Abstract
Background:
Acute consumption of cannabis or its primary psychoactive ingredient Δ9-tetrahydrocannabinol has been shown to impair memory, reaction time, time perception, and attention. However, it is difficult to measure these impairments in a brief test that can be used in a non-laboratory setting.
Aims:
We aim to develop and validate a prototype for a mobile phone application to measure Δ9-tetrahydrocannabinol-induced cognitive impairment.
Methods:
We conducted two double-blind, within-subjects studies examining impairments after oral doses of Δ9-tetrahydrocannabinol (0, 7.5, 15 mg) using both standardized computer-based tasks and our novel phone-based tasks. The tasks measured cognitive speed, reaction time, fine motor ability, and working memory and, in the second study, time perception. Study 1 (n=24) provided initial data, and Study 2 (n=24) was designed to refine the measures. In both studies, healthy non-daily cannabis users participated in three four-hour experimental sessions in which they received capsules containing Δ9-tetrahydrocannabinol (7.5, 15 mg) or placebo. Subjective and cardiovascular measures were obtained at regular intervals, and at the time of peak drug effect subjects completed both standardized, computer-based and brief, phone-based tasks.
Results:
Δ9-Tetrahydrocannabinol-induced impairment was detected on most of the computer tasks, but was not evident on most of the phone tasks.
Conclusions:
The phone tasks were brief, to facilitate use in a non-laboratory setting, but it is likely that this made them less sensitive to the impairing effects of Δ9-tetrahydrocannabinol. These findings confirm that Δ9-tetrahydrocannabinol impairs performance on several tasks at two recreationally relevant doses, but raises question about the feasibility of designing a phone application as a sensitive field sobriety test for cannabis.
Keywords: Cannabis, marijuana, dronabinol, Δ9-tetrahydrocannabinol, driving
Introduction
Cannabis is widely used for nonmedical purposes, and to a more limited degree also for therapeutic reasons (National Instituate of Drug Abuse, 2018). In 2017, an estimated 45% of Americans over the age of 12 years reported having used cannabis within their lifetime and use is expected to rise as the drug becomes increasingly available (Hasin et al., 2015). The escalation in use is likely to be accompanied by increased risk of accidents or impaired driving performance (Desrosiers et al., 2015; Hartman and Huestis, 2013; Kalant 2016). Driving under the influence of cannabis increases the risk of accidents by approximately two-fold, and cannabis is the most common illicit substance implicated in motor vehicle fatalities (Martin et al., 2017). Controlled studies (Hartman and Huestis, 2013) indicate that the drug impairs reaction time, headway maintenance, headway variability, road tracking, speed, speed variability, divided attention, visual search, and working memory.
The effects of cannabis are attributed to its primary psychoactive compound Δ9-tetrahydrocannabinol (THC), which acts as a partial agonist at cannabinoid (CB1) receptors (Broyd et al., 2016; Desrosiers et al., 2015; Sewell et al., 2009; Solowij et al., 1995). CB1 receptors are distributed within brain areas directly relevant to psychomotor performance, including the caudate nucleus, putamen, globus pallidus, hippocampus, and cerebellum (Glass et al., 1997; Mechoulam and Parker, 2013). For reasons that are not fully understood, the psychomotor-impairing effects of cannabis and THC are greater in occasional users of cannabis compared to more frequent or chronic users (Hartman and Huestis, 2013; Ramaekers et al., 2009).
Although impairments induced by cannabis and its active constituents have been demonstrated in controlled laboratory studies (Desrosiers et al., 2015; Oomen et al., 2018), we lack sensitive methods to assess cannabis-related impairment in real-world situations. A rapid and sensitive behavioral measure of impairment would be of value to law enforcement personnel, employers, and to the drug users themselves. Thus, there is a need for a quick and convenient behavioral task battery that can be used to measure cannabis-induced psychomotor impairment as a predictor of impaired ability in any setting, such as a phone-based application (app).
There are several challenges in developing a sensitive phone-based measure. Most importantly, the tasks should be sensitive and directly relevant to cognitive and psychomotor functions relevant in daily functioning, but brief and engaging enough to be convenient to use in real-life situations. This is a particular challenge for detecting impairments with cannabis because these impairments are relatively subtle, they may be overcome with effort and may only be evident under sustained conditions (Bondallaz et al., 2016). Tasks in a phone-based measure should also be robust enough to withstand noise from other sources (e.g. distractions from environmental factors, and extraneous noise or interference). Finally, they should have demonstrable sensitivity to the effects of THC under controlled conditions (e.g. blinded drug administration, placebo-control, verified abstinence from recent use of cannabis or other drugs). The goal in the present study was to develop a phone-based app with brief tasks that are sensitive to the effects of THC, similar to the sensitivity of standardized computer-based tasks.
To achieve this goal, we conducted two studies using both computer and phone-based app tasks to examine effects of THC (0, 7.5, and 15 mg oral), administered under controlled, double-blind conditions. Participants were occasional cannabis users who were drug-free at the time of testing. After ingesting the drug, they completed standardized, computer-based tasks as well as briefer, phone-based tasks that might be conveniently administered outside the laboratory. The standardized computer tasks included a two-column addition task, a reaction time task, a fine motor task, and a working memory span task. The two-column addition task evaluated cognitive processing speed, how quickly an individual can comprehend a situation and take in relevant information. This task was chosen because impaired processing speed increases the probability of accidents, including automobile accidents (Edwards et al., 2008). A reaction time task was chosen because it is directly relevant to driving as well as other skilled actions (Guo et al., 2016). A fine motor coordination task was chosen because impairments in coordination affect the ability to react efficiently and effectively. Lastly, a working memory task was chosen because spatial working memory ability is critical for efficient cognitive performance, including driving (Endsley and Garland, 2000). All of the standardized computer-based tasks were sensitive to THC-induced impairments in cognitive performance, reaction time, and motor responses (Desrosiers et al., 2015; Hartman and Huestis, 2013; Oomen et al., 2018). Phone-based tasks were chosen or developed using the software framework ResearchKit (Apple Inc.) to measure the same underlying processes represented in the computer tasks. In the first study the phone-based tasks included a Paced Visual Serial Addition Task (PVSAT), a reaction time task, a fine motor finger tapping coordination task, and a spatial working memory task. Because the phone-based app tasks were relatively insensitive to THC in the first study, we modified these tasks in the second study, by adjusting the length and difficulty and adding a time perception task. Across the two studies we describe our process of improving the phone-based app tasks.
Methods
Design
Studies 1 and 2 were conducted sequentially, in separate groups of subjects (n=24 and n=24 respectively). Both studies utilized three-session double-blind within-subjects designs in which healthy occasional cannabis users received oral THC (0, 7.5, and 15 mg) before completing tasks on a computer and on a phone. Abstinence from recent drug use was verified at the beginning of the sessions. The four-hour sessions were conducted in comfortably furnished study rooms that resembled living room settings, with couches, coffee tables, TVs, books, art work, and windows with natural light to provide a setting conducive to experiencing drug effects. Sessions were separated by at least seven days. The studies were approved by the University of Chicago Institutional Review Board.
Study 1
Participants
Healthy occasional cannabis users (n=24; 50% males, 18–34 years) were recruited via printed and online advertisements. Eligibility criteria included cannabis use at least four times with no adverse events, and non-daily use. Screening included a physical examination, an electrocardiogram and a semi-structured psychiatric interview by a clinical psychologist. Subjects provided detailed information on their current and lifetime drug use. Exclusion criteria included current Axis I Diagnostic and Statistical Manual of Mental Disorders, 5th Edition disorder (American Psychiatric Association, 2013), including substance use disorder, history of psychosis or mania, less than a high school education, lack of English fluency, a body mass index outside 19–30 kg/m2, high blood pressure (>140/90), abnormal electrocardiogram, daily use of any medication other than birth control, pregnancy, or lactating. All participants were right hand dominant.
Drug
Dronabinol (7.5 or 15 mg; Marinol; Solvay Pharmaceuticals) was encapsulated in opaque red capsules with dextrose filler. Placebo capsules contained only dextrose. These doses of THC produce plasma levels similar to those attained with recreational cannabis use, and they are known to produce reliable and stable subjective, behavioral, and cognitive effects (Ballard et al., 2012; Curran et al., 2002; Wachtel and de Wit, 2000). The 7.5 and 15 mg doses produce blood plasma THC levels of roughly 4 and 8 ng/mL respectively, and their effects peak at about the time the study tasks were administered (Wachtel et al., 2002). We chose to study THC rather than cannabis because THC is known to be the primary psychoactive ingredient of cannabis, and in order to minimize variability related to plant products. Several lines of evidence support the idea that psychomotor effects of cannabis are mediated by THC. Cannabis and matched doses of THC produce similar subjective and physiological effects (Wachtel et al., 2002). Drugs that block the CB1 receptors (where THC acts) block the psychological and physiological effects of smoked cannabis (Huestis et al. 2001), and oral THC effectively and dose-dependently suppresses cannabis withdrawal symptoms in daily cannabis users (Budney et al. 2007; Haney et al. 2004; Haney et al. 2008).
Procedure
Pre-session instructions.
Participants were advised to get their normal amounts of sleep and not to eat for two hours before each experimental session. They were told to consume their normal amounts of caffeine and nicotine before sessions but to abstain from using alcohol, prescription drugs (except contraceptives), over-the-counter drugs, cannabis, and other illicit drugs for 48 h before each session. Participants were informed that they would be tested for recent drug use before each session and that sessions would be cancelled if they tested positive. To minimize expectancy, participants were informed that they could receive a stimulant, sedative, cannabinoid, or placebo.
Study sessions.
Sessions were conducted from 09:00–13:00. Subjects provided breath samples for recent alcohol use (Alco-sensor III; Intoximeters, St Louis, Missouri, USA), urine samples for recent drug use (ToxCup, Branan Medical, Irvine, California, USA), and a pregnancy test (females only; Aimstrip, Craig Medical, Vista, California, USA). They were rescheduled or dropped if they tested positive. Then, baseline cardiovascular and mood measures were obtained (see below), and subjects consumed a capsule containing placebo or THC (7.5 or 15 mg) under double-blind conditions. For the following two hours subjects relaxed with magazines, and mood and cardiovascular measures were obtained every 30 min. The tasks began two hours post-capsule, at the time when plasma levels were expected to peak (Curran et al., 2002; Karschner et al., 2012). Subjects completed several tasks on both a desktop computer and on a phone-based app. The order of computer vs phone-based app tasks was counterbalanced, but the sequence of tasks within each modality was kept constant across all participants. The computer-based tasks, described below, included the Two Column Addition Task, Simple Response Time Task, Finger Tapping Task, and the Memory Span tasks. These tasks took 7, 5, 3, and 15 min, respectively (total 30 min). The phone-based tasks included the PVSAT, the Reaction Time Task, the Finger Tapping Task, and the Spatial Memory Task. These tasks took 0.5, 0.5, 1, and 3 min, respectively (total five minutes). After completing the tasks, participants were permitted to relax for 30 min, and then completed the tasks a second time, at three hours post capsule. This second administration was designed to capture individual differences in absorption time, and also provided a measure of test-retest reliability. After completing all sessions, participants were debriefed and financially compensated.
Cardiovascular measures
Heart rate and blood pressure were monitored with portable monitors (Omron 10 Plus, Omron Healthcare) six times throughout the session (0, 30, 60, 90, 150, 210 min post capsule).
Self-report measures
Addiction Research Center Inventory (ARCI; Chait et al., 1985; Martin et al., 1971).
The 53-item true/false ARCI, included the original 49 items and four additional items to form a 12-item Marijuana (Mar) subscale specific to cannabis (Chait et al., 1985). We focused our analyses on the Mar scale.
Drug Effects Questionnaire (DEQ; Morean et al., 2013).
The DEQ consisted of six questions on a visual analog scale (VAS) assessing subjective drug effects. Subjects were asked to rate the extent they felt a drug effect, whether they liked or disliked the drug effect, if they felt high, and if given a choice, would they want more of the drug. Each VAS item was presented on a 100 mm line labeled “not at all” to “extremely” (or “very strong”; “very much”).
Performance measures
Computer-based tasks.
These tasks were obtained from Psychology Experimental Building Language (PEBL; Mueller and Piper, 2014).
Two Column Addition Task.
The Two Column Addition Task is a measure of cognitive processing speed. Participants are asked to perform mathematical calculations as rapidly as possible. Three two-digit numbers are shown on the screen and the individual has 15 s to type in the sum starting with the left-most digit. They begin with three practice trials followed by 45 included trials. The primary outcome was accuracy of responses. Total duration of this task is about seven minutes.
Response Time Task.
The simple Response Time Task measures reaction time to a visually presented stimulus. A single stimulus (an ‘X’) appears at a specifiable delay (250–2500 ms), and the individual is instructed to press the “X” key in response to the stimulus. It contains four blocks of 50 trials; with a break between blocks. The primary outcome measure was mean response time over all trials.
Finger Tapping Task.
The Finger Tapping Task is a measure of psychomotor performance and coordination fine motor speed. Individuals are instructed to tap the letter “A” on their keyboard with the index finger as rapidly as possible for eight trials of 10 s. This task is completed once with the dominant hand and once with the non-dominant hand. Mean number of taps is the primary outcome measure.
Memory Span Task.
The Memory Span Task is a measure of working memory. Individuals are asked to recall sequences of labeled images in order from a minimum length of two to a maximum of nine. The span is automatically varied during the task, increasing after successful completion of a sequence, and decreasing back down to two new images after failures. Average length of correct sequence, labeled memory span, was the primary outcome measure.
Phone-based app tasks (“Am I Stoned?”: ResearchKit Tasks (Apple Inc. 2015).
Paced Visual Serial Addition Task (PVSAT).
The PVSAT measures cognitive function including visual information processing speed and the ability to conduct arithmetic calculation. Single digits are presented every two or three seconds and the individual must add each new digit to the one immediately before. The primary outcome is the total number correct answers out of the 10 possible correct answers. Total duration of this task is about one minute.
Reaction Time Task.
The Reaction Time Task measures response time to a presented stimulus. The subject is instructed to shake the device faster than a set threshold acceleration three times in response to a visual cue presented on the screen. After the third trial, the three response times are then averaged to represent average reaction time over the entire task. Total duration of this task is about 30 s.
Finger Tapping Task.
The Finger Tapping Task is a measure of psychomotor performance and coordination. Participants are instructed to tap on targets presented on the screen, alternating between the index and middle finger on each target as fast as they can, for a total of 20 s. They complete the task using first the dominant hand and then the non-dominant hand. Total number of correct taps is the primary outcome measure. Total duration of this task is about one and a half minutes.
Spatial Memory Task.
The Spatial Memory task is a measure of working spatial memory span. Participants are asked to observe and then recall pattern sequences of increasing length. The span length is automatically varied during the task, in a range from 3–9, increasing after successful completion of a sequence, and decreasing after failures. The game finishes either when seven tests have been completed, or when the participant makes two consecutive failures in a row. Average memory span success rate is the primary outcome measure. Total duration of this task is about one to two minutes.
Data analysis
The primary outcome measure of Study 1 was performance on the phone-based app tasks: Paced Visual Serial Addition, Response Time, Finger Tapping, and Spatial Memory. The secondary measures of Study 1 included performance on the computer-based tasks: Two Column Addition, Response Time, Finger Tapping, and Working Memory, and the physiological and subjective measures. First, we used Bonferroni corrected repeated measures analyses of variance (ANOVAs) with Helmert contrasts (placebo v. THC, and then 7.5 v. 15 mg) to confirm the expected effects of THC on physiological measures, including increase in heart rate and ratings of subjective drug effect measures. Then we confirmed that there was no improvement or decline in performance on the tasks (computer and phone-based) across the three sessions and that there were no order effects of completing computer tasks first or phone tasks first. Performance did not differ systematically across the three study sessions. On most measures task performance was not different at the two testing times, two and three hours after the capsule. On computer-based reaction time, reaction times were longer at three hours compared to two hours, regardless of what drug subjects received (F(1,20)=9.28, p-value <0.005), probably due to fatigue. Because there were no systematic differences, task performance data were averaged across the two time-points for further analyses. We used Bonferroni corrected repeated measures ANOVAs with Helmert contrasts (placebo v. THC, and then 7.5 v. 15 mg) on average computer and phone-based task performance.
Study 1 results
Participant characteristics
Demographic information and recent substance use history of the participants are listed in Table 1.
Table 1.
Study 1 participant characteristics.
| Gender, (M:F) | 12:12 |
| Age, mean (SEM) | 23.4 (0.9) |
| Education, mean (SEM) | 15.5 (0.3) |
| Racea | |
| Caucasian | 12 |
| African American | 1 |
| Other | 5 |
| More than one race | 6 |
| Recent (past month) substance use, mean (SEM), nb | |
| Caffeine (cups/day) | 1.7 (0.2), 23 |
| Tobacco cigarettes (cigs/week) | 2.8 (0.7), 5 |
| Alcohol (drinks/week) | 2.1 (0.5), 23 |
| Cannabis (times/month) | 8.6 (1.9), 13 |
| Lifetime cannabis use, mean (SEM) | 614.9 (449.8) |
SEM: standard error of the mean.
Race is number of individuals who identify as such (“Other” is mainly Asian).
Mean and SEM were calculated using only subjects who reported any recent use of the drug.
Physiological and subjective measures
Physiological and subjective measures are listed in Table 2. THC increased heart rate but did not alter blood pressure. THC increased subjective ratings of DEQ “Feel”, “High”, “Dislike”, “Like”, and the ARCI Mar Scale.
Table 2.
Study 1 physiological and subjective measures.
| Measure | Placebo v. THC Fa, p | 7.5 mg v. 15 mg F, p |
|---|---|---|
| Heart rate | 36.1, <0.001b, η2c=0.44 | 26.5, <0.001b, η2=0.36 |
| Mean arterial pressure | ns | ns |
| DEQ | ||
| “Feel” | 30.0, <0.001b, η2=0.58 | 15.0, 0.001d, η2=0.41 |
| “Like” | 25.3, <0.001b, η2=0.54 | ns |
| “Dislike” | 11.4, 0.003d, η2=0.34 | 10.3, 0.004d, η2=0.32 |
| “High” | 35.0, <0.001b, η2=0.61 | 10.4, 0.004, η2=0.32 |
| “Want more” | ns | 6.2, 0.020d, η2=0.22 |
| ARCI Marijuana Scale, mean (SEM) | 31.2, <0.001b, η2=0.59 | 9.7, 0.005d, η2=0.31 |
ANOVA: analysis of variance; ARCI: Addiction Research Center Inventory; DEQ: Drug Effects Questionnaire; SEM: standard error of the mean; THC: Δ9-tetrahydrocannabinol.
F statistic and p-value from Bonferroni corrected repeated measures ANOVA with Helmert contrasts drug condition*time interaction;
significant at α=0.0025;
partial eta squared;
significant at α=0.025,
Computer and phone-based app task performance
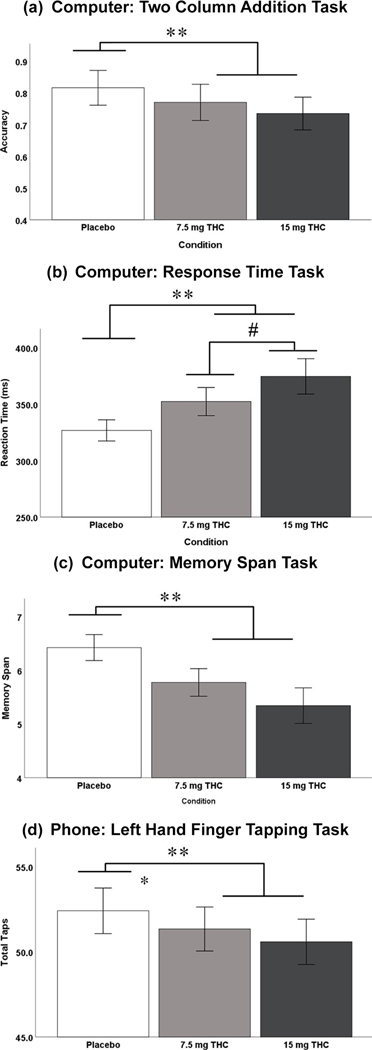
Computer and phone-based app task performance results are listed in Table 3. Three out of the four computer tasks revealed a significant drug condition effect (placebo v. THC): Two Column Addition, Reaction Time, and the Working Memory Task (Figure 1(a)–(c)). Only the reaction time task performance revealed a significant dose dependent effect (7.5 mg v. 15 mg). Only one out of the four phone-based app tasks revealed a significant drug condition effect (placebo v. THC): non-dominant hand finger tapping (Figure 1(d)).
Table 3.
Study 1 computer and phone-based task performance.
| Placebo v. THC Fa, p | 7.5 mg v. 15 mg F, p | |
|---|---|---|
| Computer | ||
| Two Column Addition | 11.78, 0.001b, η2c=0.20 | ns |
| Simple Reaction Time | 14.51, 0.001b, η2=0.42 | 5.56, 0.029d, η2=0.22 |
| Finger Tapping | ns | ns |
| Working Memory | 11.99, 0.002b, η2=0.38 | ns |
| Phone | ||
| Paced Visual Serial Addition | ns | ns |
| Simple Reaction Time | ns | ns |
| Finger Tapping | 7.99, 0.010b, η2=0.28 | ns |
| Spatial Memory | ns | ns |
ANOVA: analysis of variance; THC: Δ9-tetrahydrocannabinol.
F statistic and p-value from Bonferroni corrected repeated measures ANOVA with Helmert contrasts for drug condition;
significant at α=0.0025;
partial eta squared;
trending.
Figure 1.
Study 1 computer and phone-based task performance. (a) Mean (±standard error of the mean (SEM)) Two Column Addition accuracy (#Correct/Total); (b) mean (±SEM) computer based response time (ms);(c) mean (±SEM) computer-based memory span (maximum of nine);(d) mean (±SEM) phone application (app)-based left hand finger tapping. SE: standard error; THC: Δ9-tetrahydrocannabinol. Error Bars: +/– 1 SE.
Study 1 discussion
We detected drug-induced impairments after oral THC on several of the tasks administered on a desktop computer, and on one of the tasks administered via phone. The study showed that in principle it is possible to administer brief phone-based tasks to detect THC-induced impairment under controlled conditions. However, the impairment on the phone app was limited to one outcome measure, a fine motor task, and the effect was not dose-dependent. We reasoned that the phone tasks may have been too short to detect drug effects. Thus, we decided to conduct a follow-up study with an updated and adjusted task battery. In Study 2, we lengthened the duration (number of trials) for all three of the insensitive phone tasks. We also added two tasks to measure time perception, time perception, and time estimation. Cannabis is known to increase estimates of time duration in time estimation tasks and decrease durations in time production tasks (McDonald et al., 2003; O’Leary et al., 2003; Sewell et al., 2013; Stone et al., 2010), and time perception is likely to affect skilled performance. We utilized the same task (Time Estimation 2.0, 2001, Brainmetric Software) used by Sewell et al. (2013) for our computer-based tasks. Our phone-based versions were developed using the ResearchKit software but were shorter in duration. Thus, Study 2 was a follow-up study to improve the sensitivity of the phone task battery to detect THC impairment.
Study 2
Participants and drug
Participants in Study 2 were recruited using the same methods and inclusion and exclusion criteria as Study 1, and they received the same drug doses.
Procedure
The procedure for Study 2 was similar to that of Study 1 with three exceptions. First, the tasks were only administered once during each session (instead of twice) two hours post capsule consumption. Second, we lengthened the phone-based tasks to improve their sensitivity, and added time perception tasks (see below). Third, we added an additional subjective effect measure, the Profile of Mood States (POMS; McNair et al., 1971).
Cardiovascular measures
Same as in Study 1.
Self-report measures
The self-report measures included those used as in Study 1, as well as the POMS (McNair et al., 1971). The POMS was added because of observed subtle anxiogenic effects of the oral doses we were administering during the first study. The POMS consists of 72 adjectives commonly used to describe momentary mood states. Participants indicate how they feel in relation to each of the 72 adjectives on a five-point scale from “not at all” (0) to “extremely” (4). The questionnaire is comprised of eight sub-scales (Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness, Elation). Two summary scales are derived from the other scales: Arousal=(Anxiety+Vigor)– (Fatigue+Confusion); Positive Mood=Elation–Depression. This well-established mood measure was used to examine changes in mood states, including anxiety, before and after the capsules.
Performance measures
Phone-based app tasks: (“Am I Stoned?”: ResearchKit Tasks (Apple Inc. 2015).
For Study 2, we used the same phone-based app tasks used in Study 1, but some tasks were lengthened to increase its sensitivity to drug effects. The PVSAT increased from 10 to 20 trials. Reaction time increased from four to 10 trials. Spatial memory increased from seven to 10 trials. In addition, we added the following two time perception tasks:
Time estimation task (TET).
The TET measures the ability to estimate the amount of time that has elapsed. The individual views a screen with an outline of a circle, which is then filled completely for a period of time from 1–30 s. Five trials (durations) are presented in random order. Subjects are asked to estimate the amount of time the circle was filled after each trial. No clock is present. The primary outcome is the ratio between the actual time interval and their estimated time interval. The duration of this task was about three minutes.
Time reproduction task (TRT).
The TRT measures the ability to reproduce a time sample. The individual is asked to hold down a button for a specified interval of time from 1–30 s. Five trials (time intervals) are presented in randomized order. No clock or other timepiece is accessible to the subject. The primary outcome is the ratio between the actual time interval and amount the time that the subject holds the button. The duration of this task was about three minutes.
Computer-based tasks.
These tasks were obtained from 2001 Brainmetric Software because they have been shown to be sensitive to acute THC administration (Sewell et al., 2013).
For Study 2, we used the same tasks as Study 1, plus two time perception tasks (see below).
Time estimation task (TETc).
The TETc measures the ability to estimate time that has passed. The task began with the computer screen flashing once. The subjects then had to count the number of “B”s appearing amongst random letters appearing sequentially at random places on the computer screen, which served as a distractor task, preventing subjects from counting to themselves. The screen then flashed a second time, at which point the subject had to indicate both how many “B”s had appeared and how much time had elapsed between the two flashes. The primary outcome was the ratio between the actual time interval and the estimated time interval. The duration of this task was about eight minutes.
Time reproduction task (TRTc).
The TRTc measures time perception. The TRTc used the same software and methodology as the TETc, but instead the subject was asked to hold down a mouse button for a random given interval of time between 1–30 s. The subjects then had to count the number of “B”s appearing amongst random letters appearing sequentially at random places on the computer screen, which served as a distractor task, preventing subjects from counting to themselves. The primary outcome was the ratio between the actual time interval and the estimated time interval. The duration of this task was about eight minutes.
Data analysis
The data analysis approach for Study 2 was the same as that described for Study 1.
Study 2 results
Participant characteristics
Demographic information and recent substance use history for participants are listed in Table 4. Participants in the two studies were similar on all measures.
Table 4.
Study 2 participant characteristics.
| Gender, (M:F) | 12:12 |
| Age, mean (SEM) | 23.7 (0.8) |
| Education, mean (SEM) | 15.4 (0.3) |
| Racea | |
| Caucasian | 13 |
| African American | 4 |
| Other | 4 |
| More than one race | 3 |
| Recent (past month) substance use, mean (SEM), nb | |
| Caffeine (cups/day) | 2.3 (0.4), 19 |
| Tobacco cigarettes (cigs/week) | 2.5 (0.3), 3 |
| Alcohol (drinks/week) | 2.8 (0.3), 23 |
| Cannabis (times/month) | 5.0 (1.3), 17 |
| Lifetime cannabis use, mean (SEM) | 268.3 (81.4) |
SEM: standard error of the mean.
Race is number of individuals who identify as such (“Other” is mainly Asian);
mean and SEM were calculated using only subjects who reported any recent use of the drug.
Physiological and subjective measures
Physiological and subjective measures are listed in Table 5. THC increased heart rate, and increased ratings on DEQ “Feel”, “High”, “Dislike”, “Like”, “Want more,” and on the ARCI Mar Scale. THC also increased ratings on POMS Anxiety, Fatigue, and Confusion.
Table 5.
Study 2 physiological and subjective measures.
| Measure | Placebo v. THC Fa, p | 7.5 mg v. 15 mg F, p |
|---|---|---|
| Heart rate | 17.1, <0.001b, η2=0.43 | 8.2, 0.009c, η2=0.26 |
| Mean arterial pressure | ns | ns |
| DEQ | ||
| “Feel” | 63.2, <0.001b, η2d=0.73 | 14.6, 0.001b, η2=0.39 |
| “Like” | 34.1, <0.001b, η2=0.60 | ns |
| “Dislike” | 25.6, <0.001b, η2=0.53 | ns |
| “High” | 74.1, <0.001b, η2=0.76 | 18.3, <0.001b, η2=0.44 |
| “Want more” | 15.8, 0.001b, η2=0.41 | ns |
| ARCI Marijuana Scale | 42.8, <0.001b, η2=0.65 | 12.3, 0.002b, η2=0.35 |
| POMS | ||
| Friendliness | ns | ns |
| Anxiety | 9.9, 0.004c, η2=0.30 | 7.6, 0.011c, η2=0.25 |
| Elation | ns | ns |
| Anger | ns | ns |
| Fatigue | 14.5, 0.001b, η2=0.39 | ns |
| Depression | ns | ns |
| Confusion | 15.8, 0.001b, η2=0.41 | 7.9, 0.010c, η2=0.26 |
| Vigor | ns | ns |
ANOVA: analysis of variance; ARCI: Addiction Research Center Inventory; DEQ: Drug Effects Questionnaire; POMS: Profile of Mood States; SEM: standard error of the mean; THC: Δ9-tetrahydrocannabinol.
F statistic and p-value from Bonferroni corrected repeated measures ANOVA with Helmert contrasts drug condition*time interaction;
significant at α=0.0025;
significant at α=0.025;
partial eta squared.
Computer and phone-based app task performance
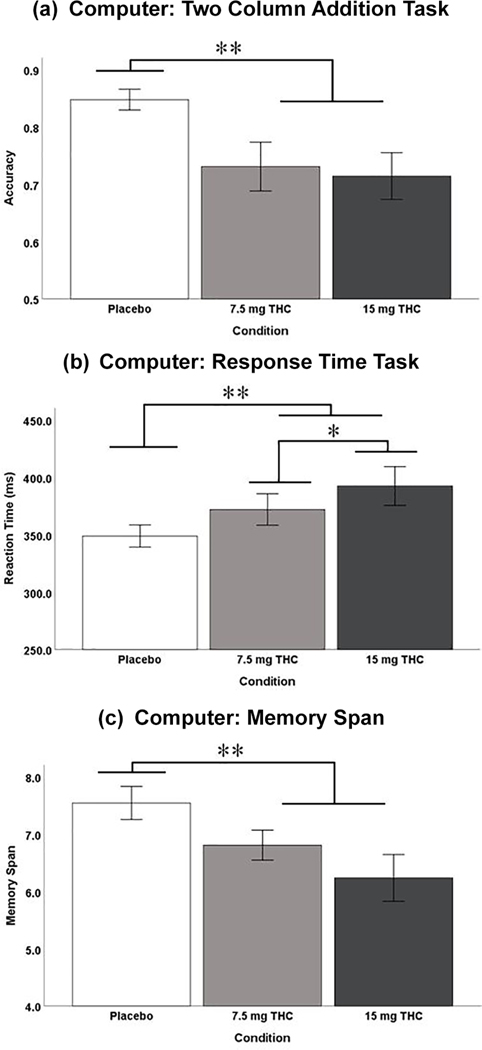
Computer and phone task performance results are listed in Table 6. Three out of the four computer tasks revealed a significant drug condition effect (placebo v. THC): Two Column Addition, Reaction Time, and the Working Memory Task (Figure 2(a)–(c)). Only the reaction time task performance revealed a significant dose dependent effect (7.5 mg v. 15 mg). None of the six phone tasks revealed a significant drug condition effect (placebo v. THC) nor a dose dependent effect (7.5 mg v. 15 mg).
Table 6.
Study 2 computer and phone-based task performance.
| Placebo v. THC Fa, pb | 7.5 mg v. 15 mg F, p | |
|---|---|---|
| Computer | ||
| Two Column Addition | 14.3, 0.001**, η2c = 0.39 | ns |
| Simple Reaction Time | 7.5, 0.012*, η2 = 0.25 | 7.1, 0.014*, η2 = 0.24 |
| Finger Tapping | ns | ns |
| Working Memory | 10.7, 0.003*, η2 = 0.32 | ns |
| Time Reproduction | ns | ns |
| Time Estimation | ns | ns |
| Phone | ||
| Paced Visual Serial Addition | ns | ns |
| Simple Reaction Time | ns | ns |
| Finger Tapping | ns | ns |
| Spatial Memory | ns | ns |
| Time Reproduction | ns | ns |
| Time Estimation | ns | ns |
F statistic and p-value from Bonferroni corrected repeated measures ANOVA with HELMERT contrasts drug condition*time interaction
significant at α = 0.025,
significant at α = 0.0025.
partial eta squared
Figure 2.
Study 2 computer-based task performance. (a) Mean (±standard error of the mean (SEM)) Two Column Addition accuracy (#Correct/Total); (b) mean (±SEM) computer based response time (ms); (c) mean (±SEM) computer-based memory span (maximum of nine). SE: standard error; THC: Δ9-tetrahydrocannabinol. Error Bars: +/– 1 SE.
Study 2 discussion
As in Study 1, Study 2 revealed impairments in performance after oral THC on several tasks administered on a desktop computer: Two Column Addition, Reaction Time, and Working Memory tasks. Unlike Study 1, none of the phone-based tasks included in Study 2 were sensitive to either dose of THC. We found no overall main effect of THC dose on time perception, both on the computer and phone-based tasks. Thus, Study 2 extended our conclusion in Study 1 that, although it may in principle be possible to administer brief phone-based tasks to detect THC-induced impairment, the sensitivity of the app tasks is too low. This difficulty in developing quick and sensitive app-based performance tests highlights the challenges in achieving the goal of a field sobriety test for recent cannabis use.
The present findings confirm that moderate doses of THC in light cannabis users impair performance on several measures of cognitive function and these findings were consistent with published reports. THC impaired performance on computer-based measures of addition, reaction time, and memory span. Previous studies using comparable doses have reported increases in reaction time, using the same simple reaction time task as ours, and deficits in working memory, and sustained attention using standardized tasks: Simple Response Time, Divided Attention, Visio-Spatial Selective Attention, and Sternberg Memory Task (Broyd et al., 2016; Desrosiers et al., 2015; Hunault et al., 2008; McDonald et al., 2003; Sewell et al., 2009; Solowij et al., 1995). In our studies, THC did not impair performance on the computer-based measure of motor control. Similar to our results, Roser et al. (2009) reported that a lower dose of oral THC (2.5 mg) did not impair performance on measures of finger tapping at two hours post-consumption. In contrast, Desrosier et al. (2015) found that smoking a 16.8% THC cigarette impaired tracking ability on a critical tracking task, a measure of fine motor ability, an hour and a half after smoking. The difference in results may be due to the smoked route of administration and differences in the peak and time course of THC plasma levels. Despite these reports that THC impairs performance under controlled conditions, it has also been noted that the impairments are relatively modest. For example, when compared to psychomotor and cognitive impairments after other commonly used drugs such as alcohol, the impairments are modest (Robbe, 1994; Sewell et al., 2009; Smiley, 1986; Sutton, 1983).
In contrast to the computer-based tasks, the effects of THC were only detected on one of the phone-based measures. In Study 1, THC impaired performance on the non-dominant finger tapping task. It is likely that the phone-based tasks are too simple and too brief to detect the relatively subtle impairments produced by the drug. Comparing the lengths of the computer-based and phone-based addition tasks, for example, the computer task consisted of 45 trials, requiring addition of three two-digit numbers within 15 s, whereas the comparable phone task consisted of 10 (Study 1) or 20 trials (Study 2) in which participants summed single digit numbers. The computer and phone-based reaction time and memory tasks also differed in duration and difficulty (e.g. reaction time tasks 200 trials vs three or 10 trials). These differences in both structure and length of the computer vs phone-based tasks likely accounted for the differences in detecting impairments across the two modalities, and illustrate the difficulty in detecting subtle impairments in brief tasks. Similar issues regarding sensitivity of brief app-based tasks evaluating psychomotor ability have been reported by researchers. For example, Honn et al. (2015) compared performance on a computer-based tasks and a smart-phone version of the psychomotor vigilance task in sleep-deprived individuals. They also found a significant loss of sensitivity when a brief version of the task was administered via phone.
These studies had several limitations. First, although the study rooms resembled a living room setting, the hospital or medical center context could have influenced the subjective drug experience. Second, the tasks used on the computer and phone were not identical, making direct comparisons difficult. We selected tasks that were thought to measure similar underlying processes but limited ourselves to standardized publicly available tasks. In addition, as noted above, the tasks differed in duration. Further, it is not clear that these results would be generalizable to a broader population of cannabis users. The participants in this study were non-daily users, who were drug-free at the time of each session. It is likely that heavier cannabis users would exhibit less impairment because of tolerance (Ramaekers et al., 2011), illustrating another challenge in developing and applying a performance-based sobriety test. Another limitation was that Study 2 was not planned, but was conducted to improve the phone-based task battery sensitivity. Unfortunately, the phone-based finger tapping impairment observed in Study 1 was not detected in Study 2.
The results of this study illustrate the challenges in developing a sensitive phone-based app. The tasks should be efficient and sensitive to the effects of THC in the broadest sample of participants. They should be short enough to maintain the user’s attention in a complex natural environment with likely distractors, but also long enough to detect the drug effect. The present findings give an idea of the minimum number of trials needed in each task to detect a drug effect. Future versions of a phone-based task might examine impairments on other cognitive functions known to be altered by THC, such as verbal memory and inhibitory control.
The development of this app raises important questions about the feasibility of any brief “sobriety” tests for cannabis. Importantly, our study was conducted under highly controlled conditions, and each subject was tested with placebo as well as drug. The placebo condition provided a measure of the individual’s non-drug performance. However, this key measure is not available in most real-world sobriety tests. Indeed, baseline (placebo) performance was highly variable across participants. Thus, without a non-drug control condition, it would be extremely difficult to detect THC-induced impairment. Moreover, at a more conceptual level, it is possible that an individual who is impaired by the drug might still perform better than another individual performs at baseline without the drug. This calls into question the value of a one-time sobriety test, at all. An alternative use of this type of app might be to personalize it for each user. That is, users could “train” the app, by completing tasks in both a drug-free state and after using the drug. Then they could query the app on future occasions to determine their own personal level of impairment. Another possible use would be to enlist registered medicinal cannabis users to provide baseline performance at the time they obtain their medical cannabis card, which could then be compared to their performance while suspected to be under the influence. Alternatively, the app might be made available to medical dispensaries to be used by customers on a voluntary basis, with data collection methods in place. This would allow for the collection of data from a wider population and increase external validity.
In sum, we have taken one of the first attempts at developing a phone app to assess cannabis-induced impairment. Although several other apps are available to measure cannabis-induced performance (see “My Canary”, National Organization for the Reform of Marijuana Laws, 2015), these apps were established based on archival evidence, without direct empirical confirmation in a controlled environment with controlled doses. To our knowledge, our study is the first to assess THC-induced app performance impairment under double-blind, placebo-controlled conditions. It may be possible to increase the sensitivity of an app like this, but it is not clear whether it can reach the standard needed for a field sobriety test for cannabis-induced impairment. Without baseline performance as comparison and knowledge of the individual’s history of cannabis use, detecting impairment roadside through an app may be difficult. Nevertheless, there may be uses for a phone-based app to detect field data under certain conditions, such as those in which baseline (drug-free) performance is available for comparison.
Acknowledgements
The authors acknowledge the assistance of Anya Bershad, Jessica Weafer and Fritz Anderson.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Institute on Drug Abuse (DA02812 supplement and T32DA043469). Elisa Pabon was supported by the University of Chicago’s Initiative for Maximizing Student Development (IMSD) program.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Psychiatric Association (2013) The Diagnostic and Statistical Manual of Mental Disorders (5th edition). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Apple Inc (2015) The HealthKit Framework. Available at: https://developer.apple.com/documentation/healthkit
- Ballard ME, Bedi G and de Wit H (2012) Effects of delta-9-tetrahydro-cannabinol on evaluation of emotional images. J Psychopharmacol 26: 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondallaz P, Favrat B, Chtioui H, et al. (2016) Cannabis and its effects on driving skills. Forensic Sci Int 268: 92–102. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, et al. (2016) Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biol Psychiatry 79: 557–567. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, et al. (2007) Oral delta-9-tetrahydro-cannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend 86(1): 22–29. DOI: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Chait LD, Fischman MW and Schuster CR (1985) ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Depend 15: 229–238. [DOI] [PubMed] [Google Scholar]
- Curran VH, Brignell C, Fletcher S, et al. (2002) Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 164: 61–70. [DOI] [PubMed] [Google Scholar]
- Desrosiers NA, Ramaekers JG, Chauchard E, et al. (2015) Smoked cannabis’ psychomotor and neurocognitive effects in occasional and frequent smokers. J Anal Toxicol 39: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Ross LA, Ackerman ML, et al. (2008) Longitudinal predictors of driving cessation among older adults from the ACTIVE clinical trial. J Gerontol B 63: P6–P12. [DOI] [PubMed] [Google Scholar]
- Endsley MR and Garland DJ (2000) Situation Awareness Analysis and Measurement. Boca Raton: CRC Press. [Google Scholar]
- Glass M, Faull RLM and Dragunow M (1997) Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradio-graphic study in the fetal, neonatal and adult human brain. Neuroscience 77: 299–318. [DOI] [PubMed] [Google Scholar]
- Guo M, Li S, Wang L, et al. (2016) Research on the relationship between reaction ability and mental state for online assessment of driving fatigue. Int J Environ Res Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, et al. (2004) Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology: Official Publication of the American College of Neuropsycho-pharmacology 29(1): 158–170. DOI: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, et al. (2008) Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology 197(1): 157–168. DOI: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RL and Huestis MA (2013) Cannabis effects on driving skills. Clin Chem 59: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, et al. (2015) Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012– 2013. JAMA Psychiatry 72: 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn KA, Riedy SM and Grant DA (2015) Validation of a portable, touch-screen psychomotor vigilance test. Aerosp Med Hum Perform 86: 428–434. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, et al. (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58(4): 322–328. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, de Vries I, et al. (2008) Delta-9-tetrahydro-cannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology (Berl) 201: 171–181. [DOI] [PubMed] [Google Scholar]
- Kalant H (2016) A critique of cannabis legalization proposals in Canada. Int J Drug Policy 34: 5–10. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Schwope DM, Schwilke EW, et al. (2012) Predictive model accuracy in estimating last Δ9-tetrahydrocannabinol (THC) intake from plasma and whole blood cannabinoid concentrations in chronic, daily cannabis smokers administered subchronic oral THC. Drug Alcohol Depend 125: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J-L, Gadegbeku B, Wu D, et al. (2017) Cannabis, alcohol and fatal road accidents. PLoS One 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, et al. (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12: 245–258. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, et al. (2003) Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 28: 1356–1365. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M and Droppleman L (1971) Profile of Mood States. San Diego: Educational and Industrial Testing Service. [Google Scholar]
- Mechoulam R and Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64: 21–47. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, et al. (2013) The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology (Berl) 227: 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller ST and Piper BJ (2014) The Psychology Experiment Building Language (PEBL) and PEBL test battery. J Neurosci Methods 222: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2018) Marijuana. North Bethesda: NIDA; Available at: https://www.drugabuse.gov/publications/drugfacts/marijuana (accessed 1 December 2017). [Google Scholar]
- National Organization for the Reform of Marijuana Laws (2015) My Canary. Washington, DC: NORML; Available at: https://appsto.re/us/QYxB7.i (accessed 3 July 2019). [Google Scholar]
- O’Leary DSC, Block RI, Turner BM, et al. (2003) Marijuana alters the human cerebellar clock. Neuroreport 14: 1145–1151. [DOI] [PubMed] [Google Scholar]
- Oomen PP, van Hell HH and Bossong MG (2018) The acute effects of cannabis on human executive function. Behav Pharmacol 29: 605–616. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen E, et al. (2009) Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23: 266–277. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Theunissen EL, de Brouwer M, et al. (2011) Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl.) 214: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe H (1994) Influence of Marijuana on Driving. Toronto: Centre for Addiction and Mental Health. [Google Scholar]
- Roser P, Gallinat J, Weinberg G, et al. (2009) Psychomotor performance in relation to acute oral administration of Δ9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. Eur Arch Psychiatry Clin Neurosci 259: 284–292. [DOI] [PubMed] [Google Scholar]
- Sewell RA, Poling J and Sofuoglu M (2009) The effect of cannabis compared with alcohol on driving. Am J Addict 18: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell RA, Schnakenberg A, Elander J, et al. (2013) Acute effects of THC on time perception in frequent and infrequent cannabis users. Psychopharmacology 226: 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley A (1986) Marijuana on-road and driving simulator studies. Alcohol Drugs Driving 2: 121–134. [Google Scholar]
- Solowij N, Michie PT and Fox AM (1995) Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry 37: 731–739. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Nottage J, et al. (2010) Delta-9-tetrahydro-cannabinol disruption of time perception and of self-timed actions. Pharmacopsychiatry 43: 236–237. [DOI] [PubMed] [Google Scholar]
- Sutton LR (1983) The effects of alcohol, marihuana and their combination on driving ability. J Stud Alcohol 44: 438–445. [DOI] [PubMed] [Google Scholar]
- Wachtel SR and de Wit H (2000) Naltrexone does not block the subjective effects of oral D9-tetrahydrocannabinol in humans. Drug Alcohol Depend 10. [DOI] [PubMed] [Google Scholar]
- Watchel S, ElSohly M, Ross S, et al. (2002) Comparison of the subjective effects of Δ 9 -tetrahydrocannabinol and marijuana in humans. Psychopharmacology 161: 331–339. [DOI] [PubMed] [Google Scholar]