearby, via viral attachment to CD21. EBV integrates the B‐cells’ nucleus and viral genome use nuclear mechanisms to express EBV nuclear antigen leader protein (EBNA‐LP) and EBNA‐2, leading to cell growth and transformation. Evasion of host surveillance is due to decreased viral protein expression on B‐cells’ surface. Also, EBV expresses nuclear antigens, small noncoding RNA and transcripts that contribute to viral genomic maintenance.6 Reduced stock and activity of cytotoxic T lymphocytes is a major issue in auto‐immunity diseases like inflammatory bowel diseases, allowing B‐cell—EBV mediated—proliferation.7 It is compounded by therapeutic strategy using immunosuppressive therapies as ciclosporin, methotrexate or more recently, anti‐TNF drugs treated with thiopurines are also at increased risk of EBV‐associated lymphomas.7, 8, 9, 10, 11 Whether risk of lymphoma in patients treated with anti‐TNF agents alone remains controversial.10 Lemaitre et al have reported in 2017, an increasing risk of lymphoma in patients treated with thiopurines (HR, 2.6 [2.0‐3.4]), anti‐TNF monotherapy (HR, 2.4 [1.6‐3.6]), and combination of thiopurines and anti‐TNF (HR, 6.1 [1.3‐4.2]).12
Epstein‐Barr virus‐positive mucocutaneous ulcer was first described in a case series of 26 patients suffering from different inflammatory diseases, treated with immunosuppressive therapy (azathioprine, cyclosporine, or methotrexate) in 2010 by Dojcinov et al1 EBVMCU is then characterized by sharply circumscribed ulcers, localized in mucosa (gastrointestinal tract and oropharynx), polymorphous infiltration of lymphocytes and immunoblasts with Reed‐Sternberg like morphology, angioinvasion, and variable tissue necrosis. The immunophenotypage shows a strong positivity for CD10, CD30, MUM1, PAX5, and variable expression of CD20, CD45, CD15, and Bcl‐6. EBER positivity demonstrates EBV proliferation.2 Affirm the diagnosis in patients suffering from inflammatory bowel diseases is not easy since EBVMCU could mimick their usual symptoms and some patients could have both affections.4 Clinical evolution is often indolent but some damages are possible: absorption dysfunction, fatigue, colic distension, perforation… Observing several biopsies at each relapse, and looking for CD30 and EBER positivity, seem to be relevant in those patients management.5
The first step of EBVMCU therapeutic strategy is based on immunosuppression reduction.2, 4 Rituximab could be tried in order to reduce lymphocytes CD20+ and also lymphoproliferation, but lacks efficacy in this indication. Recently, composed monoclonal antibody brentuximab vedotin was developed to target CD30. Brentuximab vedotin binds to CD30 on cell surface and then vedotin is transported into nucleus cell, leading to cell cycle dysfunction and to apoptosis.13, 14, 15
To our knowledge, here is the first case of EBVMCU treated by anti‐CD30 antibody that allowed to clinical, biological, and endoscopic efficacy. These interesting results attest that CD30 could be a new target in EBVMCU.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
LM and DL wrote the manuscript; ET and DC realized histology analysis; CY, CD, MF, and JPM revised the manuscript.
Supporting information
Montes L, Tredez E, Yzet C, et al. Epstein‐Barr Virus‐related mucocutaneous ulcer lymphoma associated with Crohn's disease, treated with monoclonal antibody anti‐CD30. Clin Case Rep. 2020;8:958–961. 10.1002/ccr3.2721
REFERENCES
- 1. Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34(3):405‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart M, Thakral B, Yohe S, et al. EBV‐positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lymphoproliferative disorder. Am J Surg Pathol. 2014;38(11):1522‐1529. [DOI] [PubMed] [Google Scholar]
- 3. Dierickx D, Habermann TM. Post‐transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549‐562. [DOI] [PubMed] [Google Scholar]
- 4. Nelson AA, Harrington AM, Kroft S, Dahar MA, Hamadani M, Dhakal B. Presentation and management of post‐allogeneic transplantation EBV‐positive mucocutaneous ulcer. Bone Marrow Transplant. 2016;51(2):300‐302. [DOI] [PubMed] [Google Scholar]
- 5. Vase MØ, Maksten EF, Bendix K, et al. Occurrence and prognostic relevance of CD30 expression in post‐transplant lymphoproliferative disorders. Leuk Lymphoma. 2015;56(6):1677‐1685. [DOI] [PubMed] [Google Scholar]
- 6. Young LS, Yap LF, Murray PG. Epstein‐barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16(12):789‐802. [DOI] [PubMed] [Google Scholar]
- 7. Nissen LHC, Nagtegaal ID, de Jong DJ, et al. Epstein‐barr virus in inflammatory bowel disease: the spectrum of intestinal lymphoproliferative disorders. J Crohns Colitis. 2015;9(5):398‐403. [DOI] [PubMed] [Google Scholar]
- 8. Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6‐mercaptopurine: a meta‐analysis. Clin Gastroenterol Hepatol. 2015;13(5):847‐858.e4. [DOI] [PubMed] [Google Scholar]
- 10. Andersen NN, Pasternak B, Basit S, et al. Association between tumor necrosis factor‐α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014;311(23):2406. [DOI] [PubMed] [Google Scholar]
- 11. Lam GY. Lymphoproliferative disorders in inflammatory bowel disease patients on immunosuppression: lessons from other inflammatory disorders. World J Gastrointest Pathophysiol. 2015;6(4):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Connor OA, Lue JK, Sawas A, et al. Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin’s lymphoma: an international, multicentre, single‐arm, phase 1–2 trial. Lancet Oncol. 2018;19(2):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood. 2018;131(15):1689‐1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem‐cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2015;385(9980):1853‐1862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


