Abstract
Background:
The meniscus is critical for the normal functioning of the knee joint. The specific aim of this study was to validate an in vitro culture model of meniscus explants for testing the impact of culture conditions on meniscus biomechanical properties. We hypothesized that culturing menisci in the presence of intermediate and high concentration of serum would have an positive effect on the compressive stiffness of the meniscus.
Methods:
Unconstrained microindentation testing was performed on porcine meniscus explants cultured with varying concentrations 1%, 5%, or 10% of fetal bovine serum media. Meniscus explants that were not cultured were used as a control. These tests quantified the Young’s Modulus of the listed groups of cultured and uncultured explant tissues.
Findings:
The Young’s modulus for 10% cultured explants were significantly higher compared to the control, 1%, and 5% cultured meniscus explants. There was no statistical significance when the Young’s modulus between control, 1%, and 5% cultured explants were compared.
Interpretation:
These results suggest that low concentrations of serum do not impart an anabolic effect on meniscus tissue explant biomechanical properties.
Keywords: Meniscus, Microindentation, culture model
Introduction
The meniscus is a C-shaped fibrocartilaginous tissue located in the knee joint. The meniscus is regarded as a highly hydrated tissue (70%–72% water) with the remaining composition of the tissue comprised of collagen variants and proteoglycans (Kawamura et al., 2003 and Markis et al., 2011). Proteoglycans are composed of glycosaminoglycans which confer compressive properties to the meniscus tissue (Kawamura et al., 2003). Collagen fibers are oriented uniquely that is important in influencing tensile functioning of the tissue (Kawamura et al., 2003 and Zhang et al., 2014). Zhang et al. examined the compositional orientation of different regions of the meniscus. The central region (body of the meniscus) contains a high concentration of glycosaminoglycans, resulting in higher compressive strength. The peripheral region (horns of the meniscus) contains high concentration of collagen, giving the region high tensile strength.
Healthy, intact meniscus tissue is essential for the normal biomechanical function of the knee because it withstands shear, tension, and compressive forces generated during normal joint motion (Wojtys et al., 2005). These functional properties depend on the anatomical and material composition of the tissue (Kawamura et al., 2003). Damage to the meniscus promotes further degenerative responses within the tissue, contributes to articular cartilage deterioration, and propagates loss of mechanical function of the knee (Kawamura et al., 2003).
The composition and structure of the meniscus is maintained through a balance of anabolic and catabolic activities. Both biological factors (cytokines and growth factors) and physical factors contribute to the control of the biological activity of the meniscal cells. In this regard, a range of different approaches has been used to study the mechanobiologic responses of the meniscus. (McNulty et al., 2015)
In vitro studies have provided important information on the biological regulation of the meniscus at the tissue and cellular level (McNulty et al., 2015). An organ culture model comparing 10% fetal bovine serum (FBS) media with a defined low serum media has been used as a wound repair assay for the meniscus (Webber et al. 1989). Numerous studies have used this model while others have used reduced serum formulations to study how growth factors and cytokines affect tissue repair in culture (Riera et al. 2011). While there are several studies investigating the biologic response of meniscus cells and tissue to growth factors and cytokines at lower serum media preparations, there is a paucity of literature investigating the optimal culture conditions to study the impact of growth factors and cytokines on the biomechanical properties of normal meniscus tissue. One study has been identified which demonstrated that culture in serum free media for 10 days resulted in a reduction in the equilibrium compression modulus when meniscus explants were tested in unconfined compression compared with uncultured tissue (Ling et al 2015).
Biomechanical research has helped characterize the physical properties and mechanical function of the meniscus within the knee joint. Indentation is a commonly used approach to nondestructively evaluate mechanical properties including the compressive stiffness of soft tissues such as articular cartilage (Lu et al., 2004). Microindentation testing allows for the testing of small tissue specimens to measure compressive stiffness in a non-destructive fashion (Li X et al. 2007). Microindentation also facilitates testing the same tissue in multiple regions without damaging the surrounding tissue. Moreover, microindentation permits testing locoregional mechanical properties of tissues that are perhaps not homogenous throughout the entirety of the structure due to functional and anatomic differences, e.g., the meniscus.
The specific aim of this study was to create an in vitro culture model of meniscus explants for testing the impact of varied serum concentrations on meniscus biomechanical properties, with the objective of determining the optimal concentration of FBS supplementation required to support the maintenance of the compressive properties of the meniscus.
Unconstrained microindentation testing was performed on porcine meniscus explants that were cultured at different concentrations of FBS supplemented culture media. Uncultured meniscus explants were used as a control. The hypothesis of this study was that culturing menisci with intermediate and high concentrations of serum would have a positive effect on the compressive stiffness of the meniscus.
Methods
Tissue Harvest and Culture
Porcine lateral menisci from eight pairs of knees were collected from three-year-old female pigs from a local abattoir. Six-millimeter diameter explants of 3mm thickness were collected using a biopsy punch from four different regions of the lateral meniscus (Fig. 1): posterior horn (PH), posterior body (PB), anterior body (AB), anterior horn (AH). Explants were washed in Dulbecco Modified Eagle Medium (DMEM) with penicillin/streptomycin (1,000ug/mL), amphotericin B (25ug/mL), and gentamicin (5ug/mL) for 1 hour. For the “uncultured” explants microindentation testing was performed immediately after 1 hour. “Cultured” explants were placed in DMEM with 1%, 5%, or 10% fetal bovine serum (FBS); penicillin/streptomycin (100ug/mL); amphotericin B (2.5ug/mL); and gentamicin (5ug/mL). FBS contains a large number of growth factors and nutritional macromolecules that are essential to cell and tissue growth which albumin is the major component (Lindl, 2002; Zheng et al., 2008). Tissues were incubated for seven days at 37°C/5% CO2. The medium was changed every 72 hours.
Figure 1:
Location of the Meniscus Explant Tissues. Six-millimeter diameter explants (3mm thickness) were taken from 4 different regions of the porcine, lateral meniscus: Anterior Horn (AH), Anterior Body (AB), Posterior Body (PB), and Posterior Horn (PH)
A total of sixty-four explants were collected. Zhang et al. observed differences in the compressive properties between the body and horns of the meniscus. In order to control for regional variation, specimens were randomly assigned, and each region of the meniscus was equally represented (Table 1). Laterality was not considered a variable as additional testing studies showed no difference between right and left sides (data not shown).
Table 1:
Culture Model Setup. Tissues were either cultured or uncultured. Cultured tissues were cultured in DMEM with 1%, 5% or 10% FBS. A total of n=64 explants were tested. Data repres ents n=4 from each location of the lateral meniscus. AH-anterior horn, AB – anterior body, PB – posterior body, PH-posterior horn.
| Uncultured | 1% FBS | 5% FBS | 10% FBS |
|---|---|---|---|
| PH (n=4) | PH (n=4) | PH(n=4) | PH (n=4) |
| PB (n=4) | PB (n=4) | PB (n=4) | PB (n=4) |
| AH (n=4) | AH (n=4) | AH (n=4) | AH (n=4) |
| AB (n=4) | AB (n=4) | AB (n=4) | AB (n=4) |
Microindentation Test
Microindentation experiments were performed to quantify the biomechanical properties of the cultured and uncultured explant tissues. Explants were pre-loaded to a force of 0.15N for 20s, then indented 500μm at a speed of 5μm/s using a Bose ElectroForce 3200 (ElectroForce®, Eden Prairie, MN, USA) (Lindburg, Willey, et al. 2013). Microindentation curves were fit to the Hertz contact model (Fig. 2), which assumes an infinitely hard sphere indents a flat, linear elastic, infinite half-space: where F is the measured force (N), E is the calculated stiffness (Pa), υ is Poisson’s ratio, and R is the spherical indenter radius (R = 0.0005m), and δ is the indentation depth (m) (Kawamura et al., 2003). The Hertz contact model was used to determine the Young’s modulus. The Young’s modulus is used to characterize the elastic deformation behavior of a solid material (Li X et al. 2007), and it is directly proportional to the stiffness of the material.
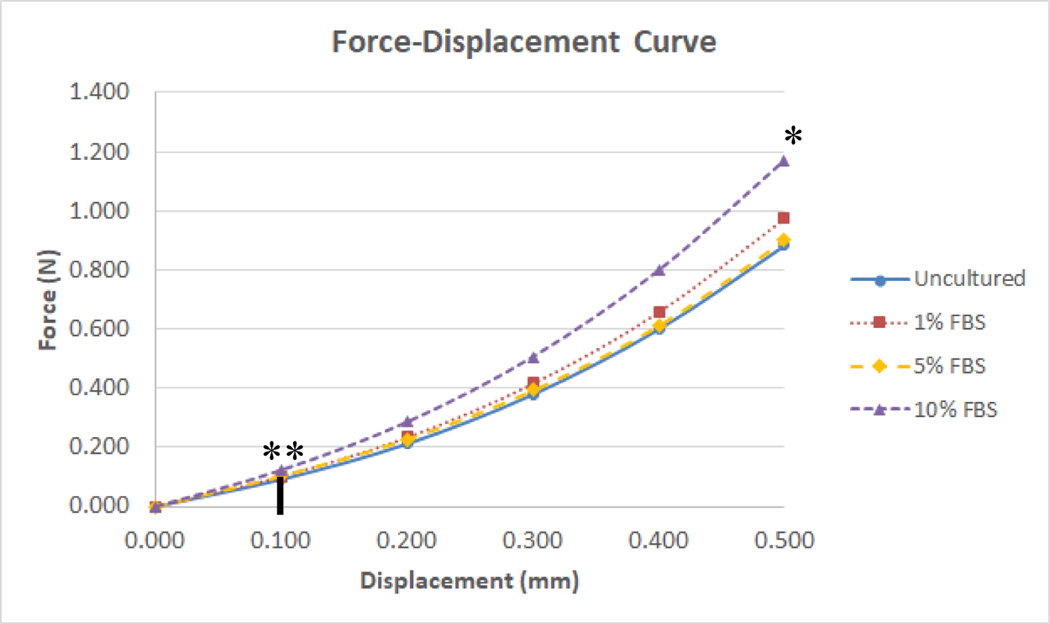
Figure 2.
Force-Displacement Curve. A scatter-plot compares the force required to produce a 0.5mm dent between uncultured tissues and tissues cultured in 1%, 5%, and 10% FBS media. There was a statistical difference in the force required to produce a 0.5mm dent in tissues between the groups (* = P < .05; Omega squared = .120). Due to the viscoelastic in nature of the meniscus, and the Hertz model assumes linear elastic behavior only the linear elastic portion of the curve, the first 100μm of indentation was used to fit the Hertz model (indicated by the black line) There was also a statistical difference in the amount of force required to produce a .1mm dent into the tissue (** = P ≤ .001; Omega squared = .203)
Because biological tissue is nearly incompressible at the indentation rates used in the present study, the Poisson’s ratio (υ) was assumed to be 0.5 (Parker et al., 1990). Due to the viscoelastic in nature of the meniscus, and the Hertz model assumes linear elastic behavior only the linear elastic portion of the curve, the first 100μm of indentation was used to fit the Hertz model. Each explant was indented at 4 different locations. An average was taken from the four locations and used for the statistical calculations.
Statistical Analysis
Data are presented as mean (standard error). One-way analysis of variance test and Fisher Lease Significant Difference post hoc test were performed using SPSS (International Business Machines Corporation, Armonk, NY 10504, USA) to determine the significant difference. Differences were considered significant at an α < 0.05. Omega squared test was performed to determine the effect size of the one-way ANOVA test, and the Cohen’s D test was performed to determine the effect size for each post hoc test.
Results
Force Analysis Between Uncultured and Cultured Meniscus Explant Tissues
Sixty-four explants were tested (sixteen tissues in each group). Force displacement curve in figure 2 represents the average force it took to put a .500mm dent in 16 explants in each group. Observing the first 100μm of indentation (Fig. 3a), it took more force to make a .100mm indent in explants cultured in 10% FBS media (mean .129 N (SE .005)) compared to uncultured explants (mean .096 N (SE .006); P < .001; Cohen’s D = 1.498) and explants cultured to in 1% (mean .105 N (SE .005); P = .004; Cohen’s D = 1.116) and 5% FBS media (mean .108 N (SE .006); P = .008; Cohen’s D = .931). There was no statistically significant difference between uncultured explants and explants cultured in 1% and 5% FBS media.
Figure 3a.
Statistical Analysis of the Force Within the first .100mm Indentation. It took statistically significant more force to make a .100mm indent on tissues cultured in 10% FBS media compared to the uncultured tissues and tissues cultured in 1% and 5% FBS. (* = P < .01)
Force within the first 100μm was analyzed within posterior and anterior region of the meniscus tissue. Within the posterior region (Fig. 4a), it took more force put a .100mm indent in explants cultured in 10% (mean .122 N (SE .007)) and 5% FBS (mean .112 N (SE .01)) media compared to the uncultured explants (mean .084 N (SE .006)) (uncultured vs 10% FBS explants P = .001 (Cohen’s D = 2.231); uncultured vs 5% FBS explants P = .009 (Cohen’s D = 1.228)). There was also a statistically significant difference between explants cultured 10% and 1% (mean .098 N (SE .005)) FBS media (P = .022; Cohen’s D = .1.449). There was no statistically significant difference between explants cultured in 10% and 5% FBS media (P = .330; Cohen’s D = .425); explants cultured in 1% and 5% FBS media (P = .160; Cohen’s D = .623); and uncultured explants and explants cultured in 1% FBS media (P = .190; Cohen’s D = .902). Within the anterior region (Fig. 5a), it took significantly more force to put an .100 mm indent in the explants cultured in 10% FBS media (mean .137 N (SE .007)) compared to uncultured explants (mean .107 N (SE .008); P = .016 (Cohen’s D = 1.330)) and explants cultured in 1% (mean .113 (SE .009); P = .049 (Cohen’s D = 1.040)) and 5% (mean .103 (SE .007); P = .007 (Cohen’s D = 1.619)) FBS media. There were no statistically significant differences between uncultured explants and explants cultured in 1% FBS and 5% FBS media.
Figure 4a.
Statistical Analysis of the Force Within the first .100mm Indentation of Explants from the Posterior Region. It took statistically significant more force to make a .100mm indent in tissues cultured 10% FBS media compared to uncultured tissues and tissues cultured in 1% and 5% FBS media. In addition, it took statistically significant more force to indent tissues cultured in 5% FBS media compared to the uncultured explant tissues (* = .001 ≤ P < .05)
Figure 5a.
Statistical Analysis of the Force Within the first .100mm Indentation of Explants from the Anterior Region of the Meniscus. It took statistically significant more force to make a .100mm indent in tissues cultured 10% FBS media compared to uncultured tissues and tissues cultured in 1% and 5% FBS media (* = P < .05). It took more force to indent explants cultured in 1% FBS compared to the explants cultured in 5% FBS, but the results are not statistically significant
Young’s Modulus Analysis Between Uncultured and Cultured Meniscus Explant Tissues
The statistical analyses on the Young’s modulus of the tissue showed comparable results to the force analyses. The Young’s modulus for 10% FBS cultured explants (mean 3.338 MPa (SE 0.113) was significantly larger compared to the uncultured (mean 2.415 MPa (SE 0.147); P < 0.001 (Cohen’s D = 1.762), 1% FBS cultured (mean 2.686 MPa (SE 0.134); P= 0.002 (Cohen’s D = 1.312)), and 5% FBS (mean 2.746 MPa (SE 0.158); P= 0.004 (Cohen’s D = 1.079)) meniscus explants (Fig. 3b). No statistically significant difference was observed in the Young’s modulus between uncultured, 1% FBS and 5% FBS cultured explants.
Young’s modulus was also analyzed between uncultured and cultured explants within the anterior and posterior regions. Explants from the posterior region of the meniscus cultured in 5% (mean 2.900 MPa (SE 0.257)) and 10% FBS (mean 3.201 MPa (SE 0.141)) had statistically significant larger compressive stiffness compared to the uncultured explants (mean 2.146 MPa (SE 0.153)) (uncultured vs. 5% FBS explants P = 0.006 (Cohen’s D = 1.260); uncultured vs. 10% FBS explants P < 0.001 (Cohen’s D = 2.537) (Figure 4b). However, there was no statistically significant difference in the Young’s modulus between the uncultured and 1% FBS cultured explants (mean 2.521 MPa (SE 0.132); P = 0.147 (Cohen’s D = .928). Explants from the anterior region of the meniscus showed similar statistical results compared to the results with both regions combined (Figure 5b). However, in these tissues, the 1% culture media had more of an effect on the tissue Young’s modulus when compared to the 5% culture media (the results were not statistically significant; P = .370 (Cohen’s D = .445).
Figure 4b.
Statistical Analysis of the Young’s Modulus of Cultured Explants from the Posterior Region of the Meniscus. Explants cultured in 10% FBS media were significantly more elastic than the uncultured explants and explants cultured in 1% FBS. In addition, explants cultured in 5% FBS were significantly stiffer than uncultured explants (* = .001 ≤ P < .05)
Figure 5b.
Statistical Analysis of the Young’s Modulus of Cultured Explants from the Anterior Region of the Meniscus. Results are similar to the trends in the combined group (figure 3b). However, the 1% culture media produced a greater effect on the tissue elasticity than the 5% culture media. (* = .005 ≤ P < .05)
Discussion
Microindentation testing demonstrated that that culture media conditions effects the stiffness of the meniscus at different concentrations. The result from this experiment indicates that culturing explants in DMEM with 10% FBS made the tissue significantly stiffer compared to tissues there were not cultured or cultured in lower serum concentration. The change in Young’s modulus appears to correlate with serum concentration of FBS. These findings support the hypothesis that intermediate and high concentrations of culture media could have an effect on the stiffness of the meniscus. As a result, the meniscus explant tissues that were cultured at 10% FBS became stiffer after being cultured for seven days. The increase in Young’s modulus may be related to an increase in organic matter within the meniscus (McQuillan et al. 1986). However, there was no significant regional variation in the response of meniscus tissue to serum aside from finding that posterior region appeared more sensitive to both intermediate (5% FBS) and high (10% FBS) serum concentration. Low serum cultivation (1% FBS) from all regions of the meniscus resulted in biomechanical properties similar to control.
Indentation testing is a commonly used approach to non-destructively measure the mechanical properties of soft tissues (Lu et al., 2004). Microindentation and nanoindentation have been widely used to test mechanical properties of the meniscus (Sweigart et al., 2005; Lu et al., 2004; Abdelgaied et al., 2015; Sweigart et al., 2005), and the changes of the mechanical properties of the meniscus in osteoarthritis (Levillain et al., 2016; Levillain et al., 2015; Baro et al., 2012). However, the impact of culture conditions on meniscus biomechanical properties has not been reported previously. This study quantified the effect of culture media on the mechanical properties of the meniscus to create a tissue culture model for use in future experiments.
Published literature of meniscus cell and tissue culture have predominantly examined the effect of serum concentrations in cell culture. Riera et al. observed serum concentration effect on meniscus cell migration and proliferation in an in vitro micro-wound healing assay performed in monolayer culture. Porcine meniscus cells were cultured in serum free, 1%, 5%, and 10% FBS conditions. Treatment with 10% FBS resulted in an increase in proliferation and in the total number of cells that migrated in the wound compared to the cells treated with serum free, 1%, and 5% FBS media. Similarly, Webber et al., 1988 compared an in vitro organ culture model for wound healing assay of meniscus cells between 10% FBS media and serum free media described in a previous study (Webber, Zitaglio et al, 1998). Cell migration was observed in culture using 10% FBS media but not in culture using serum free media. In the current study, tissues cultured in 10% FBS exerted an effect represented by an increase in tissue Young’s modulus whereas 1% FBS (low serum) had no observable effect. We did not demonstrate the mechanism of this effect in this study, but it is plausible that serum conditions stimulated collagen and GAG production which increased the compressive properties of the meniscus, whereas low serum conditions prevented loss of GAG content that could occur in completely serum free conditions. GAGs have been shown to play an important role in enhancing the compressive properties of the middle and inner regions of the meniscus compared with the peripheral regions, and also reduce the circumferential tensile properties (Sanchez Adams et al 2011). Fetal calf serum (FCS) has been shown to support optimal meniscus cell metabolism in meniscus tissue culture as demonstrated by studies of sulphate incorporation to measure proteoglycan synthesis in the presence of 20% FCS supplementation (Verbruggen et al 1996).
Other studies have researched the biomechanical characteristics between different regions within the meniscus. Sweigart and Athanasiou, 2004 looked at the biomechanical characteristics between different regions of the medial and lateral porcine meniscus. Whole porcine menisci were collected and divided into anterior, central, and posterior regions, and a microindentation test was performed. The anterior region in the medial meniscus had statistically highest Young’s modulus compared to other regions. There was no difference between the regions in the lateral meniscus. Base on that study, the lateral meniscus was used in this study because there was no variability between the regions. Sweigart eta al., 2004 did a similar biomechanical study on the medial meniscus observing the intraspecias and interspecies compressive properties and observe similar results. However, these tissues were not incubated in culture media before performing indentation testing. In addition, the tested tissues were stored at −20°C and unthawed before testing. The uncultured tissues in this study were tested after being harvest.
Abdelgaied et al., 2015 compared the biomechanical characteristics of native porcine medial meniscus to decellularized porcine meniscus. Their study observed compression and stretching force applied on the tissue, and the purpose of the experiment is to see if the decellularized porcine meniscus is an appropriate biological scaffold for partial meniscus replacement. This study, on the other hand, only compared the Young’s modulus between uncultured menisci and menisci cultured in different concentration of culture media. In addition, Abdelgaid study also froze their tissues before testing. This study did not freeze the meniscus tissue.
Cultivation of tissues in low serum concentration resulted in the maintenance of the biomechanical properties of the native tissue after seven days of culturing. It is possible the seven-day cultivation period was not long enough for phenotypic tissue changes to translate into a decline in native meniscus biomechanical properties. Also, this study did not include a no serum or supplemented serum free medium for comparison to determine if cultivation in the absence of serum is detrimental to meniscus biomechanical properties. Future studies using this model to further evaluate the impact of culture conditions, cytokines, and growth factors on meniscus biomechanical properties and use will determine if low serum conditions effect tissue compressive properties.
Figure 3b.
Statistical Analysis of the Young’s Modulus.Tissues cultured in 10% FBS media were statistically significant stiffer compared to the uncultured tissues and tissues cultured in 1% FBS and 5% FBS media (* = P < .005)
Highlights.
Determine serum concentration without affecting meniscus mechanical properties
Young’s Modulus was compared between cultured and uncultured tissues
No difference between uncultured and lower concentrated tissue culture
Low serum concentration does not impact meniscus biomechanical properties
Acknowledgement
This project was supported by the Institutional Research and Academic Career Development Awards, Grant Number: NIH/NIGMS K12-GM102773.
Grant support: Institutional Research and Academic Career Development Awards, Grant Number: NIH/NIGMS K12-GM102773
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelgaied A, Stanley M, Galfe M, Berry H, Ingham E, Fisher J. Comparison of the Biomechanical Tensile and Compressive Properties of Decellularised and natural Porcine Meniscus. Journal of Biomechanics. 2015; 48: 1389–1396. (DOI: 10.1016/j.jbiomech.2015.02.044) [DOI] [PubMed] [Google Scholar]
- Baro V, Bonnevie E, Lai X, Price C, Burris D, Wang L. Functional Characterization of Normal and Degraded Bovine Meniscus: Rate-Dependent Indentation and Friction Studies. Bone. 2012; 51: 232–240. (DOI: 10.1016/j.bone.2012.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Lotito K, Rodeo S. Biomechanics and Healing Response of the Meniscus. Operative Techniques in Sports Medicine. 2003; 11: 68–76. (DOI: 10.1053/otsm.2003.35899) [DOI] [Google Scholar]
- Levillain A, Boulocher C, Kaderli S, Viguier E, Hannouche D, Hoc T, Magoariec H 2015. Meniscal Biomechanical Alterations in an ACLT Rabbit Model of Early Osteoarthritis. Osteoarthritis and Cartilage. 2015; 23: 1186–1193. (DOI: 10.1016/j.joca.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Levillain A, Magoariec H, Boulocher C, Decambron A, Viateau V, Hoc T. Viscoelastic properties of rabbit osteoarthritic menisci: A correlation with matrix alterations. Journal of the Mechanical Behavior of Biomedical Materials. 2016; 65: 1–10. (DOI: 10.1016/j.jmbbm.2016.08.015) [DOI] [PubMed] [Google Scholar]
- Li Q, Doyran B, Gamer L, Lu X, Qin L, Ortiz C, Grodzinsky A, Rosen V, Han L. Biomechanical Properties of Murine Meniscus Surface via AFM-Based Nanoindentation. Journal of Biomechanics. 2015; 48: 1364–1370. (DOI: 10.1016/j.jbiomech.2015.02.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, An Y, Wu Y, Song Y, Chao Y, Chien C. Microindentational Test for Assessing the Mechanical Properties of Cartilaginous Tissues. Journal of Biomedical Material Research. 2007; 80B: 25–31. (DOI: 10.1002/jbm.b.30564) [DOI] [PubMed] [Google Scholar]
- Lindl T. Zell-und Gewebekultur. 5th ed. Spektrum Akademischer Verlag, Heidelberg [Google Scholar]
- Ling CH, Lai JH, Wong IJ, Levenston ME. Bovine meniscal tissue exhibits age-and interleukin1 dose-dependent degradation patterns and composition-function relationships. Journal of Orthopedic Research. 2015; 34: 801–811 (DOI: 10.1002/jor.23096) [DOI] [PubMed] [Google Scholar]
- Lindburg C, Willey J, Dean D. Effects of Low Dose X-ray Irradiation on Porcine Articular Cartilage Explants. Journal of Orthopaedic Research. 2013; 31: 1780–1785. (DOI: 10.1002/jor.22406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zheng Y. Indentation Test of Soft Tissues With Curved Substrates: A Finite Element Study. Medical & Biological Engineering & Computing. 2004; 42: 535–540. [DOI] [PubMed] [Google Scholar]
- Markis E, Hadidi P, Athanasiou K. The Knee Meniscus: Structure-Function, Pathophysiology, Current Repair Techniques, and Prospects for Regeneration. Biomaterials. 2011; 32: 7411–7431. (DOI: 10.1016/j.biomaterials.2011.06.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty A, Guilak F. Mechanobiology of the Meniscus. Journal of Biomechanics. 2015; 48: 1469–1478. (DOI: 10.1016/j.jbiomech.2015.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D, Handley C, Robinson H. Control of Proteoglycan Biosynthesis Further Studies on the Effect of Serum on Culture Bovine Articular Cartilage. Journal of Biochemistry. 1986; 237: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K, Huang S, Musulin R. Tissue Response to Mechanical Vibration for Sonoelasticity Imaging. Ultrasound in Medicine and Biology. 1990; 16: 241–246. [DOI] [PubMed] [Google Scholar]
- Riera K, [Google Scholar]
- Rothfusz N, Wilusz R, Weinberg J, Guilak F.Interleukin-1, Tumor Necrosis FactorAlpha, and Transforming Growth Factor-Beta 1 and Integrative Meniscal Repair: Influences on Meniscal Cell proliferation and Migration. Arthritis Research and Therapy. 2011; 13: 187–207. (DOI: 10.1186/ar3515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Adams J, Willard VP, Athanasiou KA. Regional Variation in the Mechanical Role of Knee Meniscus Glycosaminoglycans. Journal of Applied Physiology. 2011; 111 (6): 1590–1596 (DOI: 10.1152/japplphysiol.00848.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweigart M, Athanasiou K. Biomechanical Characteristics of the Normal Medial and Lateral Porcine Knee Menisci. Journal of Engineering in Medicine. 2005; 219: 53–62. (DOI: 10.1243/095441105X9174) [DOI] [PubMed] [Google Scholar]
- Sweigart M, Athanasiou K. Tensile and Compressive Properties of the medial Rabbit Meniscus. Journal of Engineering in Medicine. 2005; 219: 337–347. (DOI: 10.1243/095441105X34329) [DOI] [PubMed] [Google Scholar]
- Verbruggen G, Verdonk R, Veys EM, Van Daele P, De Smet P, Van den Abbeele K, Claus B, Baeten D. Human meniscal proteoglycan metabolism in long-term tissue culture. Knee Surgery, Sports Traumatology, Arthroscopy. 1996; 4(1): 57–63 [DOI] [PubMed] [Google Scholar]
- Webber J, York J, Vanderschilden J. An Organ Culture Model for Assaying Wound Repair of the Fibrocartilaginous Knee Joint Meniscus. American Journal of Sports Medicine. 1989; 17: 393–400. (DOI: 10.1177/036354658901700314) [DOI] [PubMed] [Google Scholar]
- Webber RJ, Zitaglio T, Hough AJ Jr. Serum-Free Culture of Rabbit Meniscal Fibrochondrocytes: Proliferative Response. Journal of Orthopedic Research. 1988; 6: 13–23 (DOI: 10.1002/jor.110006010) [DOI] [PubMed] [Google Scholar]
- Wojtys E, Chan D. Meniscus structure and function. Instructional Course Lectures. 2005; 54: 323–30. [PubMed] [Google Scholar]
- Zhang X, Aoyama T, Ito A, Tajino J, Nagai M, Yamaguchi S, Iijima H, Kuroki H. Regional Comparison of Porcine Menisci. Journal of Orthopaedic Research. 2014; 32(12):1602–1611 (DOI: 10.1002/jor.22687) [DOI] [PubMed] [Google Scholar]
- Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E. Proteomic Analysis for the Assessment of Different Lots of Fetal Bovine Serum as a Raw Material for Cell Culture. Part IV. Application of Proteomics to the Manufacture of Biological Drugs. Biotechnology Progress. 2008; 22 (5): 1294–3000 (DOI: 10.1021/bp060121o) [DOI] [PubMed] [Google Scholar]