This cohort study investigates the association between genotype and phenotype in individuals with retinoblastoma in France.
Key Points
Question
What is the association between genotype and phenotype in a cohort of consecutive, unrelated, documented individuals with retinoblastoma?
Findings
In this cohort study of 1404 individuals, compared with germline pathogenic variants maintaining retinoblastoma protein, germline pathogenic variants inducing the absence of retinoblastoma protein were associated with more severe disease. Female predominance was observed among nongermline carriers, and males were at higher risk of bilateral retinoblastoma compared with females.
Meaning
These results suggest that retinoblastoma risk is associated with the germline pathogenic variant and with maintenance of retinoblastoma protein and that there is a sex-linked mechanism for nongermline carriers.
Abstract
Importance
Retinoblastoma (RB) is the most common pediatric intraocular neoplasm. RB is a complex model in which atypical pathogenic variants, modifier genes, imprinting, and mosaicism are known to be associated with the phenotype. In-depth understanding of RB therefore requires large genotype-phenotype studies.
Objective
To assess the association between genotype and phenotype in patients with RB.
Design, Setting, and Participants
This single-center, retrospective cohort study, conducted from January 1, 2000, to September 30, 2017, enrolled 1404 consecutive ascertained patients with RB who consulted an oncogeneticist. All patients had their genotype and phenotype recorded. Statistical analysis was performed from July 1, 2018, to December 31, 2018.
Main Outcomes and Measures
RB1 germline and somatic pathogenic variant types, family history, and disease presentation characteristics (ie, age at diagnosis, sex, laterality, and International Intraocular Retinoblastoma Classification group).
Results
Among 1404 patients with RB (734 [52.3%] female; mean [SD] age, 20.2 [21.2] months), 866 cases (61.7%) were unilateral and 538 cases (38.3%) were bilateral. Loss of function variants were found throughout the coding sequence, with 259 of 272 (95.2%) somatic pathogenic variants and 537 of 606 (88.6%) germline pathogenic variants (difference, 6.6%; 95% CI, 4.0%-9.2%; P < .001) after excluding tumor-specific pathogenic variants (ie, promoter methylation and loss of heterozygosity); a novel low-penetrance region was identified in exon 24. Compared with germline pathogenic variants estimated to retain RB protein expression, germline pathogenic variants estimated to abrogate RB protein expression were associated with an earlier mean (SD) age at diagnosis (12.3 [11.3] months among 457 patients vs 16.3 [13.2] months among 55 patients; difference, 4 months; 95% CI, 1.9-6.1 months; P = .01), more frequent bilateral involvement (84.2% among 452 patients vs 65.2% among 45 patients; difference, 18.9%; 95% CI, 14.5%-23.3%; P < .001), and more advanced International Intraocular Retinoblastoma Classification group (85.3% among 339 patients vs 73.9% among 34 patients; difference: 11.4%; 95% CI, 6.5%-16.3%; P = .047). Among the 765 nongermline carriers of an RB1 pathogenic variant, most were female (419 females [54.8%] vs 346 males [45.2%]; P = .008), and males were more likely to have bilateral RB (23 males [71.4%] vs 12 females [34.3%]; P = .01).
Conclusions and Relevance
These results suggest that RB risk is associated with the germline pathogenic variant and with maintenance of RB protein and that there is a sex-linked mechanism for nongermline carriers.
Introduction
Retinoblastoma (RB) is the most common pediatric intraocular neoplasm and occurs in 1 of every 20 000 births.1 It results from biallelic inactivation of the RB1 tumor-suppressor gene (OMIM 180200), located on 13q14.2 The RB1 gene encodes the nuclear phosphoprotein RB protein, which plays a prominent role during the G1/S phase transition.3 Both RB1 alleles can be inactivated in tumors via diverse loss of function mechanisms including point pathogenic variants, large rearrangements (ie, large deletions or duplications from a single exon to the entire gene), promoter hypermethylation, and loss of the second allele by loss of heterozygosity. In nonhereditary RB, both RB1 pathogenic variants are somatic and occur in the same retinal cell that develops into a tumor. In contrast, in hereditary RB, the germline pathogenic variant of 1 allele is associated with RB. The germline pathogenic variant is either inherited from an affected parent or acquired during fetal development (de novo), whereas the second pathogenic variant on the other allele is somatic, usually acquired during early childhood.
Although RB1 may be regarded as the prototype tumor-suppressor gene with a simple model, previous research has provided an increasingly complex picture of RB,4 in which atypical pathogenic variants,5,6 modifier genes,7,8 imprinting,9,10,11 and mosaicism12 are known to be associated with RB phenotype. In-depth understanding of RB therefore requires large-scale genotype-phenotype studies. However, previous genotype-phenotype studies13,14,15,16,17,18,19,20 have mainly been based on series of selected families or were derived from variant screening series comprising a limited number of patients with RB. In addition, large registry-based studies21,22,23,24,25,26,27,28,29 lack detailed ocular and genotype descriptions and mainly focus on survival and treatment issues.
We conducted a retrospective study on 1404 consecutive RB cases that were well documented in terms of genotype and phenotype. We assessed the association between genotype and phenotype to be used for clinical treatment of families with RB.
Methods
Patient Cohort
This cohort study was performed at the Institut Curie, a reference center for clinical treatment of patients with RB. At the center, diagnosis of RB is established by an ophthalmologic bilateral examination under general anesthesia (anterior segment to the fundus fully dilated for a careful examination of the retinal periphery), ocular ultrasound, magnetic resonance imaging, and, when treatment involves enucleation, histologic criteria. All patients with RB are offered genetic counseling and variant analysis of the RB1 gene in germline and tumor DNA, when available. Ethical approval was obtained from the Institut Curie institutional review board. Patients were not compensated or offered incentives to participate in the study. Appropriate individual written consent for genetic analysis was obtained from all participating patients or their legal guardians.
Unrelated RB index cases consecutively referred for genetic consultation from January 1, 2000, to September 30, 2017, were included in this retrospective study (n = 1404). The cohort included patients who received a diagnosis and treatment during this period as well as older patients interested in genetic counseling because of the progress of RB genetics in routine clinical care. Familial cases were defined as families with at least 2 germline carriers of a RB1 pathogenic variant.
Phenotypic Description
The phenotypic description was based on commonly used demographic data (ie, age at diagnosis, sex, laterality, and the International Intraocular Retinoblastoma Classification [IIRC] group) at diagnosis. The IIRC is based on the natural history of intraocular RB and on the risk of loss of the eye after primary therapy.30 The IIRC comprises 5 groups that rate risk of loss of the eye (A, very low; B, low; C, moderate; D, high; and E, very high). For this study, groups A, B, and C were pooled and compared with groups D and E. The eTable in the Supplement describes the IIRC according to Murphree.30 For bilateral cases with different IIRC groups, the data on the most advanced eye were collected.
RB1 Analyses
Genetic screening was performed on DNA extracted from blood or tumor tissue samples. When a tumor sample was available after enucleation, first-line tumor screening was followed by germline targeted testing on the identified tumor pathogenic variant. Germline screening was performed when no tumor DNA was available. Analytical strategies varied according to the year of analysis but were always designed to detect the whole spectrum of RB1 pathogenic variations, including point variations, large rearrangements, promoter methylation (both at the constitutional and tumor levels), allelic loss in tumors, splice alterations, and chromothripsis. Various combinations of denaturing high-performance liquid chromatography, quantitative multiplex polymerase chain reaction of short fluorescent fragments, methylation-restriction polymerase chain reaction, multiplex ligation probe amplification (MLPA), methylation specific MLPA, comparative genomic hybridization, Sanger sequencing, and next generation sequencing were used to screen the promoter and the exons with their flanking intronic sequences.31,32,33 For the first-line screening strategy, promoter methylation was tested in both tumor and blood DNA.
Penetrance and Pathogenic Variant at the Protein Level
Germline carriers usually develop bilateral or multifocal tumors. However, some families exhibit low penetrance and variable expressivity of the disease because bilaterally affected, unilaterally affected, and unaffected pathogenic variant carriers are known to coexist. Low-penetrance pathogenic variants lead to a reduced amount of wild-type RB protein or a partially functional RB protein variant.34 According to previous studies, promoter pathogenic variants, nonsense pathogenic variants occurring in exons 1 and 2, previously described missense pathogenic variants, and in-frame splice pathogenic variants were consequently classified as presence of RB protein. Nonsense variants in exons 1 and 2 are low-penetrance pathogenic variants through alternative translation initiation resulting in truncated RB protein with suspected tumor suppressor activity.5 Because promoter methylation is known to occur at the gene expression level and therefore at the resulting protein, it was considered to indicate the presence of RB protein. Truncating pathogenic variants (including out-of-frame splice variants), large rearrangements, and RB1 allelic loss leading to loss of heterozygosity were classified as absence of RB protein.
Statistical Analysis
Statistical analysis was performed from July 1, 2018, to December 31, 2018. All analyses were performed with statistical programming language R, version 3.5.2 (R Project for Statistical Computing).35 A contingency analysis of associations between genotypes and phenotypes was performed using likelihood ratio χ2 statistics. The strength of the association was estimated by calculating the odds ratios (ORs) and 95% CIs. A nonparametric Wilcoxon test was performed to compare 2 distributions. We used a univariate logistic regression model to study the association between RB laterality and type of RB1 pathogenic variant. All tests were bilateral and performed with a 2-sided significance level of P < .05. No adjustment for multiple testing was performed.
Results
Phenotypic Description of the Cohort
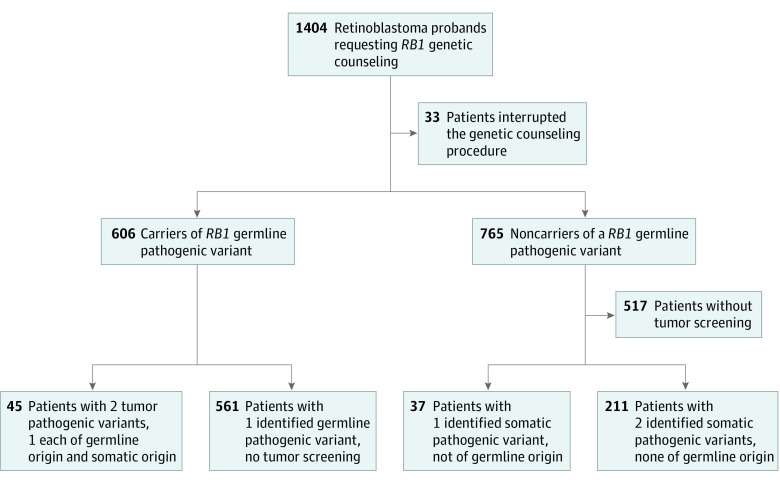
From January 1, 2000, to September 30, 2017, 1404 RB probands requested RB1 genetic counseling in the Institut Curie genetics department. Among 1404 patients with RB, 734 (52.3%) were female with mean (SD) age of 20.2 (21.2) months. Unilateral RBs were diagnosed in 866 patients (61.7%) and bilateral RBs in 538 patients (38.3%). Thirty-three patients (2.3%) discontinued the genetic counseling process. A germline pathogenic variant of the RB1 gene was identified in 606 patients (43.2%), 497 (82.0%) of whom had bilateral RB (Table 1 and eFigure 1 in the Supplement).
Table 1. Clinical Characteristics of 1404 Patients With Retinoblastoma.
| Characteristic | Affected eye with retinoblastoma | |
|---|---|---|
| Unilateral (n = 866) | Bilateral (n = 538) | |
| Age at diagnosis | ||
| Mean (SD), mo | 25.7 (24.4) | 11.9 (11.0) |
| Median (range) [IQR], mo | 22 (1-372) [11-34] | 9 (0-104) [5-15] |
| Not available, No.(%) | 181 (20.9) | 73 (13.6) |
| Sex, No. (%) | ||
| Male | 387 (44.7) | 283 (52.6) |
| Female | 479 (55.3) | 255 (47.4) |
| Familial form, No. (%) | ||
| Yes | 22 (2.5) | 96 (17.8) |
| No | 844 (97.5) | 442 (82.2) |
| IIRC group at diagnosis, No. (%)a | ||
| A | 2 (0.3) | 1 (0.3) |
| B | 37 (5.9) | 30 (7.6) |
| C | 38 (6.1) | 30 (7.6) |
| D | 429 (68.8) | 253 (64.4) |
| E | 118 (18.9) | 79 (20.1) |
| Not available | 242 (27.9) | 145 (26.9) |
Abbreviations: IIRC, International Intraocular Retinoblastoma Classification; IQR, interquartile range.
The most affected eye in bilateral cases.
Known for 1150 patients, median age at diagnosis was 15 months (range, 0-372 months), with a younger age for patients with bilateral RB (median age at diagnosis was 9 months [range, 0-104 months] for 465 patients with bilateral RB and 22 months [range, 1-372 months] for 685 patients with unilateral RB, P < .001) (Table 1). Eye involvement differed according to age at diagnosis: bilateral cases were more frequent between birth and 12 months of age but were as frequent as unilateral RB between 6 months and 15 months of age. Inversely, unilateral RBs were more frequent than bilateral RBs after 15 months of age (eFigure 2 in the Supplement). Among the 118 patients (8.4%) with a family history of RB, 96 (81.4%) had bilateral RB. The cohort included 670 males (47.7%) and 734 females (52.3%). Males were more likely to have bilateral RB than were females (283 [52.6%] vs 255 [47.4%]; OR, 1.37; 95% CI, 1.11-1.70; P = .004) (Table 1).
The IIRC group of the only involved eye in unilateral RB or of the most affected eye in bilateral RB at diagnosis was known for 1017 patients (72.4%) and was distributed as follows: group A, 3 (0.3%); group B, 67 (6.6%); group C, 68 (6.7%); group D, 682 (67.1%); and group E, 197 (19.4%). No difference in IIRC group was observed according to RB laterality (Table 1).
RB1 Pathogenic Variants
A total of 1110 RB1 pathogenic variants were identified. The first-line tumor screening strategy (n = 293) identified 45 patients with 2 tumor pathogenic variants, one of which was of germline origin; 211 patients with 2 identified tumor-only pathogenic variants, none of which was of germline origin (ie, somatic), and 37 patients with a unique identified somatic pathogenic variant (no germline origin). When tumor DNA was not available, initial germline screening identified 561 patients with 1 germline pathogenic variant (Figure). We identified 28 mosaic patients considered as harboring a germline variation in 14 unilateral and 14 bilateral RBs.
Figure. Study Flow Diagram.
The distribution of 606 germline and 504 somatic RB1 pathogenic variants, by type, associated with presence or absence of RB protein and according to laterality is detailed in Table 2. All missense variants were tested at the RNA level and were considered to be splice pathogenic when an association with splicing was demonstrated. Splice-neutral missense variants were classified as pathogenic according to previous literature10 (eg, p.Arg661Trp). The 4 most frequent types of the 606 germline pathogenic variants were nonsense (222 [36.6%]), frameshift (140 [23.0%]), out-of-frame splice variants (110 [18.2%]), and large rearrangements (65 [10.8%]). The 4 most frequent types of 504 somatic pathogenic variants were allelic loss leading to loss of heterozygosity (205 [40.7%]), nonsense (124 [24.6%]), frameshift (57 [11.3%]), and large rearrangements (46 [9.1%]). Promoter methylation was exclusively found in tumor DNA, with a frequency of 5.3% (27 of 504). Loss of function variants were found throughout the coding sequence, with 259 of 272 (95.2%) somatic pathogenic variants and 537 of 606 (88.6%) germline pathogenic variants (difference, 6.6%; 95% CI, 4.0%-9.2%; P < .001) after excluding tumor-specific pathogenic variants (ie, promoter methylation and loss of heterozygosity). Somatic pathogenic variants were more frequently associated with the absence of RB protein (537 of 796, 67.4%) than were germline pathogenic variants (259 of 796, 32.6%) (OR, 2.56; 95% CI, 1.42-4.63; P = .002).
Table 2. Characterization of 606 Germline and 504 Somatic RB1 Pathogenic Variants From 854 Retinoblastoma (RB) Probands.
| Pathogenic variant type | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Germline pathogenic variants | Somatic pathogenic variants | Total identified pathogenic variants (n = 1110) | |||||
| Unilateral (n = 109) | Bilateral (n = 497) | Total (n = 606) | Unilateral (n = 484) | Bilateral (n = 20) | Total (n = 504) | ||
| Presence of RB protein | |||||||
| Missense | 13 (11.9) | 12 (2.4) | 25 (4.1) | 2 (0.4) | 0 | 2 (0.4) | 27 (2.4) |
| In-frame deletion | 1 (0.9) | 3 (0.6) | 4 (0.7) | 2 (0.4) | 0 | 2 (0.4) | 6 (0.5) |
| Splice pathogenic variant in frame, exonic | 0 | 2 (0.4) | 2 (0.3) | 1 (0.2) | 0 | 1 (0.2) | 3 (0.3) |
| Splice pathogenic variant in frame, intronic | 8 (7.4) | 25 (5.1) | 33 (5.5) | 7 (1.4) | 0 | 7 (1.4) | 40 (3.6) |
| Promoter methylation | 0 | 0 | 0 | 27 (5.4) | 0 | 27 (5.3) | 27 (2.4) |
| Promoter sequence pathogenic variants | 2 (1.8) | 3 (0.6) | 5 (0.8) | 1 (0.2) | 0 | 1 (0.2) | 6 (0.5) |
| Total | 24 (22.0) | 45 (9.1) | 69 (11.4) | 40 (8.0) | 0 | 40 (7.9) | 109 (9.8) |
| Absence of RB protein | |||||||
| Frameshift deletion | 20 (18.4) | 90 (18.1) | 110 (18.2) | 43 (8.9) | 1 (5.0) | 44 (8.7) | 154 (13.9) |
| Frameshift delinsa | 0 | 0 | 0 | 1 (0.2) | 0 | 1 (0.2) | 1 (0.1) |
| Frameshift insertion | 5 (4.6) | 25 (5.0) | 30 (4.8) | 12 (2.5) | 0 | 12 (2.4) | 42 (3.8) |
| Nonsense | 24 (22.0) | 198 (39.8) | 222 (36.6) | 118 (24.5) | 6 (30.0) | 124 (24.6) | 346 (31.2) |
| Large rearrangementb | 17 (15.6) | 48 (9.7) | 65 (10.8) | 45 (9.4) | 1 (5.0) | 46 (9.1) | 111 (10.0) |
| Loss of heterozygosityc | 0 | 0 | 0 | 194 (40.1) | 11 (55.0) | 205 (40.7) | 205 (18.5) |
| Splice out of frame, exonic | 1 (0.9) | 10 (2.0) | 11 (1.8) | 2 (0.4) | 0 (0) | 2 (0.4) | 13 (1.2) |
| Splice out of frame, intronic | 18 (16.5) | 81 (16.3) | 99 (16.4) | 29 (6.0) | 1 (5.0) | 30 (6.0) | 129 (11.6) |
| Total | 85 (78.0) | 452 (90.9) | 537 (88.6) | 444 (92.0) | 20 (100) | 464 (92.1) | 1001 (90.2) |
Described as a variant event combining deletion and insertion at the same time.
Large deletions or duplications from a single exon to the entire gene.
Loss of the wild-type allele by different mechanisms (ie, deletions with or without duplication of the mutant allele or mitotic recombination [second hit]).
Location of Pathogenic Variants
A RB1 region (eg, R1) (eFigure 1 in the Supplement) was defined as an exonic sequence with the flanking intronic regions, making a total of 27 regions downstream from the promoter sequence. We identified 780 pathogenic variants associated with a single region excluding large rearrangements and loss of heterozygosity (ie, 551 germline and 229 somatic pathogenic variants). Point variations (ie, excluding promoter methylation, large rearrangements, and loss of heterozygosity) were scattered from the promoter to region 25, with the exception of region 5 (no somatic pathogenic variant). The greatest number of 551 germline pathogenic variants were found in regions 8 (45 [8.2%]), 17 (45 [8.2%]), 14 (45 [8.2%]), 20 (39 [7.1%]), 23 (34 [6.2%]), and 10 (34 [6.2%]). Although regions 17 (29 [12.6%]), 14 (24 [10.5%]), and 8 (16 [7.0%]), also frequently harbored 229 somatic pathogenic variants, comparison of germline and somatic variation spectra showed differential variant regions (ie, regions 1, 2, 6, 18, 20, and 24). This difference was observed for region 1 (21 germline pathogenic variants [3.8%] vs 2 somatic pathogenic variants [0.8%]; OR, 5.03; 95% CI, 1.35-18.73; P = .02), region 2 (24 [4.4%] vs 4 [1.6%]; OR, 2.99; 95% CI, 1.08-8.26; P = .03), and region 24 (17 [3.1%] vs 2 [0.8%]; OR, 4.04; 95% CI, 1.04-15.76; P = .04). Large rearrangements (excluding loss of heterozygosity) were found throughout the gene at both the germline and somatic levels without difference in terms of size distribution (52 germline large rearrangements with a median [SD] size of 14.6 [10.65] regions vs 27 somatic rearrangements with a median [SD] size of 12.5 [7.9] regions; P = .41).
Genotype-Phenotype Associations
Germline pathogenic variants were more frequent in bilateral RB (497 of 538; 92.4% sensitivity of detection) than in unilateral RB (109 of 866; 12.6% sensitivity of detection; OR, 84.19; 95% CI, 62.61-113.20; P < .001). Bilateral RB was more commonly associated with RB1 germline pathogenic variants associated with elimination of RB protein expression than was unilateral RB (452 of 537 [84.2%] vs 45 of 69 [65.2%]; difference, 18.9%; 95% CI, 14.5%-23.3%; P<.001) (Table 3). Compared with nonsense variants, missense variants, large rearrangements, and in-frame splice variants were associated with a lower risk of bilateral RB (Table 4). Nonsense variants more frequently occurred in bilateral RB than in unilateral RB (198 of 497 [39.8%] vs 24 of 109 [22.0%]; P < .001) (Table 4), whereas similar proportions were found for frameshift variants (115 [23.1%] vs 25 [22.9%]; P = .96) (Table 4).
Table 3. Associations Between Phenotype and Germline Pathogenic Variant Identification Among Probands With a Germline Pathogenic Variant by Its Theoretical Association With RB Protein.
| Characteristic | Germline pathogenic variant | |||||
|---|---|---|---|---|---|---|
| Identification | P value | Probands by theoretical association with RB protein | P value | |||
| Yes (n = 606) | No (n = 765) | Absence of RB protein (n = 537) | Presence of RB protein (n = 69) | |||
| Age at diagnosis | ||||||
| Mean (SD), mo | 12.7 (11.5) | 26.4 (25.1) | <.001a | 12.3 (11.3) (n = 457) | 16.3 (13.2) (n = 55) | .01a |
| Median (IQR), mo | 10 (5.0-17.0) | 23 (12.0-34.0) | NA | 9.0 (5.0-16.0) | 12 (7.5- 24.0) | NA |
| NA, No. (%) | 94 (15.5) | 160 (20.9) | NA | 80 (14.9) | 14 (20.3) | NA |
| Sex, No. (%) | ||||||
| Male | 304 (50.2) | 346 (45.2) | .07b | 270 (50.3) | 34 (49.3) | .87b |
| Female | 302 (49.8) | 419 (54.8) | 267 (49.7) | 35 (50.7) | ||
| Laterality, No. (%) | ||||||
| Unilateral | 109 (18.0) | 730 (95.4) | <.001b | 85 (15.8) | 24 (34.8) | <.001b |
| Bilateral | 497 (82.0) | 35 (4.6) | 452 (84.2) | 45 (65.2) | ||
| IIRC at diagnosis, No. (%) | ||||||
| Group A, B, or C | 71 (15.9) | 66 (12.1) | .08b | 59 (14.7) | 12 (26.1) | .047b |
| Group D or E | 375 (84.1) | 480 (87.9) | 341 (85.3) | 34 (73.9) | ||
| NA | 160 (26.4) | 219 (36.5) | NA | 137 (25.5) | 23 (33.3) | NA |
Abbreviations: IIRC, International Intraocular Retinoblastoma Classification; IQR, interquartile range; NA, not available; RB, retinoblastoma.
Wilcoxon test.
χ2 Test.
Table 4. Univariate Logistic Regression Model of RB Laterality According to the RB1 Germline Pathogenic Variant Types.
| Germline pathogenic variant types | RB laterality, No. (%) | Univariate analysis | |||
|---|---|---|---|---|---|
| Unilateral (n = 109) | Bilateral (n = 497) | Total (n = 606) | Odds ratio (95% CI) | P value | |
| Absence of RB protein | |||||
| Nonsense pathogenic variants | 24 (22.0) | 198 (39.8) | 222 (36.6) | 1 [Reference] | NA |
| Frameshift | 25 (22.9) | 115 (23.1) | 140 (23.1) | 0.56 (0.30-1.02) | .06 |
| Large rearrangementa | 17 (15.6) | 48 (9.7) | 65 (10.7) | 0.34 (0.17-0.69) | <.01 |
| Splice pathogenic variant out of frame | 19 (17.4) | 91 (18.3) | 110 (18.2) | 0.58 (0.30-1.12) | .10 |
| Presence of RB protein | |||||
| Missense pathogenic variants | 13 (11.9) | 12 (2.4) | 25 (4.1) | 0.11 (0.05-0.27) | <.001 |
| In-frame deletion | 1 (0.9) | 3 (0.6) | 4 (0.7) | 0.36 (0.04-7.51) | .39 |
| Splice pathogenic variant in frame | 8 (7.3) | 27 (5.4) | 35 (5.8) | 0.41 (0.17-1.05) | .05 |
| Promoter sequence pathogenic variants | 2 (1.8) | 3 (0.6) | 5 (0.8) | 0.18 (0.03-1.43) | .07 |
Abbreviations: NA, not applicable; RB, retinoblastoma.
Large deletions or duplications, from a single exon to the entire gene.
Retinoblastoma was diagnosed at a younger age for germline carriers (mean [SD] age: 12.7 [11.5] months for 512 carriers vs 26.4 [25.1] months for 605 noncarriers; P < .001). Among 512 germline carriers, the presence of RB protein was associated with differences in median age at diagnosis (12.0 months [range, 1.0-72.0] among 55 individuals), whereas the absence of RB protein was associated with differences in median age at diagnosis (9.0 months [range, 0-104.0] among 457 individuals) (mean [SD]: 16.3 [13.2] vs 12.3 [11.2] months; difference, 4 months; 95% CI, 1.9-6.1 months; P = .01). A higher proportion of carrier patients was observed between birth and 15 months of age, and a lower proportion was observed for patients 15 months or older (eFigure 3 in the Supplement).
Neither the proportion of germline carriers nor the putative association with RB protein differed according to sex (Table 3). However, a female predominance was observed among the 765 patients without an identified germline pathogenic variant (419 females [54.8%] vs 346 males [45.2%] compared with a theoretical 50/50 distribution; P = .008), and males were more likely to have bilateral RB than were females (23 males [71.4%] vs 12 females [34.3%]; P = .01). No sex difference was demonstrated for patients with an identified germline pathogenic variant (256 [51.5%] males vs 241 [48.5%] females; OR, 1.35; 95% CI, 0.89-2.05; P = .16).
No difference in IIRC group was observed according to the presence or absence of a germline pathogenic variant (Table 3). The proportion of RB groups D and E (in the most affected eye for bilateral RB) was similar between germline carriers and noncarriers (375 of 446 [84.1%] vs 480 of 546 [87.9%]) (Table 3). However, among germline carriers, patients with absent RB protein were more often classified in advanced IIRC groups than were carriers with present RB protein (341 of 400 [85.3%] vs 34 of 46 [73.9%]; difference, 11.4%; 95% CI, 6.5%-16.3%; P = .047) (Table 3). No sex differences were found in this subpopulation (Table 3).
Discussion
Loss of function variants were found throughout the coding sequence at both germline and somatic levels with a difference between the somatic and germline spectra (95.2% vs 88.6%, P < .001, excluding promoter methylation and loss of heterozygosity). More specifically, somatic pathogenic variants were more likely to be associated with RB protein loss, and regions 1, 2, and 24 harbored more germline variants than tumor variants. These results support the low penetrance of 5′ pathogenic variants in RB15 as well as another low penetrance region in exon 24. Because exons 26 and 27 are devoid of pathogenic variants, this novel low-penetrance region may encompass exon 25, but insufficient data were available to demonstrate an association. A plausible explanation would be a maintained although shorter RB1 messenger RNA escaping nonsense-mediated decay used to generate a 3′ shorter RB protein with intact pocket domains and putative tumor suppressor activity. These data showed that tumor pathogenic variants have a more severe association with RB protein than germline pathogenic variants. Similarly, missense TP53 variants, known to have a more severe biologic effect than other pathogenic variants, are enriched at the somatic level.36 These overall observations could be explained by the fact that somatic alterations should be associated with a growth advantage to be selected.
Germline pathogenic variants were predominantly associated with bilateral RB protein rather than unilateral RB, and the risk depends on the nature of the germline variant. Missense and in-frame splice pathogenic variants were associated with a lower risk of bilateral RB because at least partially active RB protein was maintained. This finding is in line with previous work6,10,34 showing that overexpressed missense alleles retain sufficient tumor suppressor activity to prevent the development of RB.
Large rearrangements were also associated with a lower risk of bilateral RB (Table 4), but the significance of this association may be related to another mechanism involving the contiguous gene MED4 (OMIM 605718) because RB RB1 −/− cells have a dependency on MED4.7
Similarly, age at diagnosis was associated with germline status and the presence or absence of RB protein. However, early diagnosis does not necessarily imply the presence of a germline pathogenic variant because almost 20% of patients diagnosed before the age of 3 months were noncarriers (eFigure 2 in the Supplement).
In contrast, germline status had no association with IIRC group, a finding supported by the absence of an association between IIRC and laterality, which would imply that once tumorigenesis has started, the ocular outcome is similar regardless of the origin of the first pathogenic variant (germline or somatic). Nevertheless, among germline carriers, pathogenic variants with absent RB protein were associated with advanced IIRC groups (in the most affected eye for bilateral RB).
Among the nongermline carriers, we found more females, suggesting a higher frequency of RB1 pathogenic variants in the female retina during the first months of life (Table 1). In addition, males were at higher risk of bilateral RB than females. This association was not found in germline carriers. Tumor was not available for these 35 bilateral cases, and because we failed to detect any RB1 germline pathogenic variant, mosaicism—with a pathogenic variant in both retinas but not in blood—is a plausible explanation. In turn, this would imply a higher rate of postzygotic pathogenic variants in male fetuses. Alternatively, the modifying effect of an X-linked gene could be proposed. The RBBP7 gene (OMIM 300825), a chromatin regulation factor that forms a complex with RB protein, could be an attractive candidate, albeit the mechanism remains elusive.37 The low number of bilateral cases without a germline pathogenic variant may warrant international efforts to gain statistical power. Our findings support RB protein maintenance and use of the RB1 gene as a candidate for exon skipping or read-through treatment strategies in the retina.38,39
Limitations
One limitation of this study concerns tumor availability. This study comprised only a limited number of genotyped tumors in bilateral cases because tumor material is rare and valuable, and we tend to start by germline analyses in bilateral cases, in which the detection rate is greater than 90%. In terms of ocular phenotype, most tumors screened were in advanced IIRC groups.
Conclusions
The results of this study suggest that RB risk is associated with the germline pathogenic variant and with maintenance of RB protein and that there is a sex-linked mechanism for nongermline carriers.
eTable. International Intraocular Retinoblastoma Classification
eFigure 1. RB1 Germline Pathogenic Variants and RB1 Somatic Pathogenic Variants
eFigure 2. Age in Months at Diagnosis of Retinoblastoma According to Laterality, Among Unilateral Cases, and Among Bilateral Cases
eFigure 3. Age in Months at Diagnosis of Retinoblastoma According to RB1 Germline Carriage, Among Noncarriers of RB1 Germline Pathogenic Variant, and Among Carriers of RB1 Germline Pathogenic Variant
References
- 1.MacCarthy A, Draper GJ, Steliarova-Foucher E, Kingston JE. Retinoblastoma incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System Project. Eur J Cancer. 2006;42(13):2092-2102. doi: 10.1016/j.ejca.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Friend SH, Bernards R, Rogelj S, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323(6089):643-646. doi: 10.1038/323643a0 [DOI] [PubMed] [Google Scholar]
- 3.Chau BN, Wang JYJ. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3(2):130-138. doi: 10.1038/nrc993 [DOI] [PubMed] [Google Scholar]
- 4.Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev. 2016;30(13):1492-1502. doi: 10.1101/gad.282145.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez-Sánchez F, Ramírez-Castillejo C, Weekes DB, et al. Attenuation of disease phenotype through alternative translation initiation in low-penetrance retinoblastoma. Hum Mutat. 2007;28(2):159-167. doi: 10.1002/humu.20394 [DOI] [PubMed] [Google Scholar]
- 6.Otterson GA, Chen Wd, Coxon AB, Khleif SN, Kaye FJ. Incomplete penetrance of familial retinoblastoma linked to germ-line mutations that result in partial loss of RB function. Proc Natl Acad Sci U S A. 1997;94(22):12036-12040. doi: 10.1073/pnas.94.22.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehainault C, Garancher A, Castéra L, et al. The survival gene MED4 explains low penetrance retinoblastoma in patients with large RB1 deletion. Hum Mol Genet. 2014;23(19):5243-5250. doi: 10.1093/hmg/ddu245 [DOI] [PubMed] [Google Scholar]
- 8.Castéra L, Sabbagh A, Dehainault C, et al. MDM2 as a modifier gene in retinoblastoma. J Natl Cancer Inst. 2010;102(23):1805-1808. doi: 10.1093/jnci/djq416 [DOI] [PubMed] [Google Scholar]
- 9.Kanber D, Buiting K, Roos C, et al. The origin of the RB1 imprint. PLoS One. 2013;8(11):e81502. doi: 10.1371/journal.pone.0081502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eloy P, Dehainault C, Sefta M, et al. A parent-of-origin effect impacts the phenotype in low penetrance retinoblastoma families segregating the c.1981C>T/p.Arg661Trp mutation of RB1. PLoS Genet. 2016;12(2):e1005888. doi: 10.1371/journal.pgen.1005888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quiñonez-Silva G, Dávalos-Salas M, Recillas-Targa F, Ostrosky-Wegman P, Aranda DA, Benítez-Bribiesca L. Monoallelic germline methylation and sequence variant in the promoter of the RB1 gene: a possible constitutive epimutation in hereditary retinoblastoma [published correction appears in Clin Epigenetics. 2017;9(27)]. Clin Epigenetics. 2016;8(1):1. doi: 10.1186/s13148-015-0167-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castéra L, Gauthier-Villars M, Dehainault C, et al. Mosaicism in clinical practice exemplified by prenatal diagnosis in retinoblastoma. Prenat Diagn. 2011;31(11):1106-1108. doi: 10.1002/pd.2837 [DOI] [PubMed] [Google Scholar]
- 13.Richter S, Vandezande K, Chen N, et al. Sensitive and efficient detection of RB1 gene mutations enhances care for families with retinoblastoma. Am J Hum Genet. 2003;72(2):253-269. doi: 10.1086/345651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor M, Dehainault C, Desjardins L, et al. Genotype-phenotype correlations in hereditary familial retinoblastoma. Hum Mutat. 2007;28(3):284-293. doi: 10.1002/humu.20443 [DOI] [PubMed] [Google Scholar]
- 15.Houdayer C, Gauthier-Villars M, Castéra L, Desjardins L, Doz F, Stoppa-Lyonnet D. Retinoblastoma–genetic counseling and molecular diagnosis In: Kumaramanickavel G, ed. Retinoblastoma: An Update on Clinical, Genetic Counseling, Epidemiology and Molecular Tumor Biology. InTechOpen; 2012:55-72. doi: 10.5772/34069 [DOI] [Google Scholar]
- 16.Tomar S, Sethi R, Sundar G, Quah TC, Quah BL, Lai PS. Mutation spectrum of RB1 mutations in retinoblastoma cases from Singapore with implications for genetic management and counselling. PLoS One. 2017;12(6):e0178776. doi: 10.1371/journal.pone.0178776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He M-Y, An Y, Gao Y-J, Qian X-W, Li G, Qian J. Screening of RB1 gene mutations in Chinese patients with retinoblastoma and preliminary exploration of genotype-phenotype correlations. Mol Vis. 2014;20:545-552. [PMC free article] [PubMed] [Google Scholar]
- 18.Lohmann DR, Gallie BL. Retinoblastoma: revisiting the model prototype of inherited cancer. Am J Med Genet C Semin Med Genet. 2004;129C(1):23-28. doi: 10.1002/ajmg.c.30024 [DOI] [PubMed] [Google Scholar]
- 19.Alonso J, García-Miguel P, Abelairas J, et al. Spectrum of germline RB1 gene mutations in Spanish retinoblastoma patients: phenotypic and molecular epidemiological implications. Hum Mutat. 2001;17(5):412-422. doi: 10.1002/humu.1117 [DOI] [PubMed] [Google Scholar]
- 20.Price EA, Price K, Kolkiewicz K, et al. Spectrum of RB1 mutations identified in 403 retinoblastoma patients. J Med Genet. 2014;51(3):208-214. doi: 10.1136/jmedgenet-2013-101821 [DOI] [PubMed] [Google Scholar]
- 21.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23(10):2272-2279. doi: 10.1200/JCO.2005.05.054 [DOI] [PubMed] [Google Scholar]
- 22.Sanders BM, Draper GJ, Kingston JE. Retinoblastoma in Great Britain 1969-80: incidence, treatment, and survival. Br J Ophthalmol. 1988;72(8):576-583. doi: 10.1136/bjo.72.8.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacCarthy A, Birch JM, Draper GJ, et al. Retinoblastoma in Great Britain 1963-2002. Br J Ophthalmol. 2009;93(1):33-37. doi: 10.1136/bjo.2008.139618 [DOI] [PubMed] [Google Scholar]
- 24.Moll AC, Kuik DJ, Bouter LM, et al. Incidence and survival of retinoblastoma in the Netherlands: a register based study 1862-1995. Br J Ophthalmol. 1997;81(7):559-562. doi: 10.1136/bjo.81.7.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93(1):21-23. doi: 10.1136/bjo.2008.138750 [DOI] [PubMed] [Google Scholar]
- 26.Seregard S, Lundell G, Svedberg H, Kivelä T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: advantages of birth cohort analysis. Ophthalmology. 2004;111(6):1228-1232. doi: 10.1016/j.ophtha.2003.10.023 [DOI] [PubMed] [Google Scholar]
- 27.Lumbroso-Le Rouic L, Savignoni A, Levy-Gabriel C, et al. Treatment of retinoblastoma: the Institut Curie experience on a series of 730 patients (1995 to 2009). J Fr Ophtalmol. 2015;38(6):535-541. doi: 10.1016/j.jfo.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 28.Temming P, Arendt M, Viehmann A, et al. How eye-preserving therapy affects long-term overall survival in heritable retinoblastoma survivors. J Clin Oncol. 2016;34(26):3183-3188. doi: 10.1200/JCO.2015.65.4012 [DOI] [PubMed] [Google Scholar]
- 29.Kleinerman RA, Tucker MA, Sigel BS, Abramson DH, Seddon JM, Morton LM. Patterns of cause-specific mortality among 2053 survivors of retinoblastoma, 1914-2016. J Natl Cancer Inst. 2019;111(9):961-969. doi: 10.1093/jnci/djy227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1):41-53, viii. doi: 10.1016/j.ohc.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 31.Dehainault C, Michaux D, Pagès-Berhouet S, et al. A deep intronic mutation in the RB1 gene leads to intronic sequence exonisation. Eur J Hum Genet. 2007;15(4):473-477. doi: 10.1038/sj.ejhg.5201787 [DOI] [PubMed] [Google Scholar]
- 32.Houdayer C, Gauthier-Villars M, Laugé A, et al. Comprehensive screening for constitutional RB1 mutations by DHPLC and QMPSF. Hum Mutat. 2004;23(2):193-202. doi: 10.1002/humu.10303 [DOI] [PubMed] [Google Scholar]
- 33.Collet A, Tarabeux J, Girard E, et al. Pros and cons of HaloPlex enrichment in cancer predisposition genetic diagnosis. AIMS Genet. 2015;2(4):263-280. doi: 10.3934/genet.2015.4.263 [DOI] [Google Scholar]
- 34.Harbour JW. Molecular basis of low-penetrance retinoblastoma. Arch Ophthalmol. 2001;119(11):1699-1704. doi: 10.1001/archopht.119.11.1699 [DOI] [PubMed] [Google Scholar]
- 35.R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. Accessed December 15, 2018. http://www.R-project.org
- 36.Bouaoun L, Sonkin D, Ardin M, et al. TP53 variations in human cancers: new lessons from the IARC TP53 Database and genomics data. Hum Mutat. 2016;37(9):865-876. doi: 10.1002/humu.23035 [DOI] [PubMed] [Google Scholar]
- 37.Guan LS, Li GC, Chen CC, Liu LQ, Wang ZY. Rb-associated protein 46 (RbAp46) suppresses the tumorigenicity of adenovirus-transformed human embryonic kidney 293 cells. Int J Cancer. 2001;93(3):333-338. doi: 10.1002/ijc.1338 [DOI] [PubMed] [Google Scholar]
- 38.Niks EH, Aartsma-Rus A. Exon skipping: a first in class strategy for Duchenne muscular dystrophy. Expert Opin Biol Ther. 2017;17(2):225-236. doi: 10.1080/14712598.2017.1271872 [DOI] [PubMed] [Google Scholar]
- 39.Pranke I, Golec A, Hinzpeter A, Edelman A, Sermet-Gaudelus I. Emerging therapeutic approaches for cystic fibrosis. from gene editing to personalized medicine. Front Pharmacol. 2019;10:121. doi: 10.3389/fphar.2019.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. International Intraocular Retinoblastoma Classification
eFigure 1. RB1 Germline Pathogenic Variants and RB1 Somatic Pathogenic Variants
eFigure 2. Age in Months at Diagnosis of Retinoblastoma According to Laterality, Among Unilateral Cases, and Among Bilateral Cases
eFigure 3. Age in Months at Diagnosis of Retinoblastoma According to RB1 Germline Carriage, Among Noncarriers of RB1 Germline Pathogenic Variant, and Among Carriers of RB1 Germline Pathogenic Variant