Abstract
Background
An increase in the aging yet active US population will continue to make total knee arthroplasty (TKA) procedures routine in the coming decades. For such joint procedures, the Centers for Medicare and Medicaid Services introduced programs such as the Comprehensive Care for Joint Replacement to emphasize accountable and efficient transitions of care. Accordingly, many studies have proposed models using risk factors for predicting readmissions after the procedure. We performed a systematic review of TKA literature to identify such models and risk factors therein using a reliable appraisal tool for their quality assessment.
Methods
Five databases were searched to identify studies that examined correlations between post-TKA readmission and risk factors using multivariate models. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis methodology and Transparent Reporting of a multivariate prediction model for Individual Prognosis Or Diagnosis criteria established for quality assessment of prognostic studies.
Results
Of 29 models in the final selection, 6 models reported performance using a C-statistic, ranging from 0.51 to 0.76, and 2 studies used a validation cohort for assessment. The average 30-day and 90-day readmission rates across the studies were 5.33% and 7.12%, respectively. Three new significant risk factors were discovered.
Conclusions
Current models for TKA readmissions lack in performance measurement and reporting when assessed with established criteria. In addition to using new techniques for better performance, work is needed to build models that follow the systematic process of calibration, external validation, and reporting for pursuing their deployment in clinical settings.
Keywords: Total knee arthroplasty, Patient readmission, Risk factors, Statistical models
Introduction
The Affordable Care Act of 2010 aimed to universally increase patient access to care, reduce overall health-care spending, and improve the quality of medical services throughout the United States. To address this goal of reduced spending for effective and efficient medical care, the Hospital Readmission Reduction Program within the Affordable Care Act altered the existing landscape of hospital-based medicine, with its emphasis on incentivizing quantifiable, inpatient health outcomes [1]. Specifically, Section 3205 enables hospitals to receive financial incentives for minimizing all-cause 30-day patient readmissions [2]. This integration of financial incentives with overall practice quality has had a great effect on a variety of procedures, with one of the most noticeable impacts being in the field of orthopaedics through total knee arthroplasties (TKAs) and total hip arthroplasties [3].
The combined cost for hospital readmissions after primary total hip arthroplasty and TKA is exorbitant, amounting to roughly 1.1 billion USD [4]. By the year 2030, more than 3.5 million TKAs will have been performed, with potential for an exponential increase, given Medicaid’s expansion qualifying more individuals for elective procedures [1,5,6]. This amounts to a greater than 600% projected increase for TKA, most of which is already covered by Medicare [3]. Thus, the Centers for Medicare and Medicaid Services (CMS) introduced the Comprehensive Care for Joint Replacement model in 2016 for total knee and hip replacements in many metropolitan areas. This model represents a departure from the typical fee-for-service model and makes hospitals responsible for a bundle of care, starting from admission to 90 days after discharge; if hospitals spend more than the precalculated cost of care determined by the CMS for hip or knee replacements, they are responsible for covering the rest of the cost, but if they spend less, they receive a financial bonus [7]. This system imposes caps on repayment and bonuses depending on the sequential year of implementation, with an ultimate goal of promoting regional competition among hospitals to implement low-cost but highly effective ways to increase quality of care and decrease readmissions. Decreasing cost associated with readmissions concurrently improves transition of care at and after discharge, thereby decreasing the likelihood of further readmissions [8,9] and increasing value for patients.
In response to the changing care landscape, many studies have proposed predictive models for readmissions after TKA to better assess risk and costs and to facilitate transitions of care. For instance, a predictive model developed in 2019 by Ali et al [10] delineated specific predictors of 30-day TKA readmissions based on an analysis of 566,273 procedures in the United Kingdom over a 10-year period. Specific predictors found to significantly increase 30-day readmissions included obesity, coagulopathy, psychosis, arrhythmias, chronic obstructive pulmonary disease, advanced age, and emergency hospital admission in the previous 12 months. Another large study by Urish et al [11] noted that the greatest predictors for TKA 30-day readmissions include congestive heart failure, hospital length of stay (LOS) greater than 4 days, and chronic renal disease, with a median cost of approximately $6753 USD per readmission. Despite these valuable findings, both studies fall short when appraised against gold-standard criteria and use of a validation cohort. These studies are representative of paucity in the literature delineating evidence-based and critically appraised risk factors contributing to 30-day TKA readmissions. Accordingly, the purpose of this study was threefold: (i) to identify validated and clinically usable statistical models predicting readmissions for patients undergoing TKA; (ii) to assess the quality of the models with a reliable appraisal tool; and (iii) to leverage the identified models to elucidate new evidence-based factors contributing to 30-day TKA readmission. We hypothesize that the majority of current studies outlining predictors for 30-day TKA readmissions lack appraisal against standard gold criteria and inclusion of held-out or external validation cohorts.
Material and methods
Search strategy and criteria
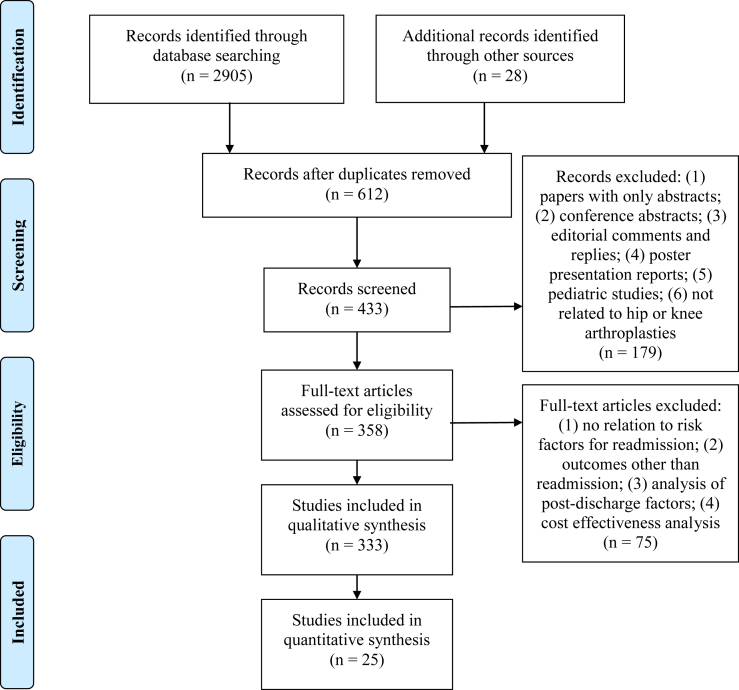
This study followed criteria set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statement [12]. The article heading, use of the PRISMA flow diagram shown in Figure 1 for study selection [13], and use of the PRISMA-P checklist in Electronic Supplementary Table 1 reflect adherence to the PRISMA-P criteria [14]. In addition, a priori protocol registration and description were performed at the PROSPERO registry site (Registration number: CRD42018108571) [15].
Figure 1.
PRISMA flow diagram for systematic review of predictive models for TKA readmissions.
The initial search began with a manual exploration of printed articles in the hospital medical library and an electronic search on Google Scholar. Important and relevant reference articles from the initial search were additionally obtained, ultimately accounting for 28 articles during manual search. Analysis of these articles helped establish an automated strategy for searching electronic databases. This systematic review describes readmission prediction models for only knee arthroplasty; however, the search criteria were formulated for both hip and knee arthroplasty procedures. This strategy was adopted for completeness because our initial search revealed 3 paradigms for cohort selection: (i) some studies had used combined cohorts of patients undergoing hip and knee arthroplasties; (ii) some others had created separate subgroups for hip and knee arthroplasties from a common cohort; and (iii) the remaining had used only patients who underwent knee arthroplasty. A medical librarian and one author (B.T.A. and S.M.M.) designed and implemented all the searches in the following databases: (1) PubMed; (2) Embase; (3) Ovid MEDLINE; (4) Cochrane Database of Systematic Reviews; and (5) Web of Science. If the database did not take the exact date for search, it was approximated to the nearest month and/or year. We searched for articles between the date of inception of each database and February 2019. Each database had slightly different search methodology; nonetheless, the search was first started with hospital readmission as the exploded Medical Subject Headings (MeSH) term, and the key words readmi∗, rehosp∗, where ∗ was used as the truncation character. Second, we searched for risk as the exploded MeSH term, and the key words were model∗, predict∗, use∗, util∗, and risk∗. Third, we performed a search that used the exploded MeSH terms arthroplasty, replacement, total, partial, prosthesis, and knee. Fourth, we performed a search that used the exploded MeSH terms arthroplasty, replacement, total, partial, hemiarthroplasty, prosthesis, and hip. Finally, we combined all the search criteria that identified our final reference set in each database.
Inclusion and exclusion
Study eligibility was determined using 3 stages of title review, abstract review, and full-article review. In addition, studies were considered eligible if they (a) used readmission as an independent or composite outcome; (b) measured readmission after index hospitalization for TKA; (c) examined the association between readmission and predictors using a multivariate statistical model; and (d) were published in the English language. Finally, if a study reported multiple models, one of which used readmission as an outcome, that model was included in this analysis.
Assessment of study quality
Figure 1 shows the steps of article selection in the PRISMA flow diagram. It also shows exclusion criteria at each step of the selection process, starting with 2933 articles and ending with 358 articles for full review.
In the final step, we excluded studies (n = 85) that (1) analyzed readmissions after generic index admission and did not have TKA as the discharge diagnosis and (2) created cohorts based on explicit subgroups such as revision arthroplasty or knee fracture. Each article selected for detailed review was appraised for risk of bias using the Transparent Reporting of a multivariate prediction model for Individual Prognosis Or Diagnosis (TRIPOD) assessment tool [16]. The TRIPOD standard is used specifically for transparent reporting of studies developing, validating, or updating a multivariate prediction model for prognostic purposes. It is implemented as a 22-item checklist that provides comprehensive criteria for ensuring that authors report the necessary information to compare, contrast, and assess the predictive capabilities of various models. Electronic Supplementary Table 2 shows the checklist applied to all the models in the final selection of studies. The TRIPOD tool factored out studies that did not analyze multivariate models (n = 213), and thus, it reduced the risk of bias in the final selection of studies. During this step, we also found other reviews (n = 10); 6 of these were related to the postoperative management and interventions after joint or knee surgery discharge and hence were excluded from our analysis. Of the remaining 4 reviews [[17], [18], [19], [20]], one review considered only postoperative factors after hip arthroplasties [17]. The 2 reviews focused on total joint arthroplasties but did not adhere to formal systematic review standards; they are literature reviews and described modifiable and nonmodifiable risk factors for patients undergoing joint arthroplasties [19,20]. One of the two studies also considered perioperative risk factors including administrative and systemic factors after the discharge and hence did not describe predictive factors for readmissions [20]. Podmore et al. (2018) [18] considered short- and long-term impacts of comorbidities in patients undergoing joint arthroplasty on many outcome variables, with readmission as one of the outcomes in their systematic review and meta-analysis. Risk factors other than comorbidities, however, were not considered in their review. Thus, none of the reviews have aimed to carry out a systematic review adhering to the specific reporting standards to understand the quantitative impact of all the risk factors on readmission after TKA.
Data collection and abstraction
Our detailed review entailed 25 studies that described 29 risk models (7 combined hip and knee arthroplasty cohort models and 22 TKA models). Cram et al. (2012) [21], Mnatzaganian et al. (2014) [22], and Swenson et al. (2018) [23] created 2, 3, and 2 models, respectively, in the same study, with subgroups based on specific predictors or outcome criteria. This analysis was carried out by at least 2 authors separately (E.K. and S.M.M.; C.N. and S.M.M.; J.B. and S.M.M.), and results of the selection process were verified for selection bias and resolution of low-confidence selections by each author. A predictive model was considered to be a statistical construct for this review, created to understand the combined effect of predictors derived from known data sources on readmission as the outcome, using a specific study design. Predictive models may also be referred to as risk stratification models—both here and in other literature—in that a predictive model is used to stratify population of patients based on their membership of specific risk categories such as high, medium, or low risk of readmission. In addition, we extracted the following data items from each study in a tabulated format in 3 separate documents: (1) the research study design, the country where the study was conducted, data source and timeframe of the data cohort, derivation and validation cohort sizes, statistical model used, and whether the risk score was created (items summarized as research study characteristics and shown in Table 1); (2) readmission as a single or composite outcome measure, whether the outcome was TKA specific or all-cause or surrogate readmission measure, timeframe used for readmission, observed readmission rate, and C-statistic or area under the curve for validation cohort (items summarized as outcome characteristics and shown in Table 2); (3) significant risk factors in each final model (items summarized as risk factor characteristics and shown in Table 3).
Table 1.
Study design characteristics for studies for readmissions for TKA.
| Study | Research study design | Data source | Year(s) of data | Derivation cohort | Validation cohort | Statistical model | Country/risk score creation |
|---|---|---|---|---|---|---|---|
| (Solomon, Chibnik et al., 2006) [24] | Retrospective | CMS files and EHR system | 2000 | 9073 | NR | HMLR | USA/Yes |
| (Higuera, Elsharkawy et al., 2011) [25] (TKA subgroup) | Prospective | Single-center EHR system | 2008 | 352 | NR | HMLR | USA/No |
| (Cram, Lu et al., 2012-a) [21] (Primary TKA only) | Retrospective | CMS files | 1991-2010 | 915,562 | NR | MLR | USA/No |
| (Cram, Lu et al., 2012-b) [21] (Revision TKA only) | Retrospective | CMS files | 1991-2010 | 74,935 | NR | MLR | USA/No |
| (Pugely, Callaghan et al., 2013) [26] (TKA subgroup) | Retrospective | ACS-NSQIP | 2011 | 11,814 | NR | MLR | USA/No |
| (Pugely, Martin et al., 2013) [27] | Retrospective | ACS-NSQIP | 2005-2010 | 14,052 | NR | MLR | USA/No |
| (Mnatzaganian, Ryan et al., 2014-a) [22] (Subset1 predictors) | Retrospective | Single-center EHR system | 1996-1999 | 819 | NR | CPHR | Australia/No |
| (Mnatzaganian, Ryan et al., 2014-b) [22] (Subset2 predictors) | Retrospective | Single-center EHR system | 1996-1999 | 819 | NR | CPHR | Australia/No |
| (Mnatzaganian, Ryan et al., 2014-c) [22] (Subset3 predictors) | Retrospective | Single-center EHR system | 1996-1999 | 819 | NR | CPHR | Australia/No |
| (Schairer, Vail et al., 2014) [28] | Retrospective | Single-center EHR system | 2005-2011 | 1408 | NR | CPHR | USA/No |
| (Belmont, Goodman et al., 2016) [29] | Retrospective | ACS-NSQIP | 2011-2012 | 1754 | NR | MLR | USA/No |
| (Feng, Lin et al., 2017) [30] | Retrospective | Single-center knee arthroplasty registry | 2008-2013 | 1542 | NR | MLR | China/No |
| (Lee, Kumar et al., 2017) [31] | Retrospective | Single-center EHR system | 2004-2013 | 3049 | NR | MLR | Korea/No |
| (Siracuse, Ippolito et al., 2017) [32] | Retrospective | HCUP for 4 states | 2006-2011 | 433,638 | 269,934 | MLR | USA/Yes |
| (Kimball, Nichols et al., 2018) [33] (TKA subgroup) | Retrospective | CMS files | 2014-2015 | 58,064 | NR | CPHR | USA/No |
| (Lehtonen, Hess et al., 2018) [34] | Retrospective | ACS-NSQIP | 2012-2015 | 137,209 | NR | MLR | USA/No |
| (Saku, Madanat et al., 2018) [35] | Retrospective | Finnish Hospital Discharge Register | 2015 | 894 | NR | MLR | Finland/No |
| (Urish, Qin et al., 2018) [11] | Retrospective | HCUP | 2014 | 224,465 | NR | MLR | USA/No |
| (Yohe, Funk et al., 2018) [36] | Retrospective | ACS-NSQIP | 2008-2014 | 12,026 | NR | MLR | USA/No |
| (Ali, Louffler et al., 2019) [10] | Retrospective | NHS ES | 2006-2016 | 566,323 | NR | MLR | UK/No |
| (Zmistowski, Restrepo et al., 2013) [37] | Retrospective | Single-center EHR system | 2004-2008 | 5426 | NR | MLR | USA/No |
| (Mesko, Bachmann et al., 2014) [38] | Retrospective | Single-center EHR system | 2010-2011 | 1291 THKA combined | 1291 (with bootstrapping of 1000 samples) | MLR | USA/Yes |
| (Tiberi, Hansen et al., 2014) [39] (Only for cirrhosis patients undergoing THKA) | Retrospective – 1:2 matched case control | Single-center EHR system | 2000-2012 | 230 THKA combined | NR | MLR | USA/Yes |
| (Ricciardi, Oi et al., 2017) [40] | Retrospective – 1:2 matched case control | Single-center EHR system | 2010-2014 | 21,864 THKA combined |
NR | MLR | USA/No |
| (Sher, Keswani et al., 2017) [41] | Retrospective | ACS-NSQIP | 2011-2014 | 7474 THKA combined | NR | MLR | USA/No |
| (Yao, Keswani et al., 2017) [42] (TKA subgroup) | Retrospective | ACS-NSQIP | 2011-2014 | 71,293 | NR | MLR | USA/No |
| (Schroer, Diesfield et al., 2018) [43] | Retrospective | Multicenter (5) EHR system | 2014-2015 | 6968 THKA combined | NR | DS | USA/No |
| (Swenson, Bastian et al., 2018-a) [23] (30 day readmission) | Retrospective | Single-center EHR system | 2013- 2015 | 622 THKA combined | NR | MLR | USA/No |
| (Swenson, Bastian et al., 2018-b) [23] (90 day readmission) | Retrospective | Single-center EHR system | 2013-2015 | 622 THKA combined | NR | MLR | USA/No |
CPHR, Cox proportional hazards regression; DS, descriptive study; HCUP, Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality; HMLR, Hierarchical Logistic Regression Model using Generalized Estimating Equations; MLR, multivariate logistic regression; NHS ES, National Health Service Hospital Episode Statistics database; NR, not reported; Subset1, subset predictors of age and the number of comorbidities; Subset2, subset predictors of age and Charlson Comorbidity Index; Subset3, Subset predictors of age and Elixhauser Comorbidity Index; THKA, total hip or knee arthroplasty.
Table 2.
Outcome characteristics for studies for readmissions for TKA.
| Study | Outcome: readmission—single measure or composite readmission/mortality or other complications requiring readmission | Readmission days | Observed readmission rate (%) | C-statistics or AUC for validation cohort |
|---|---|---|---|---|
| (Solomon, Chibnik et al., 2006) [24] | Composite | 90 | 3.6 | 0.62 |
| (Higuera, Elsharkawy et al., 2011) [25] | Composite–complications | 90 | 9.4 | NR |
| (Cram, Lu et al., 2012-a) [21] | Single | 30 | 5.0 | NR |
| (Cram, Lu et al., 2012-b) [21] | Single | 30 | 8.9 | NR |
| (Pugely, Callaghan et al., 2013) [26] | Composite–complications | 30 | 4.6 | NR |
| (Pugely, Martin et al., 2013) [27] | Composite–complications | 30 | 10.7 & 12.3a | NR |
| (Mnatzaganian, Ryan et al., 2014-a) [22] | Single | 90 | NR | 0.51 |
| (Mnatzaganian, Ryan et al., 2014-b) [22] | Single | 90 | NR | 0.54 |
| (Mnatzaganian, Ryan et al., 2014-c) [22] | Single | 90 | NR | 0.61 |
| (Schairer, Vail et al., 2014) [28] | Composite–complications | 30 & 90 | 4.0 & 8.0 | NR |
| (Belmont, Goodman et al., 2016) [29] | Single | 30 | 6.2 | 0.75 |
| (Feng, Lin et al., 2017) [30] | Composite–complications | 30 | 8.9 | NR |
| (Lee, Kumar et al., 2017) [31] | Single | 30 and 90b | 1.9 and 3.3 | NR |
| (Siracuse, Ippolito et al., 2017) [32] | Single | 30 | 5.1 | NR |
| (Kimball, Nichols et al., 2018) [33] | Single | 30 | 5.4 | NR |
| (Lehtonen, Hess et al., 2018) [34] | Single | 30 | 3.4 | NR |
| (Saku, Madanat et al., 2018) [35] | Single | 90 | 8.0 | NR |
| (Urish, Qin et al., 2018) [11] | Composite–complications | 30 | 4.0 | NR |
| (Yohe, Funk et al., 2018) [36] | Composite–complications | 30 | 4.7 | NR |
| (Ali, Louffler et al., 2019) [10] | Single | 30 | 6.0 | NR |
| (Zmistowski, Restrepo et al., 2013) [37] | Composite–complications | 30 & 90 | 3.1 & 5.3 | NR |
| (Mesko, Bachmann et al., 2014) [38] | Single | 30 | 3.6 | 0.76 |
| (Tiberi, Hansen et al., 2014) [39] | Single | 90 | 10.0 | NR |
| (Ricciardi, Oi et al., 2017) [40] | Single | 30 | NR | NR |
| (Sher, Keswani et al., 2017) [41] | Composite–complications | 30 | 1.9 | NR |
| (Yao, Keswani et al., 2017) [42] | Composite–complications | 30 | 3.5 | NR |
| (Schroer, Diesfield et al., 2018) [43] | Composite–complications | 90 | 8.4 | NR |
| (Swenson, Bastian et al., 2017-a) [23] | Composite–complications | 30 | 3.4 | NR |
| (Swenson, Bastian et al., 2017-b) [23] | Composite–complications | 90 | 8.1 | NR |
AUC, area under the curve; NR, not reported.
: For spinal and general anesthesia, respectively.
Not reported which outcome was used for modeling.
Table 3.
Risk factor characteristics for studies for readmissions for TKA.
| Predictors (level) | Unit of measure and comparison | Effect size [CI] (wrt reference)a,b | Study |
|---|---|---|---|
| Demographics | |||
| Age (patient) | 65-70/71-80/81-95 years | OR: 1.3 [1.0-1.6] (71-80 wrt 65-70) OR: 1.6 [1.1-2.4] (81-95 wrt 65-70) |
(Solomon, Chibnik et al., 2006) [24] |
| Age (patient) | 65-74/75-84/85+ years | RR: 1.43 [1.14-1.80] (75-84 wrt 65-74) RR: 1.25 [0.79-1.98] (85 + wrt 65-74) |
(Higuera, Elsharkawy et al., 2011) [25] |
| Age (patient) | 65-74/75-84/85+ years | OR: 1.4 [1.4-1.4] (75-84 wrt 65-74) OR: 1.8 [1.8-1.9] (85 + wrt 65-74) |
(Cram, Lu et al., 2012-a) [21] |
| Age (patient) | 65-74/75-84/85+ years | OR: 1.2 [1.1-1.2] (75-84 wrt 65-74) OR: 1.4 [1.3-1.5] (85 + wrt 65-74) |
(Cram, Lu et al., 2012-b) [21] |
| Age (patient) | <45/46-55/56-65/66-75/76-85/>85 years | OR: 2.59 [1.44-4.67] (<45 wrt 56-65) OR: 1.42 [1.08-1.85] (76-85 wrt 56-65) OR: 1.79 [1.09-2.97] (>85 wrt 56-65) |
(Pugely, Callaghan et al., 2013) [26] |
| Age (patient) | 50-59/60-69/70-79/>=80 years | OR: 1.53 [1.26-1.87] (70-79 wrt 50-59) OR: 2.17 [1.73-2.74] (>=80 wrt 50-59) |
(Pugely, Martin et al., 2013) [27] |
| Age (patient) | Continuous years | HR: 1.02 [0.98-1.06] | (Mnatzaganian, Ryan et al., 2014-b) [22] |
| Age (patient) | 41-50/51-60/61-70/71-80/81-90 years | OR: 1.13 [1.05-1.22] (41-50 wrt 51-60) OR: 1.01 [0.97-1.06] (61-70 wrt 51-60) OR: 1.21 [1.15-1.28] (71-80 wrt 51-60) OR: 1.70 [1.61-1.81] (81-90 wrt 51-60) |
(Siracuse, Ippolito et al., 2017) [32] |
| Age (patient) | 50-59/60-69/70-79/>80 | OR: 1.40 [0.72-2.69] (50-59 wrt <50) OR: 1.66 [0.87-3.16] (60-69 wrt <50) OR: 2.33 [1.18-4.59] (70-79 wrt <50) OR: 4.17 [1.18-4.59] (>80 wrt <50) |
(Sher, Keswani et al., 2017) [41] |
| Age (patient) | Continuous years | OR: 1.01 [1.01-1.02] | (Yao, Keswani et al., 2017) [42] |
| Race (patient) | White/African/Hispanic-Asian-Native (other) | OR: 1.2 [1.2-1.3] (African wrt white) OR: 0.9 [0.9-1.0] (Other wrt white) |
(Cram, Lu et al., 2012-a) [21] |
| Race (patient) | White/African/Hispanic-Asian-Native (Other) | OR: 1.1 [1.0-1.2] (African wrt white) OR: 0.9 [0.8-1.1] (Other wrt white) |
(Cram, Lu et al., 2012-b) [21] |
| Race (patient) | African/white | OR: 1.68 [1.35-2.09] (African wrt white) | (Pugely, Martin et al., 2013) [27] |
| Race (patient) | White/African/Hispanic-Asian-Native (other) | OR: 1.37 [1.30-1.44] (African wrt white) OR: 1.08 [1.04-1.13] (Other wrt white) |
(Siracuse, Ippolito et al., 2017) [32] |
| Sex (patient) | Male/female | OR: 1.6 [1.3-2.1] (Male wrt female) | (Solomon, Chibnik et al., 2006) [24] |
| Sex (patient) | Male/female | OR: 1.3 [1.2-1.3] (Male wrt female) | (Cram, Lu et al., 2012-a) [21] |
| Sex (patient) | Male/female | OR: 1.1 [1.0-1.2] (Male wrt female) | (Cram, Lu et al., 2012-b) [21] |
| Sex (patient) | Male/female | OR: 1.25 [1.03-1.53] (Male wrt female) | (Pugely, Callaghan et al., 2013) [26] |
| Sex (patient) | Male/female | OR: 1.18 [1.35-2.09] (Female wrt male) | (Pugely, Martin et al., 2013) [27] |
| Sex (patient) | Male/female | OR: 1.75 [1.15-2.68] (Female wrt male) | (Belmont, Goodman et al., 2016) [29] |
| Sex (patient) | Male/female | OR: 1.19 [1.16-1.23] (Male wrt female) | (Siracuse, Ippolito et al., 2017) [32] |
| Sex (patient) | Male/female | OR: 1.31 [1.21-1.42] (Male wrt female) | (Yao, Keswani et al., 2017) [42] |
| Sex (patient) | Male/female | OR: 3.44 [2.838-4.042] (Male wrt female) | (Swensen, Bastian et al., 2018-a) [23] |
| Sex (patient) | Male/female | OR: 10.6 [9.67-11.53] (Male wrt female) | (Swensen, Bastian et al., 2018-b) [23] |
| BMI (patient) | BMI >40 kg/m2 | OR: 1.25 [0.73-2.16] (BMI >40 wrt no) | (Sher, Keswani et al., 2017) [41] |
| BMI (patient) | BMI >40 kg/m2 | OR: 1.09 [0.96-1.23] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Obesity (patient) | BMI >45 kg/m2 | DS: 11.3% | (Schroer, Diesfield et al., 2018) [43] |
| Administrative | |||
| Cluster 5 (patient) | Age 59.17/BMI 41.25/LOS 59.33/pain 33.47/symptoms 34.91/function daily life 33.3/function sports and leisure 7.89/QOL 10.43 | OR: 4.52 [3.779-5.261] | (Swensen, Bastian et al., 2018-a) [23] |
| Discharge HHC (system) | Yes/no | OR: 23.5 [22.06-24.94] (Yes wrt no) | (Swensen, Bastian et al., 2018-b) [23] |
| Discharge other location (system) | Other than HHC, SNF, and IRF: Yes/no | OR: 31.3 [30.183-32.417] (Yes wrt no) | (Swensen, Bastian et al., 2018-a) [23] |
| Discharge other location (system) | Other than HHC, SNF, and IRF: Yes/no | OR: 48.1 [46.45-49.75] (Yes wrt no) | (Swensen, Bastian et al., 2018-b) [23] |
| Disposition (system) | Home/IRF | OR: 1.99 [1.50-2.64] (IRF wrt home) | (Zmistowski, Restrepo et al., 2013) [37] |
| Disposition (system) | Home or IRF/SNF | HR: 1.62 [1.11-1.24] (SNF wrt home or IRF) | (Schairer, Vail et al., 2014) [28] |
| Dedicated orthopaedic operating room for >75% time (system) | Yes/no | OR: 1.3 [1.0-1.7] (No wrt yes) | (Solomon, Chibnik et al., 2006) [24] |
| Hospital teaching status (system) | Major/minor/nonteaching | OR: 0.9 [0.9-1.0] (Minor wrt major) OR: 0.9 [0.9-0.9] (Nonteaching wrt major) |
(Cram, Lu et al., 2012-a) [21] |
| Hospital teaching status (system) | Major/minor/nonteaching | OR: 0.9 [0.9-1.0] (Minor wrt major) OR: 0.9 [0.9-1.0] (Nonteaching wrt major) |
(Cram, Lu et al., 2012-b) [21] |
| Hospital volume for primary TKA (system) | Quartile1/ Quartile2/ Quartile3/ Quartile4 | OR: 0.9 [0.8-0.9] (Quartile2 wrt Quartile1) OR: 0.8 [0.8-0.9] (Quartile3 wrt Quartile1) OR: 0.8 [0.7-0.8] (Quartile4 wrt Quartile1) |
(Cram, Lu et al., 2012-a) [21] |
| Hospital volume for revision TKA (system) | Quartile1/ Quartile2/ Quartile3/ Quartile4 | OR: 1.0 [0.8-1.1] (Quartile2 wrt Quartile1) OR: 1.0 [0.8-1.1] (Quartile3 wrt Quartile1) OR: 0.9 [0.8-1.1] (Quartile4 wrt Quartile1) |
(Cram, Lu et al., 2012-b) [21] |
| Hospital volume for TKR (system) | High (≥23)/low (<23) | OR: 1.6 [1.1-2.5] (Low wrt high) | (Solomon, Chibnik et al., 2006) [24] |
| Income (patient) | First/second/third/fourth quartile | OR: 0.99 [0.95-1.04] (First wrt fourth) OR: 0.98 [0.94-1.02] (Second wrt fourth) OR: 0.97 [0.94-1.01] (Third wrt fourth) |
(Siracuse, Ippolito et al., 2017) [32] |
| Insurance (patient) | Private/Medicare/Medicaid/self-pay | OR: 1.27 [1.22-1.32] (Medicare wrt private) OR: 1.43 [1.33-1.54] (Medicaid wrt private) OR: 0.70 [0.52-0.93] (Self-pay wrt private) |
(Siracuse, Ippolito et al., 2017) [32] |
| LOS - log (patient) | Continuous days | OR: 1.9 [1.9-2.0] | (Cram, Lu et al., 2012-a) [21] |
| LOS - log (patient) | Continuous days | OR: 2.1 [2.0-2.2] | (Cram, Lu et al., 2012-b) [21] |
| LOS (patient) | ≤5/>5 days | HR: 1.94 [1.3-2.9] (>5 wrt <=5) | (Schairer, Vail et al., 2014) [28] |
| LOS (patient) | Continuous days | OR 1.19 [1.00-1.40] | (Saku, Madanat et al. 2018) [35] |
| Preoperative teaching program (system) | Yes/no | OR: 1.8 [1.2-2.6] (No wrt yes) | (Solomon, Chibnik et al., 2006) [24] |
| Type of anesthesia (provider) | Spinal/other | RR: 0.65 [0.51-0.81] (Spinal wrt other) | (Higuera, Elsharkawy et al., 2011) [25] |
| Type of anesthesia (provider) | General/spinal | OR: 1.13 [1.00-1.27] (General wrt spinal) | (Pugely, Martin et al., 2013) [27] |
| Type of anesthesia (provider) | General/other (spinal-epidural-regional) | OR: 1.74 [1.09-2.79] (General wrt other) | (Belmont, Goodman et al., 2016) [29] |
| Type of surgery - revision (provider) | Primary/revision/AS TKA | HR: 2.0 [1.3-3.0] (Revision wrt primary) HR: 1.79 [1.0-3.1] (ACS wrt primary) |
(Schairer, Vail et al., 2014) [28] |
| Type of surgery - unilateral or bilateral (provider) | Unilateral/bilateral TKA | RR: 1.66 [1.18-2.35] (Bilateral wrt unilateral) | (Higuera, Elsharkawy et al., 2011) [25] |
| Comorbidities | |||
| Anemia (patient) | Yes/no | OR: 1.19 [1.14-1.23] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Anemia (patient) | Hemoglobin <10 g/dL | DS: 20% | (Schroer, Diesfield et al., 2018) [43] |
| Ischemic heart disease/arrhythmia (patient) | Yes/no | RR: 1.73 [1.36-2.21] (Yes wrt no) | (Higuera, Elsharkawy et al., 2011) [25] |
| Arrhythmia (patient) | Yes/no | OR: 11.3 [10.25-12.35] (Yes wrt no) | (Swensen, Bastian et al., 2018-b) [23] |
| ASA level (patient) | Class 4/class 1-2 | OR: 1.42 [1.15-1.74] (4 wrt 1-2) | (Pugely, Callaghan et al., 2013) [26] |
| ASA level (patient) | Class 3-4/not 3-4 | OR: 1.20 [1.06-1.37] (3-4 wrt not 3-4) | (Pugely, Martin et al., 2013) [27] |
| ASA class (patient) | Class 3-4/1-2 | OR: 1.42 [1.01-2.00] (Class 3-4 wrt 1-2) | (Sher, Keswani et al., 2017) [41] |
| ASA class (patient) | Class 3 or 4: Yes/no | OR: 1.37 [1.25-1.50] (Yes wrt no) | (Yao, Keswani et al. 2017) [42] |
| Asthma (patient) | Yes/no | OR: 2.60 [1.30-5.21] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| COPD (patient) | Yes/no | OR: 1.29 [1.24-1.34] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Avascular necrosis etiology (patient) | Yes/no | OR: 0.69 [0.22-2.19] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Bleeding disorder (patient) | Yes/no | OR: 2.01 [1.34-3.01] (Yes wrt no) | (Pugely, Callaghan et al., 2013) [26] |
| Bleeding disorder (patient) | Yes/no | OR: 1.22 [1.11-1.34] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Bleeding disorders (patient) | Current bleeding-causing disorder (Yes/no) | OR: 2.56 [1.22-5.38] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Bleeding disorders (patient) | Yes/no | OR: 1.63 [1.33-2.01] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Cancer (patient) | Yes/no | OR: 11.73 [1.93-71.30] (Yes wrt no) | (Pugely, Callaghan et al. 2013) [26] |
| Lymphoma (patient) | Yes/no | OR: 23.6 [22.25-24.95] (Yes wrt no) | (Swensen, Bastian et al., 2018-b) [23] |
| CCI (patient) | 0.8 | RR: 1.18 [1.11-1.26] (Per index point) | (Higuera, Elsharkawy et al., 2011) [25] |
| CCI (patient) | Continuous | HR: 1.09 [0.98-1.19] | (Mnatzaganian, Ryan et al., 2014-b) [22] |
| Number of comorbidities (patient) | Continuous | OR: 2.20 [1.94-2.46] | (Swensen, Bastian et al., 2018-a) [23] |
| Cardiac disease (patient) | Chronic heart failure in 30 days before surgery, myocardial infarction within 6 months of surgery, previous percutaneous coronary intervention, or history of angina within 1 month of surgery: Yes/no | OR: 1.44 [0.73-2.84] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Congestive heart failure (patient) | Yes/no | OR: 1.64 [1.53-1.76] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Chronic heart failure (patient) | Yes/no | OR: 1.14 [0.56-2.35] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Deep venous thrombosis (patient) | Yes/no | OR: 8.59 [2.36-31.24] (Yes wrt no) | (Belmont, Goodman et al., 2016) [29] |
| Diabetes (patient) | Yes/no | OR: 1.19 [1.15-1.23] (Yes wrt no) | (Siracuse, Ippolito et al. 2017) [32] |
| Diabetes (patient) | Yes/no | OR: 1.28 [0.82-2.01] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Diabetes (patient) | Yes/no | DS: 11% | (Schroer, Diesfield et al., 2018) [43] |
| Diabetes (patient) | Yes/no | OR: 1.03 [0.92-1.15] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| ECI (patient) | 0/1-2/3-4/>4 | OR: 1.3 [1.2-1.3] (1-2 wrt 0) OR: 1.8 [1.7-1.8] (3-4 wrt 0) OR: 2.3 [2.2-2.5] (>4 wrt 0) |
(Cram, Lu et al., 2012-a) [21] |
| ECI (patient) | 0/1-2/3-4/>4 | OR: 1.2 [1.0-1.3] (1-2 wrt 0) OR: 1.4 [1.3-1.6] (3-4 wrt 0) OR: 1.8 [1.6-2.1] (>4 wrt 0) |
(Cram, Lu et al., 2012-b) [21] |
| Epilepsy (patient) | Yes/no | OR: 5.36 [1.17-24.62] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| Fluid and electrolyte disorders (patient) | Yes/no | HR: 1.80 [1.12-1.27] (Yes wrt no) | (Schairer, Vail et al., 2014) [28] |
| Fluid and electrolyte disorders (patient) | Yes/no | OR: 1.25 [1.19-1.32] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| High number of drugs (patient) | Yes/no | OR: 1.11 [1.04-1.19] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| Hypertension (patient) | Yes/no | OR: 1.10 [1.07-1.14] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Hypertension (patient) | Yes/no | OR: 2.10 [1.14-3.87] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| Hypertension-managed (patient) | Yes/no | OR: 0.61 [0.31-0.96] (Yes wrt no) | (Belmont, Goodman et al., 2016) [29] |
| Hypertension (patient) | Yes/no | OR: 1.28 [0.91-1.79] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Hypertension (patient) | Yes/no | OR: 23.6 [22.4-24.8] (Yes wrt no) | (Swensen, Bastian et al., 2018-b) [23] |
| Hypertension (patient) | Yes/no | OR: 1.18 [1.08-1.30] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Liver disease (patient) | Yes/no | OR: 1.27 [1.13-1.43] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Malnutrition (patient) | Albumin <3.4 g/dL | DS: 8.8% | (Schroer, Diesfield et al., 2018) [43] |
| Narcotic use (patient) | Narcotic prescription filled within 3 months of surgery | DS: 10.7% | (Schroer, Diesfield et al., 2018) [43] |
| Preoperative knee flexion (patient) | <110 degrees (Yes/no) | OR: 1.86 [1.03-3.36] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| Preoperative tibiofemoral angle (patient) | Angle <0/0-4 /11-15/>15 | OR: 1.16 [0.51-2.65] (Angle <0 wrt 5-10) OR: 1.18 [0.46-3.04] (Angle 0-4 wrt 5-10) OR: 2.94 [1.19-7.27] (Angle 11-15 wrt 5-10) OR: 1.27 [0.39-4.12] (Angle >15 wrt 5-10) |
(Saku, Madanat et al., 2018) [35] |
| Preoperative serum BUN level (patient) | Continuous mg/dL | OR: 1.02 [1.01-1.03] | (Pugely, Callaghan et al., 2013) [26] |
| Preoperative serum creatinine level (patient) | Continuous mg/dL | OR: 1.48 [1.24-1.75] | (Pugely, Martin et al., 2013) [27] |
| Psychiatric disease (patient) | Yes/no | OR: 2.97 [1.30-6.81] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| Walking aid (patient) | Yes/no | OR: 2.26: [1.03-4.94] (Yes wrt no) | (Saku, Madanat et al., 2018) [35] |
| Pulmonary disease (patient) | Yes/no | OR: 1.36 [0.65-2.86] (Yes wrt no) | (Sher, Keswani et al. 2017) [41] |
| Pulmonary disease (patient) | Yes/no | OR: 1.43 [0.56-2.35] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Smoking (patient) | Yes/no | DS: 7.6% | (Schroer, Diesfield et al., 2018) [43] |
| Smoking (patient) | Yes/no | OR: 1.62 [1.06-2.46] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Smoking (patient) | Yes/no | OR: 1.43 [1.25-1.63] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Steroid use (patient) | Yes/no | OR: 1.79 [0.86-3.74] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Steroids for chronic disease (patient) | Yes/no | OR: 1.35 [1.11-1.65] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Operative variables | |||
| Severe adverse event before discharge (patient) | Yes/no | OR: 13.13 [5.1-33.79] (Yes wrt no) | (Sher, Keswani et al., 2017) [41] |
| Severe adverse event before discharge (patient) | Yes/no | OR: 3.69 [2.79-4.86] (Yes wrt no) | (Yao, Keswani et al., 2017) [42] |
| Postoperative medical complications | |||
| MELD score (patient) | <10/≥10 | OR: 3.16 [1.35-7.39] (≥10 wrt <10) | (Tiberi, Hansen et al., 2014) [39] |
| Number of significant risk factors (patient) | 0/1/2/3/≥4 | OR: 1.43 [1.09-1.88] (1 wrt 0) OR: 2.08 [1.61-2.69] (2 wrt 0) OR: 2.82 [2.18-3.64] (3 wrt 0) OR: 4.36 [3.37-5.66] (>4 wrt 0) |
(Yao, Keswani et al., 2017) [42] |
| Postoperative surgical complications | |||
| RCI (patient) | 0/1/2+ | OR: 1.5 [1.2-1.9] (1 wrt 0) OR: 1.7 [1.2-2.3] (2 + wrt 0) |
(Solomon, Chibnik et al., 2006) [24] |
| MELD score (patient) | <10/≥10 | OR: 4.75 [1.45-15.56] (≥10 wrt <10) | (Tiberi, Hansen et al., 2014) [39] |
| Renal disease (patient) | Yes/no | OR: 1.33 [1.25-1.42] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Rheumatoid arthritis (patient) | Yes/no | OR: 1.14 [1.06-1.23] (Yes wrt no) | (Siracuse, Ippolito et al., 2017) [32] |
| Stroke or cerebrovascular accident (patient) | Yes/no | OR: 3.47 [1.30-9.25] (Yes wrt no) | (Belmont, Goodman et al., 2016) [29] |
| Superficial surgical site infection (patient) | Yes/no | OR: 16.57 [5.82-47.22] (Yes wrt no) | (Belmont, Goodman et al., 2016) [29] |
| Deep or incisional or organ space surgical site infection (patient) | Yes/no | OR: 15.09 [5.57-40.91] (Yes wrt no) | (Belmont, Goodman et al., 2016) [29] |
| Urinary tract infection (patient) | Yes/no | OR: 3.41 [1.04-11.22] (Yes wrt no) | (Belmont, Goodman et al., 2016) [29] |
wrt, with respect to; AS, antibiotic spacer; ASA, American Society of Anesthesiologists patient fitness level before surgery; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DS, descriptive statistics; HHC home health care; IRF, Inpatient Rehabilitation Facility; MELD, Model for End-stage Liver Disease; QOL quality of life; RCI, Replacement Composite Index; SNF, Skilled Nursing Facility; TKR, total knee replacement.
Effect size reported as adjusted odds ratio (OR) or hazards ratio (HR) or relative risks ratio (RR) typically at P < .05 (some ratios significant at higher P values are reported by some authors).
Odds ratio for continuous variables is reported as change in readmission odds for unit change in continuous variable.
Results
The earliest predictive model for TKA readmissions was published in 2006 with the majority published between 2011 and 2019 (Table 1). Of the total, 22 of 29, or 76%, were built in the United States [11,21,[23], [24], [25], [26], [27], [28], [29],[32], [33], [34],[36], [37], [38], [39], [40], [41], [42], [43]]. Three models were built in Australia [22], and one each in China [30], Finland [35], Korea [31], and the United Kingdom [10]. One model was based on the prospective design [25], and the remaining (97%) were retrospective designs with 2 models applying additional case control [39,40]. Most studies used either the Electronic Health Record (EHR) database at the facility (13 of 29, or 44%) [22,23,25,28,31,37,38,39,40,43] or the American College of Surgeons–National Surgical Quality Improvement Program (ACS NSQIP) database (7 of 29, or 24%) [26,27,29,34,36,41,42] for building models. The other data sources used were insurance files, health databases, and surgical procedure registers at a national level (7 of 29, or 24%) [10,21,24,30,33,35] and health databases at a state level (2 of 29, or 7%) [11,32]. The models were built with a minimum of 230 patients and a maximum of 566,323 patients. Most researchers used multiple logistic regression (23 of 29, or 79%) [10,11,21,[23], [24], [25], [26], [27],29,[30], [31], [32],[34], [35], [36], [37], [38], [39], [40], [41], [42]], with 2 studies [24,25] using its hierarchical version. Five of 29 models (17%) [22,28,33] used Cox proportional hazards regression, whereas one study [43] provided descriptive statistics with frequency percentages for various risk factors in the sample cohort.
All the studies determined any- or all-cause readmission after TKA in accordance with the CMS definition of 30-day readmission, as opposed to only TKA-related readmissions (Table 2). 13 of 29 models (45%) [11,23,[25], [26], [27],30,36,37,[41], [42], [43], [44]] used various types of postsurgical complications as surrogate outcomes for readmission. Most models focused on 30-day readmission (17 of 29, or 59%) [10,11,21,23,26,27,29,30,[32], [33], [34],36,38,[40], [41], [42]]. Nine models (31%) [[22], [23], [24], [25],35,39,43] used 90-day readmission as an outcome measure, whereas 3 models (10%) [28,31,37] used both 30-day and 90-day outcome measures for readmissions. The cohort-specific empirical readmission rates varied across the studies, and 2 studies, one with 3 models [22] and the other with one model [40], did not report readmission rates in their cohorts. In the remaining studies, 20 models built with 30-day readmission as an outcome had an average readmission rate of 5.33% (min: 1.90%; max: 12.30%; standard deviation: 2.82). The remaining 9 models built on 90-day readmission as an outcome variable had an average readmission rate of 7.12% (min: 3.30%; max: 10.00%; standard deviation: 2.45). Thus, in the pooled sample, the average 90-day readmission rate is 34% higher than the average 30-day readmission rate. All the models presented adjusted odds ratios (ORs) for significant predictors where applicable; however, only 6 of 29 models across 4 studies [22,24,29,38] presented an aggregate performance C-statistic that ranged from a minimum of 0.51 to a maximum of 0.76.
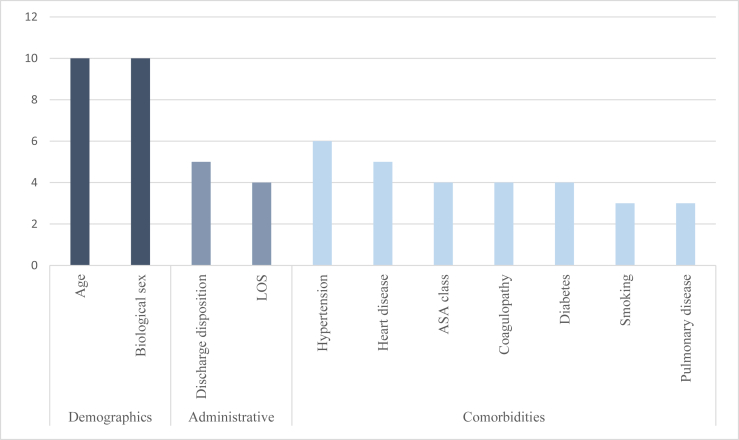
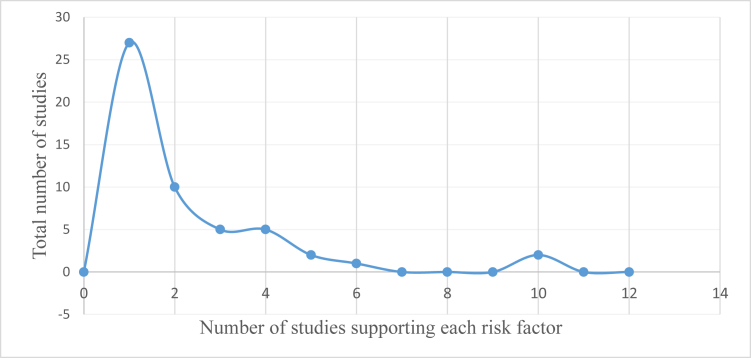
We classified the risk factors into overarching categories to understand their impact on post-TKA readmissions (Table 3). Accepting only risk factors in the 75th percentile of the number of references predicting readmission, the following factors were found to be significant: age, sex, discharge disposition, LOS, hypertension, heart disease, American Society of Anesthesiologists (ASA) physical status class, coagulopathy, diabetes, smoking, and pulmonary disease (Fig. 2). The 75th percentile cutoff was chosen for what was considered substantial support because of the right-skewed distribution: selecting those above average 50th percentile would have included many risk factors with weak support from merely one or 2 studies (Fig. 3). Note that Figure 3 depicts only significant factors of readmission found across the reviewed publications. Meta-analysis on the risk factors across the studies was not attempted because of inconsistencies in the factor and outcome definitions. For example, some studies used age as continuous variable [22,42], whereas others created bins for age as a categorical variable as per differing cutoff points [[24], [25], [26], [27],32]. Similarly, some studies presented ORs for 30-day readmission as an outcome [10,11,21,26,30,32,33,34,36], whereas others for 90-day readmission as an outcome [[23], [24], [25],35,43].
Figure 2.
Number of references associated with each significant risk factor.
Figure 3.
Right-skewed distribution in the number of references associated with each risk factor.
Discussion
Study design characteristics
All the studies except one in the final selection of the review used single-arm, retrospective, and observational design for cohort selection. This is expected because no interventions were considered in any study while quantifying and predicting the risk of future readmission, and given that the main objective of all selected studies was to understand factors associated with the risk of post-TKA readmission. Many studies used ACS-NSQIP data sets to take advantage of the large sample size and diverse geographic representation of patients across multiple years. This cohort setup is useful for deriving statistical models; however, only 2 models [32,38] of 29 used it to create separate test data sets for validation of the derived models. A large percentage of studies used data sets derived from the facility EHR systems. This preference for building models and understanding the risk factors based on the local data sets represents an interesting divergence from using national data sources. Nonetheless, support for significant risk factors for post-TKA readmission appears to be impervious to specific data origin. Finally, none of the models used newer and more advanced methods—such as machine learning and deep learning methods—for building predictive models. This lack of use of new tools might represent a scarcity of professionals with advanced computational and statistical backgrounds and signifies a missed opportunity to implement better-performing predictive models for eventual deployment in clinical practice.
Outcome characteristics
The studies in our review used differing outcome measures, perhaps because the Readmission Reduction Program uses a 30-day readmission measure, whereas the bundled payment model uses a 90-day readmission measure. Another finding is that a majority of studies have not reported any predictive performance measures. This is concerning in terms of incomplete reporting, but more importantly, the absence of any reportable performance measure makes it hard to compare and understand the influence of the combined risk factors on the predicted outcome. This is a requirement if one wants to deploy the models in clinical practice for assessing risk of future events.
Previously established risk factors for readmission after TKA
A risk factor was deemed previously established if it was referenced by reviews before the beginning of our analysis. Of the factors in the 75th percentile of the number of supporting references, 8 of 11 factors had been previously established as risk factors for readmission after TKA: age, sex, LOS, hypertension, heart disease, diabetes, smoking, and pulmonary disease.
Sveom et al. [20] previously referenced age and found that there were moderate increases in readmission in older patients. We found that among new studies included in our review (Table 3), older age was a risk factor for readmission after TKA. However, there were 2 studies that also showed a slight uptick in readmission in a younger population group. Pugely et al. found that patients younger than 45 years had a significantly increased risk of readmission after TKA (OR: 2.59 [1.44, 4.67]) [26]. Siracuse et al. backed this finding with a slightly higher risk in patients aged 41-50 years (OR: 1.13 [1.05, 1.22]) [32]. Both used 50-60 years of age as a reference range. However, the remaining 8 studies that referenced age as a risk factor indicated that increased age was associated with increased risk [21,22,24,25,27,41,42]. Increased age has also been shown to be associated with an increased number of comorbidities, which can result in poorer wound healing, poorer rehabilitation potential, and increased recovery time after a major joint replacement [45].
Sveom et al. also referenced sex as a risk factor previously [20], finding that males were at higher risk for readmission. Among the 10 studies in our review that referenced sex as a risk factor for readmission (Table 3), 8 studies found male sex as a significant risk factor for readmission. Pugely et al. and Belmont et al. both found that female sex was associated with a higher risk for readmission [29,27]. However, a second study by Pugely et al. found that male sex was associated with a higher risk for readmission [26]. Despite these conflicting data, the vast majority of studies indicated male sex as a risk factor for readmission after TKA [21,23,24,26,32,42]. Many studies have previously documented the variable manifestations of common diseases between male and female patients based on hormone composition, such as the influence of testosterone and the protective benefits of estrogen in bone health and joint recovery [46]. Thus, it is plausible that a lack of estrogen might contribute to the slightly increased risk for readmission among male patients.
Sveom et al. [20] found that an increased LOS was associated with a higher risk of readmission. Three other studies in our review also identified LOS as a risk factor for readmission (Table 3) [21,28,35]. Similar to the effects of age and sex, a longer LOS has been previously correlated with a higher number of comorbidities, such as chronic obstructive pulmonary disease, congestive heart failure, and diabetes [47,48]. The downstream manifestations of these chronic medical conditions lead to less-robust postsurgical recovery, thereby causing higher readmission risks for patients with longer LOS, given a higher likelihood of complications including hospital-acquired infections.
Hypertension has been previously indicated as a risk factor by Podmore et al. for contributing to increased risk of TKA readmissions [18]. Furthermore, Belmont et al. found that hypertension treated with medication decreased the risk of readmission after TKA (OR: 0.61 [0.39, 0.96]) [29], making it a tractable and modifiable risk factor. Overall, hypertension has been indicated as a risk factor for readmission by 6 studies (Table 3) [23,29,32,35,41,42]. Hypertension is associated with numerous complications as it is often secondary to atherosclerosis, especially in elderly patients. In such situations, patients may experience decreased overall perfusion, leading to diminished oxygen transport and blood flow to recovering tissues after joint replacement surgery.
Diabetes has been extensively indicated as a risk factor for readmission after TKA [[18], [19], [20],49]. Four studies in our review indicated diabetes as a risk factor (Table 3) [32,[41], [42], [43]]. Diabetes has been well characterized as leading to delayed wound healing because of the effect of glucose in hindering immune cell activation and transport through an osmotic effect [50]. Specifically, diabetes creates a proinflammatory state in the body, with cytokines further contributing to erroneous signaling during postsurgical recovery. Thus, delayed wound healing can lead to further complications, most notably wound infection, that lead to increased readmission risk.
Smoking has also been extensively indicated as a risk factor for readmission after TKA [19,20,49]. Three studies in our review also indicated smoking as a risk factor for readmission (Table 3) [[41], [42], [43]]. The combination of nicotine and carbon monoxide from cigarettes causes vasoconstriction and decreased oxygen saturation, both of which lead to decreased oxygen supply to healing tissue. Smoking also negatively affects lipid profiles by increasing low-density lipoprotein and decreasing high-density lipoprotein [51]. With this vasoconstriction and alteration in lipid profiling, decreased overall perfusion from smoking delays wound healing and increases risk of readmission.
Sveom et al. and Podmore et al. have characterized heart disease as a risk factor for readmission [18,20]. Five studies included in our review also characterized heart disease as a risk factor for readmission (Table 3) [23,25,32,41,42]. Sveom et al. and Podmore et al. [18,20] have also identified pulmonary disease as a risk factor for readmission. Four studies in our review characterized pulmonary disease as a risk factor for readmission (Table 3) [32,35,41,42]. Because heart disease and pulmonary disease are generic risk factors, it is difficult to pinpoint an exact mechanism by which they increase the risk of readmission. However, they are both likely related to decreased oxygen perfusion, longer recovery times, and increased risk of infection.
New risk factors for readmission after TKA
Among the factors in the 75th percentile of the number of references in our review, coagulopathy, ASA class, and discharge disposition have not yet been referenced as risk factors previously for post-TKA readmission.
Coagulopathies were referenced 4 times as risk factors for readmission after TKA (Table 3). Patients with a coagulopathy were indicated to be at a higher risk for readmission [26,32,41,42]. Coagulopathies are comorbidities that predispose patients to bleeding, which could lead to postoperative complications requiring readmission. Because TKA disrupts well-vascularized tissue, bleeding is common. Rosenburg describes 3 complications from bleeding: hemarthrosis, hematoma, and wound drainage [52]. All 3 complications need careful observation if mild but severe bleeding could lead to clinical symptoms that require treatment [52]. By predisposing patients to bleeding, coagulopathies increase the risk for severe complications requiring treatment.
The ASA class was referenced 4 times as a risk factor for readmission after TKA (Table 3). ASA class 1 is indicative of a normal, healthy patient, and ASA class 2 is indicative of a patient with mild systemic disease [53]. Patients are classified as ASA class 3 if they have a severe systemic disease that is not life-threatening and class 4 if they have a severe systemic disease that is a constant threat to life. The studies in our review identified ASA classes 3 and 4 as significant risk factors for readmission when compared with ASA classes 1 and 2 [26,27,41,42]. Patients categorized as ASA class 5 require surgery to survive, such as in cases of ruptured abdominal aortic aneurysm or intracranial hemorrhage with mass effect; similarly, patients categorized as ASA class 6 are brain dead and undergoing surgery for organ donation [53]. TKA, being an elective surgery with the goal of improving quality of life, is not typically performed on patients considered ASA class 5 or 6, so these were likely not included for the analysis. ASA classes 3 and 4 are risk factors for readmission after TKA when compared with classes 1 and 2 because patients with severe systemic disease have a higher likelihood of presenting with exacerbations of their systemic disease or further complications.
Discharge disposition was cited as a risk factor for readmission by 3 studies. However, the studies in our review had differing results when it came to which discharge dispositions were considered as risk factors (Table 3) [23,28,37]. Swenson et al. found that discharge to nonrehabilitation centers, including discharge on home health support, was associated with an increased risk of readmission when compared with not being discharged on home health [23]. In contrast, Zmistowski et al. and Schairer et al. found that discharge to home was associated with a decreased risk of readmission when compared with discharge to an inpatient rehabilitation facility or skilled nursing facility [28,37]. One hypothesis is that these differences in outcomes could be attributed to the individualized circumstances related to discharge disposition. Some patients are discharged to an inpatient rehabilitation facility if they have multiple comorbidities that need to be comanaged in the presence of insufficient familial support, in which case these patients have a higher baseline risk of readmission. Some patients operate best in the familiarity of their homes, so having a structured routine could improve outcomes.
Clinical implications
There are several risk factors highlighted in our analysis that can be addressed to improve future clinical outcomes: age, diabetes, hypertension, ASA class, and coagulopathy. Age is a nonmodifiable risk factor for readmission that could still have clinical importance through the lens of decreasing readmission rates. Although elderly patients between the age of 65 and 84 years are the primary age group for elective TKA [54], risk for readmission after TKA increases with increasing age. Surgeons have been and should continue to be wary of age when discussing eligibility for surgery. Diabetes is a modifiable risk factor that could be managed with diet and medication. Previous studies have shown that well-managed diabetes decreases the risk for readmission after noncritical care admissions [55]. We expect that extrapolating this result to TKA would have similar results. Similarly, hypertension is also a modifiable risk factor that could be managed with diet and medication. Belmont et al. found that adequate management of hypertension decreased the risk for readmission after TKA [29]. Adequate management of hypertension by collaborating with primary care before surgery should help alleviate this risk factor. Similarly, well-managed comorbidities by the primary care team would result in lower ASA class assignment before surgery, thereby reducing the likelihood of post-TKA readmission. Coagulopathy is a modifiable risk factor that can predispose patients to postoperative complications such as hemarthrosis, hematoma, or wound drainage [52]. Adequate control of coagulopathy in patients before and after TKA may improve patient outcomes and reduce risk of readmission.
Limitations
Despite adhering to a rigorous process and applying multiauthor vetting for the article selection process, there is a possibility of selection bias in our review. We may have missed some studies written in languages other than English without known available translations. Because we focused on primary TKA cohort studies in our review, some additional nuances related to revision arthroplasties and knee fracture cohorts are missing in our analyses. As all the studies in our review are single-arm retrospective observational cohorts, there is inherent systemic bias in their results, despite the evidence for many new and motivating risk factors. Our review is also limited by the poor adherence to standard TRIPOD development and reporting criteria and a lack of use of widely accepted procedures such as testing the models with external or bootstrapped validation cohorts. Indeed, even with partial credit being awarded to several criteria during evaluation, the average TRIPOD score was 69% (Electronic Supplementary Table 3). Because the TRIPOD statement is intended to be a checklist for the essential components for transparent reporting, studies should ideally have a TRIPOD score closer to 100%. However, the studies in our review scored from 56% to 83%, with a median of 71%. The interquartile range of 65% to 74% indicates that most studies either failed the TRIPOD evaluation or were borderline passing. It is also worth noting that the TRIPOD standard was published in 2015—therefore, it is possible that authors publishing studies before the 2015-2016 timeframe did not have access to the checklist when reporting study results. Nonetheless, certain aspects of the TRIPOD standard were widely espoused before its publication by precedent standards such as the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies checklist [56] and Critical Appraisal Skills Programme tool [57]. For example, discriminative statistics reporting (C-statistics or area under the curve) was widely promoted by earlier guidelines but was found to be lacking in many of the models covered by this analysis. Authors of predictive model studies should be mindful to conduct reporting based on whatever prevalent standard is available at the time of publication.
Conclusions
Our review of predictive models for the risk of readmission after TKA entailed 29 models from detailed analyses of 25 studies using the TRIPOD quality assessment criteria. Most models were built over the last decade in the United States, either using a facility EHR system or the ACS-NSQIP data sets. Despite 2 validated models showing moderate predictive capabilities, most studies did not use new statistical techniques for building models. Most studies neither measured nor validated the model performance using an external, held-out, or bootstrapped cohort. This review emphasizes that authors of future studies must be mindful of adhering to accepted reporting practices to maximize clinical utility of predictive models, especially given that clinical applicability is not recommended until these steps have been taken into account. Some comorbidities—coagulopathies, hypertension, heart disease, and diabetes—were found to be risk factors with strong support for the risk of post-TKA readmission. In particular, coagulopathy, ASA class, and discharge disposition were not only mentioned in previous systematic reviews but also found to be strong risk factors for readmission. The clinical ramifications of these findings should be noted and acted upon by care teams in practice.
Conflict of interest
The authors declare there are no conflicts of interest.
Supplementary data
Appendix
Electronic Supplementary Table 1.
PRISMA-P checklist for systematic review of studies for readmissions for TKA.
| Section and topic | Item No | Checklist item | (Page no.#) |
|---|---|---|---|
| Administrative information | |||
| Title | |||
| Identification | 1a | Identify the report as a protocol of a systematic review | 1 |
| Update | 1b | If the protocol is for an update of a previous systematic review, identify as such | N/A |
| Registration | 2 | If registered, provide the name of the registry (such as PROSPERO) and registration number | 5 |
| Authors | |||
| Contact | 3a | Provide the name, institutional affiliation, e-mail address of all protocol authors; provide the physical mailing address of the corresponding author | Title page |
| Contributions | 3b | Describe contributions of protocol authors and identify the guarantor of the review | N/A |
| Amendments | 4 | If the protocol represents an amendment of a previously completed or published protocol, identify as such and list changes; otherwise, state the plan for documenting important protocol amendments | N/A |
| Support | |||
| Sources | 5a | Indicate sources of financial or other support for the review | Title page |
| Sponsor | 5b | Provide name for the review funder and/or sponsor | |
| Role of the sponsor or funder | 5c | Describe roles of funder(s), sponsor(s), and/or institution(s), if any, in developing the protocol | |
| Introduction | |||
| Rationale | 6 | Describe the rationale for the review in the context of what is already known | 3-4 |
| Objectives | 7 | Provide an explicit statement of the question(s) the review will address with reference to participants, interventions, comparators, and outcomes (PICO) | 3-4 |
| Methods | |||
| Eligibility criteria | 8 | Specify the study characteristics (such as PICO, study design, setting, time frame) and report characteristics (such as years considered, language, publication status) to be used as criteria for eligibility for the review | 6-7 |
| Information sources | 9 | Describe all intended information sources (such as electronic databases, contact with study authors, trial registers, or other gray literature sources) with planned dates of coverage | 5-6 |
| Search strategy | 10 | Present draft of search strategy to be used for at least one electronic database, including planned limits, such that it could be repeated | 5-6 |
| Study records | |||
| Data management | 11a | Describe the mechanism(s) that will be used to manage records and data throughout the review | 8-9 |
| Selection process | 11b | State the process that will be used for selecting studies (such as 2 independent reviewers) through each phase of the review (ie, screening, eligibility, and inclusion in meta-analysis) | 8-9 |
| Data collection process | 11c | Describe planned method of extracting data from reports (such as piloting forms, done independently, in duplicate), any processes for obtaining and confirming data from investigators | 8-9 |
| Data items | 12 | List and define all variables for which data will be sought (such as PICO items, funding sources), any preplanned data assumptions and simplifications | 8-9 |
| Outcomes and prioritization | 13 | List and define all outcomes for which data will be sought, including prioritization of main and additional outcomes, with rationale | Table 1, Table 2, Table 3 |
| Risk of bias in individual studies | 14 | Describe anticipated methods for assessing risk of bias of individual studies, including whether this will be performed at the outcome or study level, or both; state how this information will be used in data synthesis | ESM 2 |
| Data synthesis | 15a | Describe criteria under which study data will be quantitatively synthesized | ESM 3 |
| 15b | If data are appropriate for quantitative synthesis, describe planned summary measures, methods of handling data, and methods of combining data from studies, including any planned exploration of consistency (such as I2, Kendall’s τ) | ||
| 15c | Describe any proposed additional analyses (such as sensitivity or subgroup analyses, meta-regression) | ||
| 15d | If quantitative synthesis is not appropriate, describe the type of summary planned | ||
| Meta-bias(es) | 16 | Specify any planned assessment of meta-bias(es) (such as publication bias across studies, selective reporting within studies) | N/A |
| Confidence in cumulative evidence | 17 | Describe how the strength of the body of evidence will be assessed (such as GRADE) | ESM 2 |
PRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol [14].
Electronic Supplementary Table 2.
TRIPOD checklist for readmission risk models for TKA.
| Section/Topic | Item | Checklist item | Page | |
|---|---|---|---|---|
| Title and abstract | ||||
| Title | 1 | D;V | Identify the study as developing and/or validating a multivariable prediction model, the target population, and the outcome to be predicted. | 1 |
| Abstract | 2 | D;V | Provide a summary of objectives, study design, setting, participants, sample size, predictors, outcome, statistical analysis, results, and conclusions. | 3-4 |
| Introduction | ||||
| Background and objectives | 3a | D;V | Explain the medical context (including whether diagnostic or prognostic) and rationale for developing or validating the multivariable prediction model, including references to existing models. | 5-6 |
| 3b | D;V | Specify the objectives, including whether the study describes the development or validation of the model or both. | 6-7 | |
| Methods | ||||
| Source of data | 4a | D;V | Describe the study design or source of data (eg, randomized trial, cohort, or registry data), separately for the development and validation data sets, if applicable. | 7-8 |
| 4b | D;V | Specify the key study dates, including the start of accrual; the end of accrual; and, if applicable, end of the follow-up. | 7 | |
| Participants | 5a | D;V | Specify key elements of the study setting (eg, primary care, secondary care, general population) including number and location of centers. | 7 |
| 5b | D;V | Describe eligibility criteria for participants. | 7-8 | |
| 5c | D;V | Give details of treatments received, if relevant. | n/a | |
| Outcome | 6a | D;V | Clearly define the outcome that is predicted by the prediction model, including how and when assessed. | 9 |
| 6b | D;V | Report any actions to blind assessment of the outcome to be predicted. | 8 | |
| Predictors | 7a | D;V | Clearly define all predictors used in developing the multivariable prediction model, including how and when they were measured. | 8 |
| 7b | D;V | Report any actions to blind assessment of predictors for the outcome and other predictors. | 8 | |
| Sample size | 8 | D;V | Explain how the study size was arrived at. | 9 |
| Missing data | 9 | D;V | Describe how missing data were handled (eg, complete-case analysis, single imputation, multiple imputation) with details of any imputation method. | 11 |
| Statistical analysis methods | 10a | D | Describe how predictors were handled in the analyses. | 10 |
| 10b | D | Specify type of model, all model-building procedures (including any predictor selection), and the method for internal validation. | 10-12 | |
| 10c | V | For validation, describe how the predictions were calculated. | n/a | |
| 10d | D;V | Specify all measures used to assess model performance and, if relevant, to compare multiple models. | 12-13 | |
| 10e | V | Describe any model updating (eg, recalibration) arising from the validation, if performed. | n/a | |
| Risk groups | 11 | D;V | Provide details on how risk groups were created, if performed. | 12-13 |
| Development vs validation | 12 | V | For validation, identify any differences from the development data in setting, eligibility criteria, outcome, and predictors. | n/a |
| Results | ||||
| Participants | 13a | D;V | Describe the flow of participants through the study, including the number of participants with and without the outcome and, if applicable, a summary of the follow-up time. A diagram may be helpful. | 13 |
| 13b | D;V | Describe the characteristics of the participants (basic demographics, clinical features, available predictors), including the number of participants with missing data for predictors and outcome. | 13-14 | |
| 13c | V | For validation, show a comparison with the development data of the distribution of important variables (demographics, predictors, and outcome). | n/a | |
| Model development | 14a | D | Specify the number of participants and outcome events in each analysis. | 14 |
| 14b | D | If performed, report the unadjusted association between each candidate predictor and outcome. | Table 2 (15) | |
| Model specification | 15a | D | Present the full prediction model to allow predictions for individuals (ie, all regression coefficients, and model intercept or baseline survival at a given time point). | 16-17 (Table 3) |
| 15b | D | Explain how to use the prediction model. | 16-18 | |
| Model performance | 16 | D;V | Report performance measures (with CIs) for the prediction model. | 18-19 |
| Model updating | 17 | V | If performed, report the results from any model updating (ie, model specification, model performance). | n/a |
| Discussion | ||||
| Limitations | 18 | D;V | Discuss any limitations of the study (such as nonrepresentative sample, few events per predictor, missing data). | 22-23 |
| Interpretation | 19a | V | For validation, discuss the results with reference to performance in the development data and any other validation data. | n/a |
| 19b | D;V | Give an overall interpretation of the results, consider objectives, limitations, results from similar studies, and other relevant evidence. | 19-22 | |
| Implications | 20 | D;V | Discuss the potential clinical use of the model and implications for future research. | 20-22 |
| Other information | ||||
| Supplementary information | 21 | D;V | Provide information about the availability of supplementary resources, such as study protocol, Web calculator, and data sets. | 10 |
| Funding | 22 | D;V | Give the source of funding and the role of the funders for the present study. | 7, 24 |
TRIPOD checklist: Prediction model development and validation.
Items relevant only to the development of a prediction model are denoted by D, items relating solely to a validation of a prediction model are denoted by V, and items relating to both are denoted D;V. We recommend using the TRIPOD Checklist in conjunction with the TRIPOD Explanation and Elaboration document.
Electronic Supplementary Table 3.
TRIPOD scoring with partial scores.
| Study | Title | Abstract | Background and objectives | Source of data | Participants | Outcome | Predictors | Sample size | Missing data | Statistical analysis methods | Risk groups | Development vs validation | Participants | Model development | Model specification | Model performance | Model updating | Limitations | Interpretation | Implications | Supplementary information | Funding | Average score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “Solomon, Chibnik et al. 2006” [24] | 0 | 0.9 | 1 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 0.75 | 1 | 0 | 0.66 | 0.5 | 0.9 | 1 | n/a | 1 | 1 | 1 | 0 | 1 | 0.65 | |
| “Higuera, Elsharkawy et al. 2011” [25] | 1 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 0 | 0.75 | 0 | 0 | 0.67 | 0.5 | 0 | 0 | n/a | 1 | 1 | 1 | 1 | 1 | 0.64 | |
| "Cram, Lu et al. 2012" | 0.67 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0.5 | 0.9 | 0 | 0 | 0.66 | 1 | 0.9 | 0 | n/a | 1 | 1 | 1 | 1 | 1 | 0.74 | |
| “Pugely, Callaghan et al. 2013” [26] | 0.67 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.6 | 0 | 1 | 0.67 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 0 | 1 | 0.73 | |
| “Pugely, Martin et al. 2013” [27] | 0.33 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 0.75 | 0 | 0 | 0 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 1 | 1 | 0.71 | |
| "Mnatzaganian, Ryan et al. 2014" | 0 | 1 | 1 | 1 | 1 | 0.5 | 0.33 | 1 | 0 | 1 | 0 | 0 | 0.66 | 0.5 | 1 | 0 | n/a | 1 | 1 | 1 | 1 | 1 | 0.67 | |
| “Schairer, Vail et al. 2014” [28] | 0 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.75 | 0 | 0 | 0.66 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 0 | 0 | 0.56 | |
| “Belmont, Goodman et al. 2016” [29] | 0.67 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.75 | 0 | 0 | 0.66 | 0.5 | 0 | 0 | n/a | 1 | 1 | 1 | 1 | 0 | 0.65 | |
| “Feng, Lin et al. 2017” [30] | 0.33 | 1 | 0.84 | 1 | 0.67 | 0.5 | 0.5 | 1 | 1 | 1 | n/a | n/a | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | n/a | 1 | 0.82 | |
| “Lee, Lumar, & Kim. 2017” [31] | 0.67 | 0.9 | 1 | 1 | 0.67 | 0.5 | 0.5 | 1 | 0 | 1 | n/a | n/a | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | n/a | 0 | 0.74 | |
| “Siracuse, Ippolito et al. 2017” [32] | 0 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.75 | 1 | 1 | 1 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 0 | 1 | 0.72 | |
| “Kimball, Nicholas et al. 2018” [33] | 0.33 | 1 | 1 | 1 | 0.67 | 0.5 | 0.5 | 1 | 1 | 1 | n/a | n/a | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | n/a | 1 | 0.83 | |
| “Lehtonen, Hess et al. 2018” [34] | 0.33 | 1 | 1 | 1 | 0.67 | 0.5 | 0.5 | 1 | 1 | 0.75 | n/a | n/a | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | n/a | 0 | 0.76 | |
| “Saku, Madanat et al., 2018” [35] | 0.67 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 0 | 0.75 | 0 | 0 | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | 0 | 1 | 0.71 | |
| “Urish, Qin, et al., 2018” [11] | 0.33 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.75 | n/a | 0 | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | 0 | 1 | 0.70 | |
| “Yohe, Funk et al. 2018” [36] | 0.67 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.75 | 0 | 0 | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | 0 | 1 | 0.69 | |
| “Ali, Loeffler et al. 2019” [10] | 0.33 | 0.9 | 1 | 1 | 0.67 | 0.5 | 0.5 | 1 | 0 | 1 | n/a | n/a | 1 | 1 | 1 | 1 | n/a | 1 | 1 | 1 | n/a | 0 | 0.77 | |
| “Zmistowski, Retrepo et al. 2013” [37] | 0 | 0.8 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.75 | 0 | 0 | 0 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 0 | 1 | 0.62 | |
| “Mesko, Bachmann et al. 2014” [38] | 1 | 0.9 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.75 | 0 | 1 | 1 | 0.5 | 1 | 0 | n/a | 1 | 1 | 1 | 0 | 1 | 0.72 | |
| “Tiberi, Hansen et al. 2014” [39] | 0.33 | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 1 | 0 | 0.75 | 0 | 0 | 0.67 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 0 | 1 | 0.60 | |
| “Ricciardi, Oi et al. 2017” [40] | 0.33 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.75 | 0 | 0 | 0.67 | 0.5 | 0.9 | 0 | n/a | 1 | 1 | 1 | 0 | 0 | 0.58 | |
| “Sher, Keswani et al. 2017” [41] | 0.33 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 1 | 0 | 0 | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | 0 | 1 | 0.68 | |
| "Swensen, Bastian et al. 2018" | 0.33 | 0.8 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 0.75 | 0 | 0 | 1 | 0.5 | 1 | 1 | n/a | 1 | 1 | 1 | 1 | 0 | 0.76 | |
| “Yao, Keswani et al. 2017” [42] | 0.67 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.75 | 1 | 0 | 1 | 0.5 | 0.5 | 1 | n/a | 1 | 1 | 1 | 0 | 0 | 0.73 | |
| “Schroer, Diesfield et al. 2018” [43] | 0.33 | 0.9 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0.33 | 1 | 0 | 1 | 0.5 | 0 | 0 | n/a | 1 | 1 | 1 | 0 | 0 | 0.57 |
References
- 1.Chambers M.C., Ei-Othmani M.M., Saleh K.J. Health care reform impact on total joint replacement. Orthop Clin North Am. 2016;47(4):645. doi: 10.1016/j.ocl.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim A.M., Nathan H., Thumma J.R., Dimick J.B. Impact of the hospital readmission reduction program on surgical readmissions among Medicare beneficiaries. Ann Surg. 2017;27:27. doi: 10.1097/SLA.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karas V., Kildow B.J., Baumgartner B.T. Preoperative patient profile in total hip and knee arthroplasty: predictive of increased Medicare payments in a bundled payment model. J Arthroplasty. 2018;33(9):2728. doi: 10.1016/j.arth.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz S.M., Lau E.C., Ong K.L., Adler E.M., Kolisek F.R., Manley M.T. Which clinical and patient factors influence the national economic burden of hospital readmissions after total joint arthroplasty? Clin Orthop Relat Res. 2017;475(12):2926. doi: 10.1007/s11999-017-5244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers M.C. Reducing 30-day readmission after joint replacement. Orthop Clin North Am. 2016;47(4):673. doi: 10.1016/j.ocl.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Levin J.M., Khlopas A., Sodhi N. The association between readmission and patient experience in a total hip arthroplasty population. J Arthroplasty. 2018;33(6):1668. doi: 10.1016/j.arth.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Ellimoottil C., Ryan A.M., Hou H., Dupree J.M., Hallstrom B., Miller D.C. Implications of the definition of an episode of care used in the comprehensive care for joint replacement model. JAMA Surg. 2017;152:49. doi: 10.1001/jamasurg.2016.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudali T., Robinson R., Bhattarai M. Reducing 30-day rehospitalization rates using a transition of care clinic model in a single medical center. Adv Med. 2017;2017:5132536. doi: 10.1155/2017/5132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhaegh K.J., MacNeil-Vroomen J.L., Eslami S., Geerlings S.E., de Rooij S.E., Buurman B.M. Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff. 2014;33(9):1531. doi: 10.1377/hlthaff.2014.0160. [DOI] [PubMed] [Google Scholar]
- 10.Ali A.M., Loeffler M.D., Aylin P., Bottle A. Predictors of 30-day readmission after total knee arthroplasty: analysis of 566,323 procedures in the United Kingdom. J Arthroplasty. 2019;34(2):242. doi: 10.1016/j.arth.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Urish K.L., Qin Y., Li B.Y. Predictors and cost of readmission in total knee arthroplasty. J Arthroplasty. 2018;33(9):2759. doi: 10.1016/j.arth.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamseer L., Moher D., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 15.Dissemination, C.f.R.a. PROSPERO: international prospective register of systematic reviews. 2011. https://www.crd.york.ac.uk/prospero/
- 16.Moons K.G., Altman D.G., Reitsma J.B. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 17.Hofstede S.N., Gademan M.G., Vlieland T.P.V., Nelissen R.G., Marang-van de Mheen P.J. Preoperative predictors for outcomes after total hip replacement in patients with osteoarthritis: a systematic review. BMC Musculoskelet Disord. 2016;17:212. doi: 10.1186/s12891-016-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podmore B., Hutchings A., van der Meulen J., Aggarwal A., Konan S. Impact of comorbid conditions on outcomes of hip and knee replacement surgery: a systematic review and meta-analysis. BMJ Open. 2018;8(7):e021784. doi: 10.1136/bmjopen-2018-021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero J.A., Jones R.E., Brown T. Modifiable risk factors and preoperative optimization of the primary total arthroplasty patient. Curr Orthop Pract. 2017;28(3):272. [Google Scholar]
- 20.Sveom D.S., Otteman M.K., Garvin K.L. Improving quality and decreasing cost by reducing re-admissions in patients undergoing total joint arthroplasty. Curr Rev Musculoskelet Med. 2017;10(3):388. doi: 10.1007/s12178-017-9424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cram P., Lu X., Kates S.L., Singh J.A., Li Y., Wolf B.R. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mnatzaganian G., Ryan P., Hiller J.E. Does co-morbidity provide significant improvement on age adjustment when predicting medical outcomes? Methods Inf Med. 2014;53(2):115. doi: 10.3414/ME13-01-0095. [DOI] [PubMed] [Google Scholar]
- 23.Swenson E.R., Bastian N.D., Nembhard H.B., Davis C.M., III Reducing cost drivers in total joint arthroplasty: understanding patient readmission risk and supply cost. Health Syst. 2018;7(2):135. doi: 10.1080/20476965.2017.1397237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon D.H., Chibnik L.B., Losina E. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54(5):1536. doi: 10.1002/art.21772. [DOI] [PubMed] [Google Scholar]
- 25.Higuera C.A., Elsharkawy K., Klika A.K., Brocone M., Barsoum W.K. 2010 Mid-America Orthopaedic Association Physician in Training Award: predictors of early adverse outcomes after knee and hip arthroplasty in geriatric patients. Clin Orthop Relat Res. 2011;469(5):1391. doi: 10.1007/s11999-011-1804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugely A.J., Callaghan J.J., Martin C.T., Cram P., Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499. doi: 10.1016/j.arth.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Pugely A.J., Martin C.T., Gao Y., Mendoza-Lattes S., Callaghan J.J. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193. doi: 10.2106/JBJS.K.01682. [DOI] [PubMed] [Google Scholar]
- 28.Schairer W.W., Vail T.P., Bozic K.J. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181. doi: 10.1007/s11999-013-3030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belmont P.J., Jr., Goodman G.P., Rodriguez M., Bader J.O., Waterman B.R., Schoenfeld A.J. Predictors of hospital readmission following revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3329. doi: 10.1007/s00167-015-3782-6. [DOI] [PubMed] [Google Scholar]
- 30.Feng B., Lin J., Jin J., Qian W.W., Wang W., Weng X.S. Thirty-day postoperative complications following primary total knee arthroplasty: a retrospective study of incidence and risk factors at a single center in China. Chin Med J. 2017;130(21):2551. doi: 10.4103/0366-6999.213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.W., Kumar G.N.K., Kim T.K. Unplanned readmissions after primary total knee arthroplasty in Korean patients: rate, causes, and risk factors. Knee. 2017;24(3):670. doi: 10.1016/j.knee.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Siracuse B.L., Ippolito J.A., Gibson P.D., Ohman-Strickland P.A., Beebe K.S. A preoperative scale for determining surgical readmission risk after total knee arthroplasty. J Bone Joint Surg Am. 2017;99(21):e112. doi: 10.2106/JBJS.16.01043. [DOI] [PubMed] [Google Scholar]
- 33.Kimball C.C., Nichols C.I., Nunley R.M., Vose J.G., Stambough J.B. Skilled nursing facility star rating, patient outcomes, and readmission risk after total joint arthroplasty. J Arthroplasty. 2018;33(10):3130. doi: 10.1016/j.arth.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Lehtonen E.J., Hess M.C., McGwin G., Jr, Shah A., Godoy-Santos A.L., Naranje S. Risk factors for early hospital readmission following total knee arthroplasty. Acta Ortop Bras. 2018;26(5):309. doi: 10.1590/1413-785220182605190790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saku S.A., Madanat R., Makinen T.J. Reasons and risk factors for ninety day re-admission following primary total knee arthroplasty in a high-volume centre. Int Orthop. 2018;42(1):95. doi: 10.1007/s00264-017-3676-y. [DOI] [PubMed] [Google Scholar]
- 36.Yohe N., Funk A., Ciminero M., Erez O., Saleh A. Complications and readmissions after total knee replacement in octogenarians and nonagenarians. Geriatr Orthop Surg Rehabil. 2018;9 doi: 10.1177/2151459318804113. 2151459318804113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zmistowski B., Restrepo C., Hess J., Adibi D., Cangoz S., Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869. doi: 10.2106/JBJS.L.00679. [DOI] [PubMed] [Google Scholar]
- 38.Mesko N.W., Bachmann K.R., Kovacevic D., LoGrasso M.E., O’Rourke C., Froimson M.I. Thirty-day readmission following total hip and knee arthroplasty - a preliminary single institution predictive model. J Arthroplasty. 2014;29(8):1532. doi: 10.1016/j.arth.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Tiberi J.V., 3rd, Hansen V., El-Abbadi N., Bedair H. Increased complication rates after hip and knee arthroplasty in patients with cirrhosis of the liver. Clin Orthop Relat Res. 2014;472(9):2774. doi: 10.1007/s11999-014-3681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricciardi B.F., Oi K.K., Daines S.B., Lee Y.Y., Joseph A.D., Westrich G.H. Patient and perioperative variables affecting 30-day readmission for surgical complications after hip and knee arthroplasties: a matched cohort study. J Arthroplasty. 2017;32(4):1074. doi: 10.1016/j.arth.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Sher A., Oi K.K., Daines S.B., Lee Y.Y., Joseph A.D., Westrich G.H. Predictors of same-day discharge in primary total joint arthroplasty patients and risk factors for post-discharge complications. J Arthroplasty. 2017;32(9):S150. doi: 10.1016/j.arth.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Yao D.H., Keswani A., Shah C.K., Sher A., Koenig K.M., Moucha C.S. Home discharge after primary elective total joint arthroplasty: postdischarge complication timing and risk factor Analysis. J Arthroplasty. 2017;32(2):375. doi: 10.1016/j.arth.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Schroer W.C., Diesfeld P.J., LeMarr A.R., Morton D.J., Reedy M.E. Modifiable risk factors in primary joint arthroplasty increase 90-day cost of care. J Arthroplasty. 2018;33(9):2740. doi: 10.1016/j.arth.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Schairer W.W., Sing D.C., Vail T.P., Bozic K.J. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464. doi: 10.1007/s11999-013-3121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divo M.J., Martinez C.H., Mannino D.M. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44(4):1055. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on sex and science. EMBO Rep. 2012;13(7):596. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuwabara K., Imanaka Y., Matsuda S. The association of the number of comorbidities and complications with length of stay, hospital mortality and LOS high outlier, based on administrative data. Environ Health Prev Med. 2008;13(3):130. doi: 10.1007/s12199-007-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munoz E., Rosner F., Friedman R., Sterman H., Goldstein J., Wise L. Financial risk, hospital cost, and complications and comorbidities in medical non-complications and comorbidity-stratified diagnosis-related groups. Am J Med. 1988;84(5):933. doi: 10.1016/0002-9343(88)90074-5. [DOI] [PubMed] [Google Scholar]
- 49.Iorio R., Osmani F.A. Strategies to prevent periprosthetic joint infection after total knee arthroplasty and lessen the risk of readmission for the patient. J Am Acad Orthop Surg. 2017;25:S13. doi: 10.5435/JAAOS-D-16-00635. [DOI] [PubMed] [Google Scholar]
- 50.Brem H., Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silverstein P. Smoking and wound healing. Am J Med. 1992;93(1a):22s. doi: 10.1016/0002-9343(92)90623-j. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg A.G. Recurrent haemarthrosis: raining on your parade. Orthop Proc. 2018;100-B(SUPP_10):103. 103. [Google Scholar]
- 53.Doyle D.J., Garmon E.H. StatPearls Publishing LLC; Treasure Island, FL: 2019. American Society of Anesthesiologists Classification (ASA class), in StatPearls. [PubMed] [Google Scholar]
- 54.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997-2004. Arthritis Rheum. 2008;59(4):481. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 55.Bansal V., Mottalib A., Pawar T.K. Inpatient diabetes management by specialized diabetes team versus primary service team in non-critical care units: impact on 30-day readmission rate and hospital cost. BMJ Open Diabetes Res Care. 2018;6(1):e000460. doi: 10.1136/bmjdrc-2017-000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moons K.G., de Groot J.A., Bouwmeester W. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744. doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.2006-2018. Critical Appraisal Skills Programme Clinical Prediction Rule Checklist. UK: CASP; 2006. [Accessed 26 June 2019] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.