Highlights
-
•
ICP0 is a viral E3 ubiquitin ligase that promotes HSV-1 infection.
-
•
ICP0 interacts with multiple component proteins of the ubiquitin pathway.
-
•
ICP0 disrupts multiple cellular processes activated in response to infection
-
•
ICP0 remodels the SUMO proteome to counteract host immune defences to infection.
-
•
ICP0 is an attractive drug target for the development of antiviral HSV-1 therapeutics.
Abbreviations: ATM, Ataxia Telangiectasia-Mutated; ATRX, Alpha Thalassemia/mental Retardation syndrome X-linked; CENP, CENtromere Protein; ChIP, Chromatin Immuno Precipitation; Daxx, Death domain Associated protein; ΔICP0, null mutant ICP0 from HSV-1; FHA, ForkHead Associated; HAUSP, Herpesvirus-Associated Ubiquitin-Specific Protease; HIRA, HIstone cell cycle Regulator defective homologue A; IFI16, IFN-γ Inducible protein 16; IFN, InterFeroN; IRF3, Interferon Regulatory Factor 3; MDC1, Mediator of DNA damage Checkpoint 1; MRE11, Meiotic Recombination 11 Homolog 1; NBS1, Nijmegen Breakage Syndrome 1 (Nibrin); NF-κB, Nuclear Factor Kappa B; NLS, nuclear localization Signal; PIAS, Protein Inhibitor of Activated STAT; PML-NBs, ProMyelocytic Leukaemia Nuclear Bodies; PTM, Post-Translational Modification; RING, Really Interesting New Gene; Sp100, Speckled 100 kDa; STAT, Signal Transducer and Activator of Transcription; STING, STimulator of IFN Genes; STUbL, SUMO-Targeted Ubiquitin Ligase; SUMO, Small Ubiquitin MOdifier; vDNA, viral DNA; TERRA, TElomere Repeat-containing RNA; TPP1, TriPeptidyl Peptidase 1; TP53BP1, Tumor Protein P53 Binding Protein 1
Keywords: HSV-1, ICP0, Ubiquitin, PML-NBs, Chromatin, Immunity
Abstract
Herpes simplex virus 1 (HSV-1) hijacks ubiquitination machinery to modify the cellular proteome to create an environment permissive for virus replication. HSV-1 encodes its own RING-finger E3 ubiquitin (Ub) ligase, Infected Cell Protein 0 (ICP0), that directly interfaces with component proteins of the Ub pathway to inactivate host immune defences and cellular processes that restrict the progression of HSV-1 infection. Consequently, ICP0 plays a critical role in the infectious cycle of HSV-1 that is required to promote the efficient onset of lytic infection and productive reactivation of viral genomes from latency. This review will describe the current knowledge regarding the biochemical properties and known substrates of ICP0 during HSV-1 infection. We will highlight the gaps in the characterization of ICP0 function and propose future areas of research required to understand fully the biological properties of this important HSV-1 regulatory protein.
1. Why are herpesviruses important?
Herpesviruses are ubiquitous viral pathogens that cause a variety of clinically important diseases on a global scale, ranging from mild skin sores and rashes to blindness, congenital birth defects, cancer, and encephalitis (Knipe and Howley, 2013). The reason for their prevalence and evolutionary success is attributable to their ability to enter into a latent state of infection that is maintained for the duration of the host’s lifespan. This latent reservoir of virus evades immune clearance, which can periodically reactivate leading to recurrent disease and transmission to new hosts. Thus, understanding the cellular processes that regulate lytic and latent infection is essential to managing and treating the clinical conditions they cause. Such studies also provide fundamental insight into the molecular mechanisms employed by viruses to evade host immune defences that influence the outcome of infection, offering new opportunities for therapeutic intervention.
2. HSV-1 interacts with and hijacks the host ubiquitin machinery
Like many viruses, herpesviruses hijack component proteins of the host ubiquitin (Ub) machinery to subvert cellular processes in order to establish a conducive environment for replication (Isaacson and Ploegh, 2009; Luo, 2016). During HSV-1 infection, these events are largely driven by ICP0, a RING-finger E3 Ub ligase expressed from the outset of nuclear infection (Boutell et al., 2002; Hagglund et al., 2002). While ICP0 is classified as a non-essential viral gene product (Stow and Stow, 1986; Sacks and Schaffer, 1987; Yao and Schaffer, 1995; Chen and Silverstein, 1992), research has established ICP0 to play an important role in modulating the intracellular environment to promote the successful onset of lytic infection and productive reactivation of viral genomes from latency (Stow and Stow, 1986; Sacks and Schaffer, 1987; Yao and Schaffer, 1995; Chen and Silverstein, 1992; Everett et al., 2004; Leib et al., 1989; Halford and Schaffer, 2001; Halford et al., 2001; Thompson and Sawtell, 2006; Cai et al., 1993). Importantly, the requirement for ICP0 during HSV-1 infection is cell type dependent, with many carcinoma cell lines being permissive to HSV-1 ΔICP0 mutant infection relative to normal diploid cells (Stow and Stow, 1986; Yao and Schaffer, 1995; Everett et al., 2004; Alandijany et al., 2018). With respect to osteosarcoma (U2OS and SAOS) cells, permissivity correlates with a defect in the recruitment of key antiviral host factors to infecting vDNA from the outset of nuclear infection (discussed below; (Yao and Schaffer, 1995; Alandijany et al., 2018)). Such differences highlight the restrictive nature of the intrinsic (pre-existing) proteome to the initiation of HSV-1 replication under low MOI conditions that can vary between infected cells (Drayman et al., 2017; Drayman et al., 2019; Cohen and Kobiler, 2016). Thus, understanding the biochemical and biological properties of ICP0 provides valuable insight into host factors and cellular processes which influence the restriction of many viral pathogens.
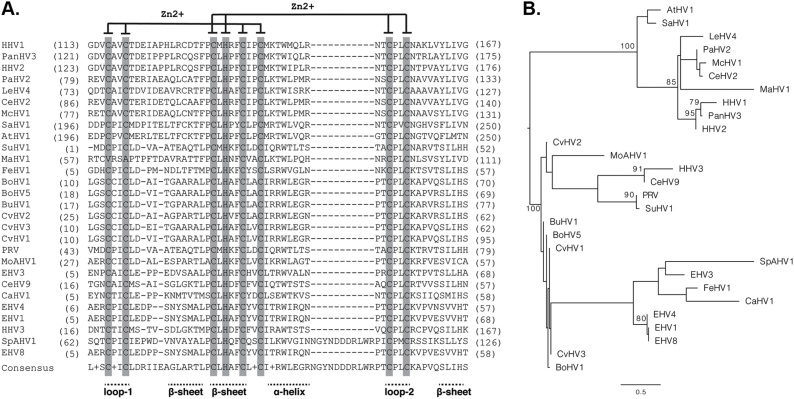
Ubiquitination of proteins occurs in a sequential cascade consisting of E1 (Ub activating, UBE1), E2 (Ub conjugating, UBE2), and E3 (Ub ligating) enzymes (Pohl and Dikic, 2019). The Ub ligase activity of ICP0 is entirely attributable to its N-terminal C3HC4 Zn2+-binding RING-finger (residues 116-156), a structural domain conserved between α-herpesvirus ICP0 orthologues (Fig. 1; (Boutell et al., 2002; Everett et al., 1993; Everett et al., 1995; Everett et al., 2010; Grant et al., 2012; Lium and Silverstein, 1997)). ICP0 interacts directly with UBE2D1-4 (UbcH5a-d) and UBE2E1-3 (UbcH6a-c) Ub conjugating enzymes (7 out of 37 known human UBE2 proteins; (Boutell et al., 2002; Gu and Roizman, 2003; Vanni et al., 2012; Michelle et al., 2009)) via amino acid (aa) residues located on loop-1, loop-2, and α-helix of its RING-finger domain (Fig. 1, Fig. 2; (Vanni et al., 2012)). ICP0 facilitates the transfer of Ub from charged UBE2 enzymes onto lysine (K) residues within target-substrate(s) with which it interacts (Everett, 2000; Boutell et al., 2003). As Ub contains seven K residues (K6, K11, K27, K29, K33, K48, and K63), anchored Ub molecules can be further ubiquitinated to form linear or branched poly-ubiquitin chains (Ohtake and Tsuchiya, 2017). Proteins conjugated with K48-linked chains are generally considered to be preferential substrates for proteasomal degradation (Braten et al., 2016; Nandi et al., 2006; Chen et al., 2016). UBE2D1-4 and UBE2E1-3 conjugating enzymes are highly conserved among Eukaryotes and known to support the K48-linked poly-ubiquitination of a wide range of substrates (Michelle et al., 2009). Accordingly, α-herpesvirus ICP0 family members have been shown to share similar biochemical properties in the presence of these UBE2 enzymes (Everett et al., 2010). However, non-primate orthologues fail to complement fully the replication defect of an HSV-1 ΔICP0 mutant, indicative of species-specific mechanisms of Ub ligase substrate targeting ((Everett et al., 1995; Everett et al., 2010); reviewed in (Boutell and Everett, 2013)). Importantly, mutation of ICP0-UBE2 interaction residues inactivate ICP0 function (Grant et al., 2012; Lium and Silverstein, 1997; Vanni et al., 2012), highlighting the importance of these host interactions in the biological lifecycle of HSV-1. As such, ICP0 represents an attractive drug target for the development of antiviral HSV-1 therapeutics (Grant et al., 2012; Deschamps et al., 2019; Boutell and Davido, 2015). It remains to be determined if ICP0 can interact with other UBE2 enzymes that may facilitate the differential ubiquitination of substrates or formation of alternative chain types, for example N-terminal methionine-linked (Met1-linked) or K63-linked poly-ubiquitin chains (Griffiths et al., 2013; Metzger et al., 2014). ICP0 can also interact with a variety of cellular E3 Ub ligases, including RNF8, RNF168, SIAH1, and TRIM27 (Fig. 3) (Lilley et al., 2010; Chaurushiya et al., 2012; Nagel et al., 2011; Conwell et al., 2015). It remains to be examined if these interactions can influence the biochemical properties of ICP0 or extend its repertoire of substrates independently of their respective degradation (discussed below; Fig. 3).
Fig. 1.
The RING-finger domain of ICP0 is conserved between α-herpesvirus orthologues. A) Alignment of α-herpesvirus (α-HV) ICP0 RING finger domains. Amino acid (aa) residues coordinating the two zinc cations (Zn2+) are highlighted in grey. Regions of secondary structure are underlined with horizontal dashes. B) Maximum likelihood phylogeny of the α-herpesvirus RING domain generated using RAxML with the LG amino acid substitution model. The phylogeny is rooted in the middle of the tree (mid-point rooted) and support for the relationships are shown at the nodes of the phylogeny using bootstrap. Only nodes with bootstrap support above 70% are shown. Abbreviation (common name), virus name, protein accession number, RING domain region: HHV1 (HSV-1), Human α-HV 1, YP_009137074.1, 113:167; PanHV3, Chimpanzee herpesvirus strain 105640, YP_009010986.1, 121:175; HHV2 (HSV-2), Human α-HV 2, YP_009137210.1, 123:176; PaHV2, Papiine α-HV 2, YP_443846.2, 79:133; LeHV4, Leporid α-HV 4, YP_009230192.1, 73:127; CeHV2, Cercopithecine α-HV 2, YP_164442.2, 86:140; McHV1, Macacine α-HV 1, NP_851859.2, 77:131; SaHV1, Saimiriine α-HV 1, YP_003933840.1, 196:250; AtHV1, Ateline α-HV 1, YP_009361938.1, 196:250; SuHV1, Suid α-HV 1, YP_068377.2, 1:52; MaHV1, Macropodid α-HV 1, YP_009227214.1, 57:111; FeHV1, Felid α-HV 1, YP_003331582.1, 5:57; BoHV1, Bovine α-HV 1, NP_045363.1, 10:62; BoHV5, Bovine α-HV 5, NP_954951.1, 18:70; BuHV1, Bubaline α-HV 1, YP_0096646811, 17:69; CvHV2, Cervid α-HV 2, AVT50781.1, 25:77; CvHV3, Cervid α-HV 3, AVT50645.1, 10:62; CvHV1, Cervid α-HV 1, AVT50711.1, 10:62; PRV, Pseudorabies virus Ea, AAG17904.1, 43:95; MoAHV1, Beluga whale α-HV 1, ASW27104.1, 27:79; EHV3, Equid α-HV 3, YP_009054966.1, 5:57; CeHV9, Cercopithecine α-HV 9, NP_077475.1, 16:68; CaHV1, Canid α-HV 1, YP_009252287.1, 5:57; EHV4, Equid α-HV 4, NP_045280.1, 6:58; EHV1, Equid α-HV 1, YP_053107.1, 5:57; HHV3 (VZV), Human α-HV 3, NP_040183.1, 16:68; SpAHV1, Sphenicid α-HV 1, YP_009342410.1, 62:126; EHV8, Equid α-HV 8, YP_006273043.1, 5:58.
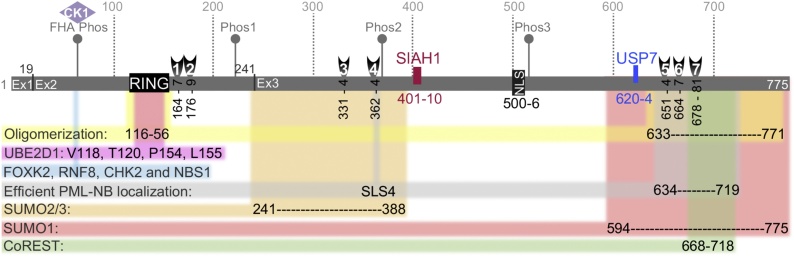
Fig. 2.
ICP0 RING-finger dependent and independent interactions. Schematic representation of the ICP0 ORF amino acid (aa) residues 1-775. Spliced exons (Ex): Ex1, aa 1-19; Ex2, aa 20-241; Ex3, aa 242-775. Ex2 harbours the RING finger domain (black box, aa 116-156) influencing ICP0 oligomerization together with aa 633-771 (highlighted yellow; (Meredith et al., 1995; Ciufo et al., 1994; Mullen et al., 1994)). ICP0 RING-finger residues required for UBE2D1 interaction (highlighted fuchsia (Vanni et al., 2012)). ICP0 phosphorylation motifs are indicated with grey pins: FHA domain (pS64 and 67-pTELF-70; (Chaurushiya et al., 2012)) recruit CK1 (lilac rhombus), FOXK2, and DDR proteins RNF8, CHK2 and NBS1 (highlighted blue; (Chaurushiya et al., 2012)). ICP0 phosphorylation regions Phos1 (S224, T226, T231, T232); Phos2 (S365, S367, S371), and Phos3 (S508, S514, S517, T518) (Davido et al., 2005). SLSs 1-7 (aa 164-167, 176-179, 331-334, 362-363, 651-654, 664-667 and 678-681, respectively) are indicated with black vertical half-moons (Boutell et al., 2011); SLS4 and aa 634-719 influence PML-NB localization (highlighted grey; (Everett et al., 2014)). SIAH binding motif (aa 401-410; (Czechowicz et al., 2018)), a NLS (aa 500-506), and USP7 binding motif (aa 620-624) are indicated with vertical rectangles. ICP0 regions of SUMO2/3 (aa 241-388, highlighted orange) and SUMO1 (aa 594-775, highlighted red) binding shown (Boutell et al., 2011; Everett et al., 2014). CoREST interaction region (aa 668-718, highlighted green; (Gu and Roizman, 2007)).
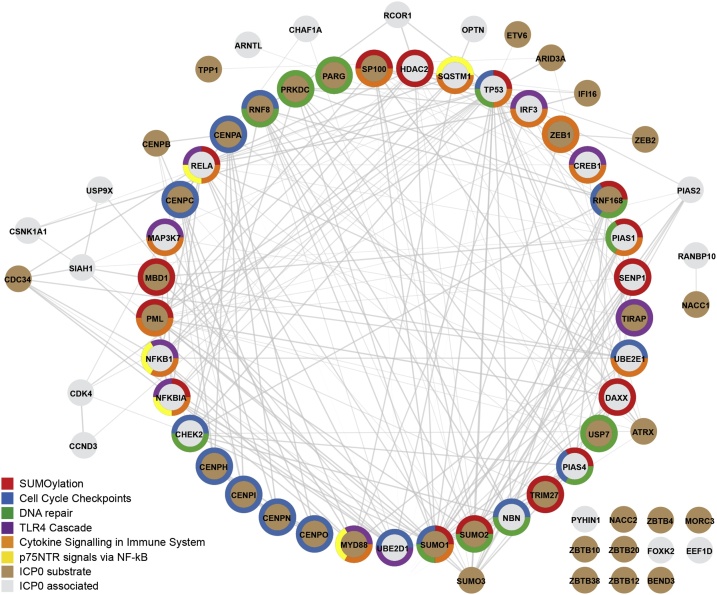
Fig. 3.
ICP0 interacts with a wide variety of overlapping host pathways. Protein interaction network of host proteins reported to associate with (grey inner circles) or to be degraded (brown inner circles) by ICP0 during HSV-1 infection. Proteins identified by manual curation of the literature. Interaction network and functional analysis was generated using STRING (high confidence threshold, 0.7). Pathway annotations based on the Reactome database. Proteins enriched in the top 6 pathways are coloured (Outer circle, as indicated). Proteins that show interconnectivity are highlighted by grey lines.
Ubiquitinated substrates can be recognized by deubiquitinase (DUB) enzymes, which cleave Ub chains from substrates (Mevissen and Komander, 2017). ICP0 can induce its own autoubiquitination leading to its proteasomal degradation (Boutell et al., 2002; Vanni et al., 2012; Canning et al., 2004). ICP0 counteracts this process by recruiting USP7 (previously known as HAUSP (Meredith et al., 1995; Everett et al., 1997); Fig. 2), which cleaves anchored Ub chains from ICP0 leading to its stabilization (Canning et al., 2004; Everett et al., 1999a). Reciprocally, ICP0 can ubiquitinate USP7 leading to its proteasomal degradation in an ICP0 phosphorylation-dependent manner (Boutell et al., 2005; Mostafa et al., 2013). Recent structural studies have solved the interaction interface between ICP0 and USP7 which has been proposed as a potential drug target (Pozhidaeva et al., 2015; Pfoh et al., 2015). It remains to be established whether ICP0’s interaction with USP7 can lead to the stabilization of other ICP0 interaction partners or influence Ub chain editing to promote substrate degradation. ICP0 can also interact with USP9X (Sato et al., 2016), although the biochemical relationship between these two proteins remains to be investigated. The HSV-1 deubiquitinase enzyme UL36 has also been reported to influence ICP0 expression levels during infection (Kattenhorn et al., 2005; Wang et al., 2013), although it remains to be determined if UL36 can catalyse the deubiquitination of ICP0 directly. Collectively, these observations demonstrate ICP0 to interact with multiple component enzymes of the Ub pathway to promote a conducive environment favourable to HSV-1 replication.
3. ICP0-mediated degradation of PML-NBs and SUMOylated host proteins
ICP0 was initially identified as an E3 Ub ligase by virtue of its ability to localize to and disrupt PML-NBs (Everett, 2000; Maul et al., 1993; Maul and Everett, 1994; Everett and Maul, 1994; Everett et al., 1998; Chelbi-Alix and de The, 1999). PML-NBs are highly dynamic nuclear substructures composed of more than 70 proteins that respond to a variety of stimuli, including heat shock, cytokine signalling, and virus infection (Lang et al., 2010; Van Damme et al., 2010; Hoischen et al., 2018; Lang et al., 2019; Bernardi and Pandolfi, 2007; Maul et al., 1995). Infecting HSV-1 genomes are rapidly entrapped by PML-NBs upon nuclear entry (Alandijany et al., 2018; Dembowski and Deluca, 2017), a host response that can lead to viral genome silencing as a component of the intrinsic antiviral immune response (discussed below) (Alandijany et al., 2018; Everett et al., 2006; Everett et al., 2008a; Glass and Everett, 2013; Cabral et al., 2018). PML, the main scaffolding protein of PML-NBs (Ishov et al., 1999), and Sp100 were among the first substrates identified to be degraded by ICP0 in a RING-finger dependent manner (Fig. 3) (Everett et al., 1998; Everett et al., 2009). Consequently, the biological role of PML-NBs during virus infection has been extensively studied, which has revealed these discrete nuclear substructures to play an important function in the spatiotemporal regulation of host immune defences to virus infection (Alandijany et al., 2018; McFarlane et al., 2019; Geoffroy and Chelbi-Alix, 2011).
The post-translational modification of proteins with SUMO plays a key role in the assembly of PML-NBs via a network of protein-protein interactions mediated between constitutively SUMOylated proteins (e.g. PML and Sp100) and resident proteins that contain SUMO Interaction Motifs (SIMs) (Ishov et al., 1999; Muller et al., 1998; Zhong et al., 2000; Shen et al., 2006; Lin et al., 2006). Inhibiting cellular ubiquitination enriches SUMO-modified transcription factors and DNA repair proteins at PML-NBs (Sha et al., 2019), highlighting the dynamic composition of PML-NBs. ICP0 contains seven SIM-Like Sequences (SLSs; Fig. 2) and shares biochemical properties similar to that of cellular SUMO Targeted Ubiquitin Ligases (STUbLs) (Everett et al., 1998; Chelbi-Alix and de The, 1999; Boutell et al., 2003; Boutell et al., 2011; Cuchet-Lourenco et al., 2012; Sloan et al., 2015). ICP0 utilizes a combination of SUMO-dependent and -independent targeting strategies to ubiquitinate and degrade PML isoforms I-VI and Sp100 leading to the disruption of PML-NBs and release of viral genomes entrapped therein (Alandijany et al., 2018; Everett et al., 2009; Boutell et al., 2003; Boutell et al., 2011; Cuchet-Lourenco et al., 2012; Everett et al., 2014). Of note, the structure of ICP0 SLS4 (residues 362-367; Fig. 2) and SUMO has recently been solved by NMR (Hembram et al., 2020), revealing cooperation between ICP0 phosphorylation domains (FHA [67-pTELF-70] and Phos2; Fig. 2) in the degradation of SUMOylated proteins (Mostafa et al., 2013; Hembram et al., 2020; Boutell et al., 2008). Importantly, this mechanism of SUMO targeting is not limited to PML-NBs, as ICP0 has been shown to induce the degradation of numerous (≥ 120) SUMOylated proteins including ARID3A/E2FBP1, BEND3, ETV6, MBD1, NACC1, NACC2, ZBTB4, ZBTB10, ZBTB38, and MORC3 (Fig. 3) (Everett et al., 1998; Chelbi-Alix and de The, 1999; Boutell et al., 2011; Sloan et al., 2015). Degradation of MORC3 by ICP0 has been proposed to inhibit the recruitment of PML-NB host factors to infecting viral genomes (Sloan et al., 2016), an observation that warrants further investigation due to the importance of PML-NBs in the intracellular restriction of viral pathogens (Geoffroy and Chelbi-Alix, 2011; Komatsu et al., 2016). In most cases, however, it remains to be determined if the degradation of these SUMOylated proteins is functionally relevant to HSV-1 infection or a consequence of collateral damage by the targeting mechanism employed by ICP0 to disrupt PML-NBs. Notably, it is becoming clear that PML-NB host factors cooperate with host SUMOylation machinery (e.g. PIAS SUMO E3 ligases) to restrict HSV-1 infection (Boutell et al., 2011; Conn et al., 2016; Brown et al., 2016). Depletion of PIAS1 and PIAS4 in combination with PML significantly alleviates the restriction of an HSV-1 ΔICP0 mutant relative to PML depletion alone (Conn et al., 2016; Brown et al., 2016). Thus, ICP0’s ability to disrupt SUMO-SIM interactions through multiple targeting mechanisms is likely to be a common strategy employed by ICP0 to remodel proteome interaction networks that facilitate or maintain the intracellular restriction of HSV-1 (Everett and Murray, 2005; Cuchet-Lourenco et al., 2011; Maroui et al., 2018).
4. Interplay between ICP0, chromatin remodelling, and intrinsic immunity
HSV-1 genomes are delivered to the nucleus of newly infected cells as linear molecules of naked DNA (Kilcher and Mercer, 2015), which immediately bind constitutively expressed host factors with pro- and antiviral cellular functions (Dembowski and DeLuca, 2018). ChIP experiments have demonstrated infecting viral genomes to rapidly associate with cellular histones, which can carry distinct epigenetic signatures that influence the progression of viral transcription (Cabral et al., 2018; Cliffe and Knipe, 2008; Kutluay and Triezenberg, 2009; Placek et al., 2009; Merkl et al., 2018; Kristie, 2015; Suzich and Cliffe, 2018). Relevant to ICP0, microscopy experiments have shown the histone H3.3 chaperone complex Daxx/ATRX and IFI16 to associate with infecting HSV-1 genomes prior to vDNA entrapment within PML-NBs (Alandijany et al., 2018; Cabral et al., 2018; Everett, 2015; Diner et al., 2016), known repositories of non-nucleosomal histone H3.3 (Delbarre et al., 2013; Corpet et al., 2014; Drane et al., 2010; Cohen et al., 2018). The recruitment of these host factors correlates with the epigenetic modification of histone H3 (H3K9me3 and H3K27me3) on viral chromatin which can lead to transcriptional silencing in the absence of ICP0 (Cabral et al., 2018; Cohen et al., 2018; Lee et al., 2016). HSV-1 mutants that fail to express ICP0, or carry mutations that abolish its Ub ligase activity, have a significantly lower probability of initiating a productive infection in restrictive cell types (approximately 1000-fold relative to WT HSV-1; (Everett et al., 2004)). Such observations have led to the hypothesis that PML-NBs may act as an axis for vDNA chromatinization and gene silencing (Cohen et al., 2018; Newhart et al., 2012), as vDNA remains stably entrapped in PML-NBs in the absence of ICP0 under low MOI conditions (Alandijany et al., 2018; Cohen et al., 2018; Everett et al., 2007; Maroui et al., 2016). In support of this, resident PML-NB proteins (PML, Daxx and ATRX) have been shown to act cooperatively with IFI16 to restrict HSV-1 ΔICP0 gene expression that correlates with repressive histone signatures (H3K9me3 and H3K27me3) on viral chromatin (Everett et al., 2008a; Cabral et al., 2018; Merkl et al., 2018; Lee et al., 2016). While Sp100 also contributes to the repression of HSV-1 ΔICP0 gene expression (Everett et al., 2008a; Glass and Everett, 2013; Everett et al., 2009), the influence of Sp100 on the epigenetic regulation of viral chromatin remains to be determined. Thus, rapid chromatinization and epigenetic modification of vDNA upon nuclear entry can restrict the initiation of productive HSV-1 infection. Importantly, this intrinsic host response to vDNA nuclear entry occurs prior to the activation of cytokine-mediated innate immune defences under low genome copy-numbers of infection (see below) (Alandijany et al., 2018; Everett et al., 2008b).
Expression of ICP0 induces the degradation and dispersal of PML-NB associated proteins from vDNA (see Section 3) (Alandijany et al., 2018; Cabral et al., 2018; Everett et al., 2013), which leads to a reduction in histone H3 loading and enhanced levels of histone H3 acetylation on vDNA to promote transcription (Cabral et al., 2018; Cliffe and Knipe, 2008; Lee et al., 2016; Ferenczy et al., 2011). ICP0 also induces the degradation of ATRX and IFI16 (Jurak et al., 2012; Orzalli et al., 2012; Orzalli et al., 2016; Cuchet-Lourenco et al., 2013; Diner et al., 2015). However, the turnover of these proteins occurs with delayed kinetics relative to that of PML degradation (Jurak et al., 2012; Cuchet-Lourenco et al., 2013). Such observations are likely to reflect the sequential degradation of host factors as infection progresses (Merkl and Knipe, 2019), a conclusion consistent with the differential accumulation of cellular factors on vDNA throughout the initiating cycle of HSV-1 infection (Dembowski and Deluca, 2017; Dembowski and DeLuca, 2015). Indeed, recent microscopy studies have identified the histone H3.3 chaperone protein HIRA to restrict HSV-1 infection following the onset of vDNA replication, a host response antagonized by ICP0 through the nuclear dispersal of HIRA (McFarlane et al., 2019). Thus, multiple histone H3.3 chaperone proteins (Daxx/ATRX and HIRA) can restrict the progress of HSV-1 ΔICP0 replication at independent phases of infection.
ICP0 has also been reported to bind CoREST, a component protein of the REST/CoREST/HDAC1,2/LSD1 nuclear repressor complex (Fig. 2, residues 668–718; (Gu et al., 2005; Gu and Roizman, 2007)). ICP0 disrupts HDAC1 binding to CoREST, leading to HDAC1 translocation to the cytoplasm (Gu et al., 2005). This ICP0-mediated action is proposed to block viral chromatin histone deacetylation and thus maintain a transcriptionally active state (Ferenczy et al., 2011; Gu and Roizman, 2007; Gu and Roizman, 2009). However, complementation assays have shown ICP0-CoREST binding mutants to have only a modest impact on the acetylation status of viral chromatin relative to that of a functionally active RING-finger domain (Ferenczy et al., 2011). Of interest, the proposed CoREST binding site lies in a region of C-terminal homology conserved between primate ICP0 orthologues (Everett et al., 2014), which plays a multi-functional role in the biological properties of ICP0, including USP7 binding and PML-NB localization (Fig. 2) (Everett et al., 2009; Everett et al., 2014). Thus, while ICP0 has the potential to influence the epigenetic modification of viral chromatin in a RING-finger independent manner, the general consensus is that ICP0’s Ub ligase activity plays a central role in its ability to transactivate viral gene expression to stimulate the progression of infection.
5. ICP0 modulation of the cellular DNA Damage Response (DDR) pathway
Like many DNA viruses, HSV-1 shows an intimate relationship with the DDR pathway which can both positively and negatively influence the outcome of infection (Wilkinson and Weller, 2004; Lilley et al., 2005; Smith and Weller, 2015; Dybas et al., 2018). Upon nuclear infection, HSV-1 induces cellular DNA double strand breaks (DSBs) that are sensed by the MRN complex (MRE11, RAD50, and NBS1) which activates ATM leading to the phosphorylation of histones H2A and H2AX (γH2AX) flanking the chromatin break (Shirata et al., 2005). This stimulates the recruitment of MDC1, that recruits the cellular Ub ligases RNF8 and RNF168 to DSBs which ubiquitinate H2A and γH2AX, a signal that promotes the recruitment of downstream repair proteins (Mailand et al., 2007; Doil et al., 2009). ICP0 targets RNF8 and RNF168 for degradation in a FHA-domain and phosphorylation-dependent manner, abrogating H2A and H2AX ubiquitination that restricts the recruitment of host DDR repair factors (e.g. TP53BP1) (Lilley et al., 2010; Chaurushiya et al., 2012). During HSV-1 ΔICP0 mutant infection, recruitment of these DDR proteins occurs in close proximity to vDNA in a PML and Sp100 independent manner (Lilley et al., 2011). Depletion of RNF8 and RNF168 partially relieves the replication defect of an HSV-1 ΔICP0 mutant, demonstrating that RNF8 and RNF168 contribute to the intrinsic antiviral restriction of HSV-1 through a mechanism antagonized by the Ub ligase activity of ICP0. Notably, SUMOylation is also known to play a key role in mediating the recruitment of DDR proteins to DSBs through SUMO-SIM interactions catalysed by PIAS SUMO ligases (Morris et al., 2009; Galanty et al., 2009). Thus, it is likely that ICP0 utilizes SUMO-dependent and -independent targeting strategies to modulate the DDR during HSV-1 infection (Conn et al., 2016; Brown et al., 2016).
DSBs can also be sensed by Ku70/Ku80, leading to the recruitment of DNA-PK which promotes non-homologous end joining (NHEJ) at DSBs. ICP0 has been shown to induce the degradation of the catalytic subunit of DNA-PK (DNA-PKcs/PRKDC; (Lees-Miller et al., 1996; Parkinson et al., 1999)). While it is clear that DNA-PKcs contributes to the cellular restriction of an HSV-1 ΔICP0 mutant, the precise mechanism of restriction remains to be defined but has been linked to viral genome circularization and regulation of innate immune defences (see below) (Jackson and DeLuca, 2003; Burleigh et al., 2020).
ICP0 also localizes to centromeres where it induces the degradation of CENP-A, CENP-B and, CENP-C (Lomonte et al., 2001; Lomonte and Morency, 2007; Everett et al., 1999b), leading to cell cycle arrest and induction of an interphase Centromere Damage Response (iCDR) (Gross et al., 2012). ICP0 has also been shown to promote remodelling of telomeres through the degradation of TPP1, leading to TERRA activation and enhanced levels of HSV-1 replication (Deng et al., 2014). Thus, ICP0’s Ub ligase activity significantly remodels the intracellular chromatin environment to promote the progression of HSV-1 infection.
6. ICP0 scrambles innate immune pathways
Detection of viral nucleic acid by host pattern recognition receptors (PRRs) plays a critical role in the activation of signalling cascades that lead to the production of pro-inflammatory cytokines, including type-I, II, and III IFNs (Unterholzner and Almine, 2019; Stempel et al., 2019; Alandijany, 2019). Secretion of IFNs leads to the induction of hundreds of IFN stimulated genes (ISGs) that generate a cellular antiviral state that limits virus propagation and spread. HSV-1 ΔICP0 mutants are hypersensitive to IFN (Everett et al., 2004; Leib et al., 1999; Mossman et al., 2000; Harle et al., 2002), highlighting a role for ICP0 in the regulation of innate immune defences to HSV-1 infection. ICP0 contributes to nuclear PRR inactivation through the degradation of IFI16 and DNA-PKcs, which signal through STING-dependent and -independent pathways, respectively (Diner et al., 2016; Orzalli et al., 2012; Orzalli et al., 2016; Cuchet-Lourenco et al., 2013; Burleigh et al., 2020; Orzalli et al., 2015). Under low MOI conditions vDNA entry into the nucleus alone is not sufficient to trigger PRR activation leading to IFN and ISG expression, which has been shown to require the onset of vDNA replication (Alandijany et al., 2018). PRR sensing by IFI16 during HSV-1 ΔICP0 infection correlates with IFI16 forming nuclear filaments on vDNA in association with PML following the saturation of PML-NBs under high genome loads (Alandijany et al., 2018; Cuchet-Lourenco et al., 2013; Merkl and Knipe, 2019). Such observations highlight a clear segregation in the regulation of intrinsic and innate immune defences that concurrently restrict the initiation and propagation of HSV-1, respectively (Alandijany et al., 2018; Cabral et al., 2018; Everett et al., 2008b). The relative spatiotemporal kinetics of IFI16 and DNA-PKcs PRR sensing of vDNA remains to be determined. Notably, PML isoforms II and IV have been shown to facilitate the loading of transcription factors (including IRF3, NF-κB, and STAT1) onto cellular gene promoters that directly influence the induction of cytokines and ISG expression that contribute to the IFN hypersensitivity of HSV-1 ΔICP0 mutants (McFarlane et al., 2019; Chen et al., 2015; Chee et al., 2003; El Asmi et al., 2014). Thus, ICP0 inactivates intrinsic and innate immune defences early in the infectious cycle through the degradation of PML and cellular PRRs. ICP0 has also been reported to influence the regulation of NF-κB signalling cascades (van Lint et al., 2010; Daubeuf et al., 2009; Zhang et al., 2013). However, the spatiotemporal regulation of this important signalling pathway under physiological infection conditions remains to be fully defined. It also remains to be determined as to what extent ICP0 reshapes the intracellular proteome upon infection of cytokine stimulated cells which express a full complement of ISG products.
7. ICP0 and HSV-1 latency
While significant progress has been made in defining the biochemical properties and cellular substrates of ICP0 during HSV-1 lytic infection (Fig. 3), significantly less is known about the Ub ligase activity of ICP0 during viral reactivation from latency. Following primary infection, HSV-1 infects the neuronal dendrites of sensory ganglia that innervate infected tissues. Retrograde transport carries viral capsids along neuronal axons to the nucleus, wherein the viral genome undergoes epigenetic silencing leading to the establishment of latency (for detailed reviews see (Wilson and Mohr, 2012; Nicoll et al., 2012; Bloom, 2016; Cliffe and Wilson, 2017)). With respect to ICP0, animal models have shown ICP0 to be dispensable for the establishment and maintenance of HSV-1 latency, but to play a critical role during viral reactivation leading to de novo virus production (Leib et al., 1989; Halford and Schaffer, 2001; Thompson and Sawtell, 2006; Cai et al., 1993). This process occurs in an ICP0 RING-finger and phosphorylation dependent manner (Thompson and Sawtell, 2006; Vanni et al., 2012; Mostafa et al., 2013; Mostafa et al., 2011), highlighting the importance of cellular ubiquitin machinery and kinases in the successful reactivation of HSV-1 from latency. Latent viral genomes can be observed to colocalize at distinct neuronal cell body sub-structures, including de novo assembled PML-NBs and centromeres (Cohen et al., 2018; Maroui et al., 2016; Catez et al., 2012), known substrates of ICP0 in mitotic cells (Fig. 3). While it’s tempting to speculate that ICP0 is required to disrupt these nuclear sub-domains that may otherwise promote or maintain vDNA in a state of transcriptional quiescence (Cohen et al., 2018), a number of key observations have been reported that remain to be resolved. Firstly, low levels of ICP0 transcription have been detected in latently infected neurones (Maillet et al., 2006; Chen et al., 2002), although it remains to be determined if ICP0 is expressed or functionally active as a Ub ligase during latency. Secondly, viral reactivation is known to occur in distinct phases; widespread reanimation of non-canonical patterns of gene expression independently of viral protein synthesis (phase-1), followed by sequential patterns of canonical gene expression driven by the transactivating protein VP16 (phase-2) (Kim et al., 2012). ICP0 is required for phase-2 reactivation in a VP16-dependent manner (Thompson and Sawtell, 2006; Thompson et al., 2009). These data indicate that phase-1 reanimation of viral transcription occurs independently of ICP0, a process that has been linked to neuronal stress and DDR pathways (Cliffe et al., 2015; Cliffe, 2019; Hu et al., 2019). Collectively, these observations raise the possibility that ICP0 may have neuronal specific functions or spatiotemporal activities out with of those identified during the initiation of lytic infection in mitotic cells. With the advent of modern cytology techniques that enable the explant or differentiation of neurones in vitro (Suzich and Cliffe, 2018; Pourchet et al., 2017; Thellman and Triezenberg, 2017; Thellman et al., 2017; D’Aiuto et al., 2019; Edwards and Bloom, 2019), the molecular function of ICP0 as a viral Ub ligase in modulating neuronal-specific processes, including host immune defences, epigenetic regulation, and the DDR, during HSV-1 latency and reactivation can now be addressed in detail.
8. Future Directions: Identification of new ICP0 substrates and host responses to viral infection
While it is clear that the Ub ligase activity of ICP0 plays a central role in the infectious cycle of HSV-1, the full repertoire of ICP0 substrates remains poorly defined. With the development of modern proteomic and bioinformatic methodologies, it is now possible to quantify the impact of ICP0 ubiquitination on both host and viral proteomes. The development of an antibody that recognizes peptides modified by Ub has heralded a significant advancement in the ability to detect, isolate, and quantify ubiquitinated substrates on a proteome-wide scale by mass spectrometry (Xu et al., 2010; Udeshi et al., 2013). Upon trypsin digestion, Ub is cleaved leaving its C-terminal di-glycine bound to K residues in the modified substrate. This di-glycine remnant can be enriched by antibody affinity capture and analyzed by mass spectrometry to identify changes in the cellular ubiquitinome between sample populations. Comparison of WT to ICP0 RING-finger or ΔICP0 mutant HSV-1 infected cells over time would provide quantitative changes in host and viral ubiquitinomes through different phases of infection. Di-glycine remnant profiling in combination with whole cell proteomics would enable the identification of concomitant changes in protein abundance to that of ubiquitination status, enabling the identification of novel ICP0 substrates, new interfaces of viral host interaction, and fundamental insights into cellular functions of ubiquitination in response to virus infection. We hypothesize that ICP0 will target a variety of substrates for ubiquitination that will have proteasome-dependent and -independent functions. Conversely, we hypothesize the host cells will utilize ubiquitination to promote the activation of host immune defences that lead to the cellular restriction of HSV-1 in the absence of ICP0. The application of such proteomic methodologies will significantly advance our understanding of the requirements for ICP0 to remodel the cellular proteome under a range of conditions pertinent to HSV-1 infection; including cell type (e.g. epithelial vs. neuronal origin) and immunological status (e.g. resting vs. cytokine stimulated). Such studies will likely reveal new and important insights into cellular processes and host factors that mediate the spatiotemporal regulation of immune defences to HSV-1 infection.
9. Conclusion
ICP0 hijacks Ub machinery to disrupt cellular pathways that play important roles in the regulation of host immunity and cellular homeostasis, which to date includes the regulation of PML-NBs and host SUMOylation, epigenetic modification, DDR, and the cell cycle (summarized in Fig. 3). The outcome of this proteome remodelling creates a favourable environment to promote the onset of HSV-1 lytic replication, propagation, and productive reactivation of viral genomes from latency. As such, the identification and development of small molecule inhibitors to ICP0 would provide significant therapeutic application in the treatment of recurrent HSV-1 infections by complementing host immune defences to block viral reactivation from latency.
Author contributions
MCR, JMD, and CB wrote the original draft. MCR, JMD, and JH prepared the figures. JH performed the bioinformatic analysis. MDW and CB edited the manuscript.
Funding
This work was supported by the Medical Research Council (https://mrc.ukri.org) grant MC_UU_12014/5 (to CB) and by grants from the National Institutes of Health (AI115104 and NS082240 to MDW, and AI147587 to JMD). The funders had no role in the study design or preparation of this manuscript.
Declaration of Competing Interest
The authors declare no conflict of interests.
Contributor Information
Matthew D. Weitzman, Email: weitzmanm@email.chop.edu.
Chris Boutell, Email: chris.boutell@glasgow.ac.uk.
References
- Alandijany T. Host Intrinsic and Innate Intracellular Immunity During Herpes Simplex Virus Type 1 (HSV-1) Infection. Front. Microbiol. 2019;10(2611) doi: 10.3389/fmicb.2019.02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alandijany T., Roberts A.P.E., Conn K.L., Loney C., McFarlane S., Orr A. Distinct temporal roles for the promyelocytic leukaemia (PML) protein in the sequential regulation of intracellular host immunity to HSV-1 infection. PLoS Pathog. 2018;14(1):e1006769. doi: 10.1371/journal.ppat.1006769. Epub 2018/01/09. doi: 10.1371/journal.ppat.1006769. PubMed PMID: 29309427; PubMed Central PMCID: PMCPMC5757968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi R., Pandolfi P.P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 2007;8(12):1006–1016. doi: 10.1038/nrm2277. Epub 2007/10/12. doi: 10.1038/nrm2277. PubMed PMID: 17928811. [DOI] [PubMed] [Google Scholar]
- Bloom D.C. Alphaherpesvirus latency: a dynamic state of transcription and reactivation. Adv. Virus Res. 2016;94:53–80. doi: 10.1016/bs.aivir.2015.10.001. Epub 2016/03/22. doi: 10.1016/bs.aivir.2015.10.001. PubMed PMID: 26997590. [DOI] [PubMed] [Google Scholar]
- Boutell C., Davido D.J. A quantitative assay to monitor HSV-1 ICP0 ubiquitin ligase activity in vitro. Methods. 2015;90:3–7. doi: 10.1016/j.ymeth.2015.04.004. Epub 2015/04/12. doi: 10.1016/j.ymeth.2015.04.004. PubMed PMID: 25862948; PubMed Central PMCID: PMCPMC4655872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutell C., Everett R.D. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J. Gen. Virol. 2013;94(Pt 3):465–481. doi: 10.1099/vir.0.048900-0. Epub 2012/12/15. doi: 10.1099/vir.0.048900-0. PubMed PMID: 23239572. [DOI] [PubMed] [Google Scholar]
- Boutell C., Sadis S., Everett R.D. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 2002;76(2):841–850. doi: 10.1128/JVI.76.2.841-850.2002. Epub 2001/12/26. doi: 10.1128/jvi.76.2.841-850.2002. PubMed PMID: 11752173; PubMed Central PMCID: PMCPMC136846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutell C., Orr A., Everett R.D. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 2003;77(16):8686–8694. doi: 10.1128/JVI.77.16.8686-8694.2003. Epub 2003/07/30. doi: 10.1128/jvi.77.16.8686-8694.2003. PubMed PMID: 12885887; PubMed Central PMCID: PMCPMC167235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutell C., Canning M., Orr A., Everett R.D. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 2005;79(19):12342–12354. doi: 10.1128/JVI.79.19.12342-12354.2005. Epub 2005/09/15. doi: 10.1128/JVI.79.19.12342-12354.2005. PubMed PMID: 16160161; PubMed Central PMCID: PMCPMC1211536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutell C., Everett R., Hilliard J., Schaffer P., Orr A., Davido D. Herpes simplex virus type 1 ICP0 phosphorylation mutants impair the E3 ubiquitin ligase activity of ICP0 in a cell type-dependent manner. J. Virol. 2008;82(21):10647–10656. doi: 10.1128/JVI.01063-08. Epub 2008/08/22. doi: 10.1128/JVI.01063-08. PubMed PMID: 18715910; PubMed Central PMCID: PMCPMC2573210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutell C., Cuchet-Lourenco D., Vanni E., Orr A., Glass M., McFarlane S. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 2011;7(9):e1002245. doi: 10.1371/journal.ppat.1002245. Epub 2011/09/29. doi: 10.1371/journal.ppat.1002245. PubMed PMID: 21949651; PubMed Central PMCID: PMCPMC3174244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braten O., Livneh I., Ziv T., Admon A., Kehat I., Caspi L.H. Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination. Proc. Nat. Acad. Sci. U S A. 2016;113(32):E4639–47. doi: 10.1073/pnas.1608644113. Epub 2016/07/08. doi: 10.1073/pnas.1608644113. PubMed PMID: 27385826; PubMed Central PMCID: PMCPMC4987823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.R., Conn K.L., Wasson P., Charman M., Tong L., Grant K. SUMO ligase protein inhibitor of activated STAT1 (PIAS1) is a constituent promyelocytic leukemia nuclear body protein that contributes to the intrinsic antiviral immune response to herpes simplex virus 1. J. Virol. 2016;90(13):5939–5952. doi: 10.1128/JVI.00426-16. Epub 2016/04/22. doi: 10.1128/JVI.00426-16. PubMed PMID: 27099310; PubMed Central PMCID: PMCPMC4907222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh K., Maltbaek J.H., Cambier S., Green R., Gale M., Jr., James R.C. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci. Immunol. 2020;5(43) doi: 10.1126/sciimmunol.aba4219. Epub 2020/01/26. doi: 10.1126/sciimmunol.aba4219. PubMed PMID: 31980485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral J.M., Oh H.S., Knipe D.M. ATRX promotes maintenance of herpes simplex virus heterochromatin during chromatin stress. Elife. 2018:7. doi: 10.7554/eLife.40228. Epub 2018/11/23. doi: 10.7554/eLife.40228. PubMed PMID: 30465651; PubMed Central PMCID: PMCPMC6307862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Astor T.L., Liptak L.M., Cho C., Coen D.M., Schaffer P.A. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 1993;67(12):7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. Epub 1993/12/01. PubMed PMID: 8230470; PubMed Central PMCID: PMCPMC238216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning M., Boutell C., Parkinson J., Everett R.D. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 2004;279(37):38160–38168. doi: 10.1074/jbc.M402885200. Epub 2004/07/13. doi: 10.1074/jbc.M402885200. PubMed PMID: 15247261. [DOI] [PubMed] [Google Scholar]
- Catez F., Picard C., Held K., Gross S., Rousseau A., Theil D. HSV-1 genome subnuclear positioning and associations with host-cell PML-NBs and centromeres regulate LAT locus transcription during latency in neurons. PLoS Pathog. 2012;8(8):e1002852. doi: 10.1371/journal.ppat.1002852. Epub 2012/08/23. doi: 10.1371/journal.ppat.1002852. PubMed PMID: 22912575; PubMed Central PMCID: PMCPMC3415458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurushiya M.S., Lilley C.E., Aslanian A., Meisenhelder J., Scott D.C., Landry S. Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain. Mol. Cell. 2012;46(1):79–90. doi: 10.1016/j.molcel.2012.02.004. Epub 2012/03/13. doi: 10.1016/j.molcel.2012.02.004. PubMed PMID: 22405594; PubMed Central PMCID: PMCPMC3648639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee A.V., Lopez P., Pandolfi P.P., Roizman B. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 2003;77(12):7101–7105. doi: 10.1128/JVI.77.12.7101-7105.2003. Epub 2003/05/28. doi: 10.1128/jvi.77.12.7101-7105.2003. PubMed PMID: 12768029; PubMed Central PMCID: PMCPMC156157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelbi-Alix M.K., de The H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene. 1999;18(4):935–941. doi: 10.1038/sj.onc.1202366. Epub 1999/02/19. doi: 10.1038/sj.onc.1202366. PubMed PMID: 10023669. [DOI] [PubMed] [Google Scholar]
- Chen J., Silverstein S. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J. Virol. 1992;66(5):2916–2927. doi: 10.1128/jvi.66.5.2916-2927.1992. Epub 1992/05/01. PubMed PMID: 1313910; PubMed Central PMCID: PMCPMC241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Lee L.Y., Garber D.A., Schaffer P.A., Knipe D.M., Coen D.M. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J. Virol. 2002;76(10):4764–4772. doi: 10.1128/JVI.76.10.4764-4772.2002. Epub 2002/04/23. doi: 10.1128/jvi.76.10.4764-4772.2002. PubMed PMID: 11967293; PubMed Central PMCID: PMCPMC136172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wright J., Meng X., Leppard K.N. Promyelocytic leukemia protein isoform II promotes transcription factor recruitment to activate interferon Beta and interferon-responsive gene expression. Mol. Cell. Biol. 2015;35(10):1660–1672. doi: 10.1128/MCB.01478-14. Epub 2015/03/04. doi: 10.1128/MCB.01478-14. PubMed PMID: 25733689; PubMed Central PMCID: PMCPMC4405644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wu J., Lu Y., Ma Y.B., Lee B.H., Yu Z. Structural basis for dynamic regulation of the human 26S proteasome. Proc. Nat. Acad. Sci. U. S. A. 2016;113(46):12991–12996. doi: 10.1073/pnas.1614614113. Epub 2016/10/30. doi: 10.1073/pnas.1614614113. PubMed PMID: 27791164; PubMed Central PMCID: PMCPMC5135334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciufo D.M., Mullen M.A., Hayward G.S. Identification of a dimerization domain in the C-terminal segment of the IE110 transactivator protein from herpes simplex virus. J. Virol. 1994;68(5):3267–3282. doi: 10.1128/jvi.68.5.3267-3282.1994. Epub 1994/05/01. PubMed PMID: 8151788; PubMed Central PMCID: PMCPMC236817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A.R. DNA damage meets neurotrophin signaling: a delicate balancing AKT to maintain virus latency. Mol. Cell. 2019;74(3):411–413. doi: 10.1016/j.molcel.2019.04.015. Epub 2019/05/06. doi: 10.1016/j.molcel.2019.04.015. PubMed PMID: 31051136. [DOI] [PubMed] [Google Scholar]
- Cliffe A.R., Knipe D.M. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J. Virol. 2008;82(24):12030–12038. doi: 10.1128/JVI.01575-08. Epub 2008/10/10. doi: 10.1128/JVI.01575-08. PubMed PMID: 18842720; PubMed Central PMCID: PMCPMC2593313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A.R., Wilson A.C. Restarting lytic gene transcription at the onset of herpes simplex virus reactivation. J. Virol. 2017;91(2) doi: 10.1128/JVI.01419-16. Epub 2016/11/04. doi: 10.1128/JVI.01419-16. PubMed PMID: 27807236; PubMed Central PMCID: PMCPMC5215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A.R., Arbuckle J.H., Vogel J.L., Geden M.J., Rothbart S.B., Cusack C.L. Neuronal stress pathway mediating a histone Methyl/Phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe. 2015;18(6):649–658. doi: 10.1016/j.chom.2015.11.007. Epub 2015/12/15. doi: 10.1016/j.chom.2015.11.007. PubMed PMID: 26651941; PubMed Central PMCID: PMCPMC4681005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E.M., Kobiler O. Gene expression correlates with the number of herpes viral genomes initiating infection in single cells. PLoS Pathog. 2016;12(12):e1006082. doi: 10.1371/journal.ppat.1006082. Epub 2016/12/07. doi: 10.1371/journal.ppat.1006082. PubMed PMID: 27923068; PubMed Central PMCID: PMCPMC5161387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Corpet A., Roubille S., Maroui M.A., Poccardi N., Rousseau A. Promyelocytic leukemia (PML) nuclear bodies (NBs) induce latent/quiescent HSV-1 genomes chromatinization through a PML NB/Histone H3.3/H3.3 Chaperone Axis. PLoS Pathog. 2018;14(9):e1007313. doi: 10.1371/journal.ppat.1007313. Epub 2018/09/21. doi: 10.1371/journal.ppat.1007313. PubMed PMID: 30235352; PubMed Central PMCID: PMCPMC6168178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn K.L., Wasson P., McFarlane S., Tong L., Brown J.R., Grant K.G. Novel role for protein inhibitor of activated STAT 4 (PIAS4) in the restriction of herpes simplex virus 1 by the cellular intrinsic antiviral immune response. J. Virol. 2016;90(9):4807–4826. doi: 10.1128/JVI.03055-15. Epub 2016/03/05. doi: 10.1128/JVI.03055-15. PubMed PMID: 26937035; PubMed Central PMCID: PMCPMC4836348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwell S.E., White A.E., Harper J.W., Knipe D.M. Identification of TRIM27 as a novel degradation target of herpes simplex virus 1 ICP0. J. Virol. 2015;89(1):220–229. doi: 10.1128/JVI.02635-14. Epub 2014/10/17. doi: 10.1128/JVI.02635-14. PubMed PMID: 25320289; PubMed Central PMCID: PMCPMC4301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet A., Olbrich T., Gwerder M., Fink D., Stucki M. Dynamics of histone H3.3 deposition in proliferating and senescent cells reveals a DAXX-dependent targeting to PML-NBs important for pericentromeric heterochromatin organization. Cell Cycle. 2014;13(2):249–267. doi: 10.4161/cc.26988. Epub 2013/11/10. doi: 10.4161/cc.26988. PubMed PMID: 24200965; PubMed Central PMCID: PMCPMC3906242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchet-Lourenco D., Boutell C., Lukashchuk V., Grant K., Sykes A., Murray J. SUMO pathway dependent recruitment of cellular repressors to herpes simplex virus type 1 genomes. PLoS Pathog. 2011;7(7):e1002123. doi: 10.1371/journal.ppat.1002123. Epub 2011/07/23. doi: 10.1371/journal.ppat.1002123. PubMed PMID: 21779164; PubMed Central PMCID: PMCPMC3136452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchet-Lourenco D., Vanni E., Glass M., Orr A., Everett R.D. Herpes simplex virus 1 ubiquitin ligase ICP0 interacts with PML isoform I and induces its SUMO-independent degradation. J. Virol. 2012;86(20):11209–11222. doi: 10.1128/JVI.01145-12. Epub 2012/08/10. doi: 10.1128/JVI.01145-12. PubMed PMID: 22875967; PubMed Central PMCID: PMCPMC3457127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchet-Lourenco D., Anderson G., Sloan E., Orr A., Everett R.D. The viral ubiquitin ligase ICP0 is neither sufficient nor necessary for degradation of the cellular DNA sensor IFI16 during herpes simplex virus 1 infection. J. Virol. 2013;87(24):13422–13432. doi: 10.1128/JVI.02474-13. Epub 2013/10/04. doi: 10.1128/JVI.02474-13. PubMed PMID: 24089555; PubMed Central PMCID: PMCPMC3838218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowicz J.S., Nagel C.H., Voges M., Spohn M., Eibl M.M., Hauber J. Interaction between the cellular E3 ubiquitin ligase SIAH-1 and the viral immediate-early protein ICP0 enables efficient replication of Herpes Simplex Virus type 2 in vivo. PLoS One. 2018;13(8):e0201880. doi: 10.1371/journal.pone.0201880. Epub 2018/08/07. doi: 10.1371/journal.pone.0201880. PubMed PMID: 30080903; PubMed Central PMCID: PMCPMC6078308 Forschungsgesellschaft mbH, Vienna, Austria) does not alter our adherence to all PLOS ONE policies on sharing data and materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto L., Bloom D.C., Naciri J.N., Smith A., Edwards T.G., McClain L. Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J. Virol. 2019;93(9) doi: 10.1128/JVI.00111-19. Epub 2019/02/23. doi: 10.1128/JVI.00111-19. PubMed PMID: 30787148; PubMed Central PMCID: PMCPMC6475775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubeuf S., Singh D., Tan Y., Liu H., Federoff H.J., Bowers W.J. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood. 2009;113(14):3264–3275. doi: 10.1182/blood-2008-07-168203. Epub 2008/10/28. doi: 10.1182/blood-2008-07-168203. PubMed PMID: 18952891; PubMed Central PMCID: PMCPMC3401030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davido D.J., von Zagorski W.F., Lane W.S., Schaffer P.A. Phosphorylation site mutations affect herpes simplex virus type 1 ICP0 function. J. Virol. 2005;79(2):1232–1243. doi: 10.1128/JVI.79.2.1232-1243.2005. Epub 2004/12/23. doi: 10.1128/JVI.79.2.1232-1243.2005. PubMed PMID: 15613350; PubMed Central PMCID: PMCPMC538545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre E., Ivanauskiene K., Kuntziger T., Collas P. DAXX-dependent supply of soluble (H3.3-H4) dimers to PML bodies pending deposition into chromatin. Genome Res. 2013;23(3):440–451. doi: 10.1101/gr.142703.112. PubMed PMID: 23222847; PubMed Central PMCID: PMCPMC3589533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembowski J.A., Deluca N.A. Purification of viral DNA for the identification of associated viral and cellular proteins. J. Vis. Exp. 2017;(126) doi: 10.3791/56374. Epub 2017/09/12. doi: 10.3791/56374. PubMed PMID: 28892026; PubMed Central PMCID: PMCPMC5614390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembowski J.A., DeLuca N.A. Selective recruitment of nuclear factors to productively replicating herpes simplex virus genomes. PLoS Pathog. 2015;11(5):e1004939. doi: 10.1371/journal.ppat.1004939. Epub 2015/05/29. doi: 10.1371/journal.ppat.1004939. PubMed PMID: 26018390; PubMed Central PMCID: PMCPMC4446364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembowski J.A., DeLuca N.A. Temporal viral genome-protein interactions define distinct stages of productive herpesviral infection. MBio. 2018;9(4) doi: 10.1128/mBio.01182-18. Epub 2018/07/19. doi: 10.1128/mBio.01182-18. PubMed PMID: 30018111; PubMed Central PMCID: PMCPMC6050965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Kim E.T., Vladimirova O., Dheekollu J., Wang Z., Newhart A. HSV-1 remodels host telomeres to facilitate viral replication. Cell Rep. 2014;9(6):2263–2278. doi: 10.1016/j.celrep.2014.11.019. Epub 2014/12/17. doi: 10.1016/j.celrep.2014.11.019. PubMed PMID: 25497088; PubMed Central PMCID: PMCPMC4356630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps T., Waisner H., Dogrammatzis C., Roy A., Chacko S., Perera C. Discovery of small-molecule inhibitors targeting the E3 ubiquitin ligase activity of the herpes simplex virus 1 ICP0 protein using an in vitro high-throughput screening assay. J. Virol. 2019;93(13) doi: 10.1128/JVI.00619-19. Epub 2019/04/19. doi: 10.1128/JVI.00619-19. PubMed PMID: 30996104; PubMed Central PMCID: PMCPMC6580980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B.A., Lum K.K., Javitt A., Cristea I.M. Interactions of the antiviral factor interferon gamma-inducible protein 16 (IFI16) mediate immune signaling and herpes simplex Virus-1 immunosuppression. Mol. Cell Proteomics. 2015;14(9):2341–2356. doi: 10.1074/mcp.M114.047068. Epub 2015/02/20. doi: 10.1074/mcp.M114.047068. PubMed PMID: 25693804; PubMed Central PMCID: PMCPMC4563720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B.A., Lum K.K., Toettcher J.E., Cristea I.M. Viral DNA sensors IFI16 and cyclic GMP-AMP synthase possess distinct functions in regulating viral gene expression, immune defenses, and apoptotic responses during herpesvirus infection. mBio. 2016;7(6) doi: 10.1128/mBio.01553-16. Epub 2016/12/10. doi: 10.1128/mBio.01553-16. PubMed PMID: 27935834; PubMed Central PMCID: PMCPMC5111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doil C., Mailand N., Bekker-Jensen S., Menard P., Larsen D.H., Pepperkok R. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136(3):435–446. doi: 10.1016/j.cell.2008.12.041. Epub 2009/02/11. doi: 10.1016/j.cell.2008.12.041. PubMed PMID: 19203579. [DOI] [PubMed] [Google Scholar]
- Drane P., Ouararhni K., Depaux A., Shuaib M., Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 2010;24(12):1253–1265. doi: 10.1101/gad.566910. PubMed PMID: 20504901; PubMed Central PMCID: PMCPMC2885661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayman N., Karin O., Mayo A., Danon T., Shapira L., Rafael D. Dynamic proteomics of herpes simplex virus infection. MBio. 2017;8(6) doi: 10.1128/mBio.01612-17. Epub 2017/11/09. doi: 10.1128/mBio.01612-17. PubMed PMID: 29114028; PubMed Central PMCID: PMCPMC5676043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayman N., Patel P., Vistain L., Tay S. HSV-1 single-cell analysis reveals the activation of anti-viral and developmental programs in distinct sub-populations. Elife. 2019;8 doi: 10.7554/eLife.46339. Epub 2019/05/16. doi: 10.7554/eLife.46339. PubMed PMID: 31090537; PubMed Central PMCID: PMCPMC6570482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybas J.M., Herrmann C., Weitzman M.D. Ubiquitination at the interface of tumor viruses and DNA damage responses. Curr. Opin. Virol. 2018;32:40–47. doi: 10.1016/j.coviro.2018.08.017. Epub 2018/09/28. doi: 10.1016/j.coviro.2018.08.017. PubMed PMID: 30261451; PubMed Central PMCID: PMCPMC6263849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T.G., Bloom D.C. Lund Human Mesencephalic (LUHMES) neuronal cell line supports herpes simplex Virus 1 latency in vitro. J. Virol. 2019;93(6) doi: 10.1128/JVI.02210-18. Epub 2019/01/04. doi: 10.1128/JVI.02210-18. PubMed PMID: 30602607; PubMed Central PMCID: PMCPMC6401467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Asmi F., Maroui M.A., Dutrieux J., Blondel D., Nisole S., Chelbi-Alix M.K. Implication of PMLIV in both intrinsic and innate immunity. PLoS Pathog. 2014;10(2):e1003975. doi: 10.1371/journal.ppat.1003975. Epub 2014/03/04. doi: 10.1371/journal.ppat.1003975. PubMed PMID: 24586174; PubMed Central PMCID: PMCPMC3937294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 2000;74(21):9994–10005. doi: 10.1128/jvi.74.21.9994-10005.2000. Epub 2000/10/12. doi: 10.1128/jvi.74.21.9994-10005.2000. PubMed PMID: 11024128; PubMed Central PMCID: PMCPMC102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D. Dynamic response of IFI16 and promyelocytic leukemia nuclear body components to herpes simplex virus 1 infection. J. Virol. 2015;90(1):167–179. doi: 10.1128/JVI.02249-15. PubMed PMID: 26468536; PubMed Central PMCID: PMCPMC4702556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Maul G.G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13(21):5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. Epub 1994/11/01. PubMed PMID: 7957072; PubMed Central PMCID: PMCPMC395452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Murray J. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 2005;79(8):5078–5089. doi: 10.1128/JVI.79.8.5078-5089.2005. Epub 2005/03/30. doi: 10.1128/JVI.79.8.5078-5089.2005. PubMed PMID: 15795293; PubMed Central PMCID: PMCPMC1069553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Barlow P., Milner A., Luisi B., Orr A., Hope G. A novel arrangement of zinc-binding residues and secondary structure in the C3HC4 motif of an alpha herpes virus protein family. J. Mol. Biol. 1993;234(4):1038–1047. doi: 10.1006/jmbi.1993.1657. Epub 1993/12/20. doi: 10.1006/jmbi.1993.1657. PubMed PMID: 8263911. [DOI] [PubMed] [Google Scholar]
- Everett R., Orr A., Elliott M. The equine herpesvirus 1 gene 63 RING finger protein partially complements Vmw110, its herpes simplex virus type 1 counterpart. J. Gen. Virol. 1995;76(Pt 9):2369–2374. doi: 10.1099/0022-1317-76-9-2369. Epub 1995/09/01. doi: 10.1099/0022-1317-76-9-2369. PubMed PMID: 7561779. [DOI] [PubMed] [Google Scholar]
- Everett R.D., Meredith M., Orr A., Cross A., Kathoria M., Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16(3):566–577. doi: 10.1093/emboj/16.3.566. Epub 1997/02/03. doi: 10.1093/emboj/16.3.566. PubMed PMID: 9034339; PubMed Central PMCID: PMCPMC1169660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Freemont P., Saitoh H., Dasso M., Orr A., Kathoria M. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 1998;72(8):6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. Epub 1998/07/11. PubMed PMID: 9658103; PubMed Central PMCID: PMCPMC109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Meredith M., Orr A. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 1999;73(1):417–426. doi: 10.1128/jvi.73.1.417-426.1999. Epub 1998/12/16. PubMed PMID: 9847347; PubMed Central PMCID: PMCPMC103848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Earnshaw W.C., Findlay J., Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18(6):1526–1538. doi: 10.1093/emboj/18.6.1526. Epub 1999/03/17. doi: 10.1093/emboj/18.6.1526. PubMed PMID: 10075924; PubMed Central PMCID: PMCPMC1171241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Boutell C., Orr A. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 2004;78(4):1763–1774. doi: 10.1128/JVI.78.4.1763-1774.2004. Epub 2004/01/30. doi: 10.1128/jvi.78.4.1763-1774.2004. PubMed PMID: 14747541; PubMed Central PMCID: PMCPMC369471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Rechter S., Papior P., Tavalai N., Stamminger T., Orr A. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 2006;80(16):7995–8005. doi: 10.1128/JVI.00734-06. Epub 2006/07/29. doi: 10.1128/JVI.00734-06. PubMed PMID: 16873256; PubMed Central PMCID: PMCPMC1563828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Murray J., Orr A., Preston C.M. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 2007;81(20):10991–11004. doi: 10.1128/JVI.00705-07. Epub 2007/08/03. doi: 10.1128/JVI.00705-07. PubMed PMID: 17670833; PubMed Central PMCID: PMCPMC2045565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Parada C., Gripon P., Sirma H., Orr A. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 2008;82(6):2661–2672. doi: 10.1128/JVI.02308-07. Epub 2007/12/28. doi: 10.1128/JVI.02308-07. PubMed PMID: 18160441; PubMed Central PMCID: PMCPMC2258993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Young D.F., Randall R.E., Orr A. STAT-1- and IRF-3-dependent pathways are not essential for repression of ICP0-null mutant herpes simplex virus type 1 in human fibroblasts. J. Virol. 2008;82(17):8871–8881. doi: 10.1128/JVI.00613-08. Epub 2008/06/27. doi: 10.1128/JVI.00613-08. PubMed PMID: 18579584; PubMed Central PMCID: PMCPMC2519674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Parsy M.L., Orr A. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 2009;83(10):4963–4977. doi: 10.1128/JVI.02593-08. Epub 2009/03/07. doi: 10.1128/JVI.02593-08. PubMed PMID: 19264778; PubMed Central PMCID: PMCPMC2682082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Boutell C., McNair C., Grant L., Orr A. Comparison of the biological and biochemical activities of several members of the alphaherpesvirus ICP0 family of proteins. J. Virol. 2010;84(7):3476–3487. doi: 10.1128/JVI.02544-09. Epub 2010/01/29. doi: 10.1128/JVI.02544-09. PubMed PMID: 20106921; PubMed Central PMCID: PMCPMC2838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D., Boutell C., Hale B.G. Interplay between viruses and host sumoylation pathways. Nat. Rev. Microbiol. 2013;11(6):400–411. doi: 10.1038/nrmicro3015. Epub 2013/04/30. doi: 10.1038/nrmicro3015. PubMed PMID: 23624814. [DOI] [PubMed] [Google Scholar]
- Everett R.D., Boutell C., Pheasant K., Cuchet-Lourenco D., Orr A. Sequences related to SUMO interaction motifs in herpes simplex virus 1 protein ICP0 act cooperatively to stimulate virus infection. J. Virol. 2014;88(5):2763–2774. doi: 10.1128/JVI.03417-13. Epub 2013/12/20. doi: 10.1128/JVI.03417-13. PubMed PMID: 24352468; PubMed Central PMCID: PMCPMC3958091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczy M.W., Ranayhossaini D.J., Deluca N.A. Activities of ICP0 involved in the reversal of silencing of quiescent herpes simplex virus 1. J. Virol. 2011;85(10):4993–5002. doi: 10.1128/JVI.02265-10. Epub 2011/03/18. doi: 10.1128/JVI.02265-10. PubMed PMID: 21411540; PubMed Central PMCID: PMCPMC3126212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanty Y., Belotserkovskaya R., Coates J., Polo S., Miller K.M., Jackson S.P. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature. 2009;462(7275):935–939. doi: 10.1038/nature08657. Epub 2009/12/18. doi: 10.1038/nature08657. PubMed PMID: 20016603; PubMed Central PMCID: PMCPMC2904806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy M.C., Chelbi-Alix M.K. Role of promyelocytic leukemia protein in host antiviral defense. J. Interferon Cytokine Res. 2011;31(1):145–158. doi: 10.1089/jir.2010.0111. Epub 2011/01/05. doi: 10.1089/jir.2010.0111. PubMed PMID: 21198351. [DOI] [PubMed] [Google Scholar]
- Glass M., Everett R.D. Components of promyelocytic leukemia nuclear bodies (ND10) act cooperatively to repress herpesvirus infection. J. Virol. 2013;87(4):2174–2185. doi: 10.1128/JVI.02950-12. Epub 2012/12/12. doi: 10.1128/JVI.02950-12. PubMed PMID: 23221561; PubMed Central PMCID: PMCPMC3571464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K., Grant L., Tong L., Boutell C. Depletion of intracellular zinc inhibits the ubiquitin ligase activity of viral regulatory protein ICP0 and restricts herpes simplex virus 1 replication in cell culture. J. Virol. 2012;86(7):4029–4033. doi: 10.1128/JVI.06962-11. Epub 2012/01/27. doi: 10.1128/JVI.06962-11. PubMed PMID: 22278229; PubMed Central PMCID: PMCPMC3302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S.J., Koegl M., Boutell C., Zenner H.L., Crump C.M., Pica F. A systematic analysis of host factors reveals a Med23-interferon-lambda regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog. 2013;9(8):e1003514. doi: 10.1371/journal.ppat.1003514. Epub 2013/08/21. doi: 10.1371/journal.ppat.1003514. PubMed PMID: 23950709; PubMed Central PMCID: PMCPMC3738494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S., Catez F., Masumoto H., Lomonte P. Centromere architecture breakdown induced by the viral E3 ubiquitin ligase ICP0 protein of herpes simplex virus type 1. PLoS One. 2012;7(9):e44227. doi: 10.1371/journal.pone.0044227. Epub 2012/10/03. doi: 10.1371/journal.pone.0044227. PubMed PMID: 23028505; PubMed Central PMCID: PMCPMC3447814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Roizman B. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Nat. Acad. Sci. U S A. 2003;100(15):8963–8968. doi: 10.1073/pnas.1533420100. Epub 2003/07/12. doi: 10.1073/pnas.1533420100. PubMed PMID: 12855769; PubMed Central PMCID: PMCPMC166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Roizman B. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Nat. Acad. Sci. U. S. A. 2007;104(43):17134–17139. doi: 10.1073/pnas.0707266104. Epub 2007/10/18. doi: 10.1073/pnas.0707266104. PubMed PMID: 17939992; PubMed Central PMCID: PMCPMC2040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Roizman B. The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J. Virol. 2009;83(1):181–187. doi: 10.1128/JVI.01940-08. Epub 2008/10/24. doi: 10.1128/JVI.01940-08. PubMed PMID: 18945770; PubMed Central PMCID: PMCPMC2612314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Liang Y., Mandel G., Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102(21):7571–7576. doi: 10.1073/pnas.0502658102. Epub 2005/05/18. doi: 10.1073/pnas.0502658102. PubMed PMID: 15897453; PubMed Central PMCID: PMCPMC1140450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagglund R., Van Sant C., Lopez P., Roizman B. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Nat. Acad. Sci. U S A. 2002;99(2):631–636. doi: 10.1073/pnas.022531599. Epub 2002/01/24. doi: 10.1073/pnas.022531599. PubMed PMID: 11805320; PubMed Central PMCID: PMCPMC117357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford W.P., Schaffer P.A. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 2001;75(7):3240–3249. doi: 10.1128/JVI.75.7.3240-3249.2001. Epub 2001/03/10. doi: 10.1128/JVI.75.7.3240-3249.2001. PubMed PMID: 11238850; PubMed Central PMCID: PMCPMC114117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford W.P., Kemp C.D., Isler J.A., Davido D.J., Schaffer P.A. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 2001;75(13):6143–6153. doi: 10.1128/JVI.75.13.6143-6153.2001. Epub 2001/06/08. doi: 10.1128/JVI.75.13.6143-6153.2001. PubMed PMID: 11390616; PubMed Central PMCID: PMCPMC114330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle P., Sainz B., Jr., Carr D.J., Halford W.P. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology. 2002;293(2):295–304. doi: 10.1006/viro.2001.1280. Epub 2002/03/12. doi: 10.1006/viro.2001.1280. PubMed PMID: 11886249. [DOI] [PubMed] [Google Scholar]
- Hembram D.S.S., Negi H., Biswas P., Tripathi V., Bhushan L., Shet D. The viral SUMO-Targeted ubiquitin ligase ICP0 is phosphorylated and activated by host kinase Chk2. J. Mol. Biol. 2020 doi: 10.1016/j.jmb.2020.01.021. Epub 2020/02/01. doi: 10.1016/j.jmb.2020.01.021. PubMed PMID: 32001251. [DOI] [PubMed] [Google Scholar]
- Hoischen C., Monajembashi S., Weisshart K., Hemmerich P. Multimodal light microscopy approaches to reveal structural and functional properties of promyelocytic leukemia nuclear bodies. Front. Oncol. 2018;8(125) doi: 10.3389/fonc.2018.00125. Epub 2018/06/12. doi: 10.3389/fonc.2018.00125. PubMed PMID: 29888200; PubMed Central PMCID: PMCPMC5980967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.L., Shiflett L.A., Kobayashi M., Chao M.V., Wilson A.C., Mohr I. TOP2beta-dependent nuclear DNA damage shapes extracellular growth factor responses via dynamic AKT phosphorylation to control virus latency. Mol. Cell. 2019;74(3):466–480. doi: 10.1016/j.molcel.2019.02.032. e4. Epub 2019/04/02. doi: 10.1016/j.molcel.2019.02.032. PubMed PMID: 30930055; PubMed Central PMCID: PMCPMC6499694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson M.K., Ploegh H.L. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5(6):559–570. doi: 10.1016/j.chom.2009.05.012. Epub 2009/06/17. doi: 10.1016/j.chom.2009.05.012. PubMed PMID: 19527883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov A.M., Sotnikov A.G., Negorev D., Vladimirova O.V., Neff N., Kamitani T. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 1999;147(2):221–234. doi: 10.1083/jcb.147.2.221. Epub 1999/10/20. doi: 10.1083/jcb.147.2.221. PubMed PMID: 10525530; PubMed Central PMCID: PMCPMC2174231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.A., DeLuca N.A. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc. Nat. Acad. Sci. U. S. A. 2003;100(13):7871–7876. doi: 10.1073/pnas.1230643100. Epub 2003/06/11. doi: 10.1073/pnas.1230643100. PubMed PMID: 12796511; PubMed Central PMCID: PMCPMC164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurak I., Silverstein L.B., Sharma M., Coen D.M. Herpes simplex virus is equipped with RNA- and protein-based mechanisms to repress expression of ATRX, an effector of intrinsic immunity. J. Virol. 2012;86(18):10093–10102. doi: 10.1128/JVI.00930-12. Epub 2012/07/13. doi: 10.1128/JVI.00930-12. PubMed PMID: 22787211; PubMed Central PMCID: PMCPMC3446562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenhorn L.M., Korbel G.A., Kessler B.M., Spooner E., Ploegh H.L. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell. 2005;19(4):547–557. doi: 10.1016/j.molcel.2005.07.003. Epub 2005/08/20. doi: 10.1016/j.molcel.2005.07.003. PubMed PMID: 16109378. [DOI] [PubMed] [Google Scholar]
- Kilcher S., Mercer J. DNA virus uncoating. Virology. 2015;479–480:578–590. doi: 10.1016/j.virol.2015.01.024. Epub 2015/03/03. doi: 10.1016/j.virol.2015.01.024. PubMed PMID: 25728300. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Mandarino A., Chao M.V., Mohr I., Wilson A.C. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog. 2012;8(2):e1002540. doi: 10.1371/journal.ppat.1002540. Epub 2012/03/03. doi: 10.1371/journal.ppat.1002540. PubMed PMID: 22383875; PubMed Central PMCID: PMCPMC3285597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D.M., Howley P.M. 6th ed. 2 volumes. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia, PA: 2013. (Fields Virology). [Google Scholar]
- Komatsu T., Nagata K., Wodrich H. The role of nuclear antiviral factors against invading DNA viruses: the immediate fate of incoming viral genomes. Viruses. 2016;8(10) doi: 10.3390/v8100290. Epub 2016/10/27. doi: 10.3390/v8100290. PubMed PMID: 27782081; PubMed Central PMCID: PMCPMC5086622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T.M. Dynamic modulation of HSV chromatin drives initiation of infection and provides targets for epigenetic therapies. Virology. 2015;479–480:555–561. doi: 10.1016/j.virol.2015.01.026. Epub 2015/02/24. doi: 10.1016/j.virol.2015.01.026. PubMed PMID: 25702087; PubMed Central PMCID: PMCPMC4424070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluay S.B., Triezenberg S.J. Regulation of histone deposition on the herpes simplex virus type 1 genome during lytic infection. J. Virol. 2009;83(11):5835–5845. doi: 10.1128/JVI.00219-09. Epub 2009/03/27. doi: 10.1128/JVI.00219-09. PubMed PMID: 19321615; PubMed Central PMCID: PMCPMC2681947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M., Jegou T., Chung I., Richter K., Munch S., Udvarhelyi A. Three-dimensional organization of promyelocytic leukemia nuclear bodies. J. Cell. Sci. 2010;123(Pt 3):392–400. doi: 10.1242/jcs.053496. Epub 2010/02/05. doi: 10.1242/jcs.053496. PubMed PMID: 20130140. [DOI] [PubMed] [Google Scholar]
- Lang A., Lang E., Boe S.O. PML bodies in mitosis. Cells. 2019;8(8) doi: 10.3390/cells8080893. Epub 2019/08/17. doi: 10.3390/cells8080893. PubMed PMID: 31416160; PubMed Central PMCID: PMCPMC6721746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Raja P., Knipe D.M. Herpesviral ICP0 protein promotes two waves of heterochromatin removal on an early viral promoter during lytic infection. mBio. 2016;7(1):e02007–15. doi: 10.1128/mBio.02007-15. Epub 2016/01/14. doi: 10.1128/mBio.02007-15. PubMed PMID: 26758183; PubMed Central PMCID: PMCPMC4725016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller S.P., Long M.C., Kilvert M.A., Lam V., Rice S.A., Spencer C.A. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 1996;70(11):7471–7477. doi: 10.1128/jvi.70.11.7471-7477.1996. Epub 1996/11/01. PubMed PMID: 8892865; PubMed Central PMCID: PMCPMC190814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D.A., Coen D.M., Bogard C.L., Hicks K.A., Yager D.R., Knipe D.M. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 1989;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. Epub 1989/02/01. PubMed PMID: 2536101; PubMed Central PMCID: PMCPMC247748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D.A., Harrison T.E., Laslo K.M., Machalek M.A., Moorman N.J., Virgin H.W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 1999;189(4):663–672. doi: 10.1084/jem.189.4.663. Epub 1999/02/17. doi: 10.1084/jem.189.4.663. PubMed PMID: 9989981; PubMed Central PMCID: PMCPMC2192939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley C.E., Carson C.T., Muotri A.R., Gage F.H., Weitzman M.D. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Nat. Acad. Sci. U. S. A. 2005;102(16):5844–5849. doi: 10.1073/pnas.0501916102. Epub 2005/04/13. doi: 10.1073/pnas.0501916102. PubMed PMID: 15824307; PubMed Central PMCID: PMCPMC556126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley C.E., Chaurushiya M.S., Boutell C., Landry S., Suh J., Panier S. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 2010;29(5):943–955. doi: 10.1038/emboj.2009.400. Epub 2010/01/16. doi: 10.1038/emboj.2009.400. PubMed PMID: 20075863; PubMed Central PMCID: PMCPMC2837166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley C.E., Chaurushiya M.S., Boutell C., Everett R.D., Weitzman M.D. The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0. PLoS Pathog. 2011;7(6):e1002084. doi: 10.1371/journal.ppat.1002084. Epub 2011/06/24. doi: 10.1371/journal.ppat.1002084. PubMed PMID: 21698222; PubMed Central PMCID: PMCPMC3116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.Y., Huang Y.S., Jeng J.C., Kuo H.Y., Chang C.C., Chao T.T. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell. 2006;24(3):341–354. doi: 10.1016/j.molcel.2006.10.019. Epub 2006/11/04. doi: 10.1016/j.molcel.2006.10.019. PubMed PMID: 17081986. [DOI] [PubMed] [Google Scholar]
- Lium E.K., Silverstein S. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 1997;71(11):8602–8614. doi: 10.1128/jvi.71.11.8602-8614.1997. Epub 1997/10/29. PubMed PMID: 9343218; PubMed Central PMCID: PMCPMC192324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte P., Morency E. Centromeric protein CENP-B proteasomal degradation induced by the viral protein ICP0. FEBS Lett. 2007;581(4):658–662. doi: 10.1016/j.febslet.2007.01.027. Epub 2007/01/30. doi: 10.1016/j.febslet.2007.01.027. PubMed PMID: 17258208. [DOI] [PubMed] [Google Scholar]
- Lomonte P., Sullivan K.F., Everett R.D. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 2001;276(8):5829–5835. doi: 10.1074/jbc.M008547200. Epub 2000/10/29. doi: 10.1074/jbc.M008547200. PubMed PMID: 11053442. [DOI] [PubMed] [Google Scholar]
- Luo H. Interplay between the virus and the ubiquitin-proteasome system: molecular mechanism of viral pathogenesis. Curr. Opin. Virol. 2016;17:1–10. doi: 10.1016/j.coviro.2015.09.005. Epub 2015/10/02. doi: 10.1016/j.coviro.2015.09.005. PubMed PMID: 26426962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N., Bekker-Jensen S., Faustrup H., Melander F., Bartek J., Lukas C. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131(5):887–900. doi: 10.1016/j.cell.2007.09.040. Epub 2007/11/16. doi: 10.1016/j.cell.2007.09.040. PubMed PMID: 18001824. [DOI] [PubMed] [Google Scholar]
- Maillet S., Naas T., Crepin S., Roque-Afonso A.M., Lafay F., Efstathiou S. Herpes simplex virus type 1 latently infected neurons differentially express latency-associated and ICP0 transcripts. J. Virol. 2006;80(18):9310–9321. doi: 10.1128/JVI.02615-05. Epub 2006/08/31. doi: 10.1128/JVI.02615-05. PubMed PMID: 16940542; PubMed Central PMCID: PMCPMC1563928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroui M.A., Calle A., Cohen C., Streichenberger N., Texier P., Takissian J. Latency entry of herpes simplex virus 1 is determined by the interaction of its genome with the nuclear environment. PLoS Pathog. 2016;12(9):e1005834. doi: 10.1371/journal.ppat.1005834. Epub 2016/09/13. doi: 10.1371/journal.ppat.1005834. PubMed PMID: 27618691; PubMed Central PMCID: PMCPMC5019400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroui M.A., Maarifi G., McManus F.P., Lamoliatte F., Thibault P., Chelbi-Alix M.K. Promyelocytic leukemia protein (PML) requirement for interferon-induced global cellular SUMOylation. Mol. Cell Proteomics. 2018;17(6):1196–1208. doi: 10.1074/mcp.RA117.000447. Epub 2018/03/15. doi: 10.1074/mcp.RA117.000447. PubMed PMID: 29535160; PubMed Central PMCID: PMCPMC5986244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G.G., Everett R.D. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 1994;75(Pt 6):1223–1233. doi: 10.1099/0022-1317-75-6-1223. Epub 1994/06/01. doi: 10.1099/0022-1317-75-6-1223. PubMed PMID: 8207389. [DOI] [PubMed] [Google Scholar]
- Maul G.G., Guldner H.H., Spivack J.G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0) J. Gen. Virol. 1993;74(Pt 12):2679–2690. doi: 10.1099/0022-1317-74-12-2679. Epub 1993/12/01. doi: 10.1099/0022-1317-74-12-2679. PubMed PMID: 8277273. [DOI] [PubMed] [Google Scholar]
- Maul G.G., Yu E., Ishov A.M., Epstein A.L. Nuclear domain 10 (ND10) associated proteins are also present in nuclear bodies and redistribute to hundreds of nuclear sites after stress. J. Cell. Biochem. 1995;59(4):498–513. doi: 10.1002/jcb.240590410. Epub 1995/12/01. doi: 10.1002/jcb.240590410. PubMed PMID: 8749719. [DOI] [PubMed] [Google Scholar]
- McFarlane S., Orr A., Roberts APE Conn K.L., Iliev V., Loney C. The histone chaperone HIRA promotes the induction of host innate immune defences in response to HSV-1 infection. PLoS Pathog. 2019;15(3):e1007667. doi: 10.1371/journal.ppat.1007667. Epub 2019/03/23. doi: 10.1371/journal.ppat.1007667. PubMed PMID: 30901352; PubMed Central PMCID: PMCPMC6472835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M., Orr A., Elliott M., Everett R. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology. 1995;209(1):174–187. doi: 10.1006/viro.1995.1241. Epub 1995/05/10. doi: 10.1006/viro.1995.1241. PubMed PMID: 7747467. [DOI] [PubMed] [Google Scholar]
- Merkl P.E., Knipe D.M. Role for a Filamentous Nuclear Assembly of IFI16, DNA, and Host Factors in Restriction of Herpesviral Infection. mBio. 2019;10(1) doi: 10.1128/mBio.02621-18. Epub 2019/01/24. doi: 10.1128/mBio.02621-18. PubMed PMID: 30670617; PubMed Central PMCID: PMCPMC6343039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkl P.E., Orzalli M.H., Knipe D.M. Mechanisms of host IFI16, PML, and daxx protein restriction of herpes simplex virus 1 replication. J. Virol. 2018;92(10) doi: 10.1128/JVI.00057-18. Epub 2018/03/02. doi: 10.1128/JVI.00057-18. PubMed PMID: 29491153; PubMed Central PMCID: PMCPMC5923075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M.B., Pruneda J.N., Klevit R.E., Weissman A.M. RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim. Biophys. Acta. 2014;1843(1):47–60. doi: 10.1016/j.bbamcr.2013.05.026. Epub 2013/06/12. doi: 10.1016/j.bbamcr.2013.05.026. PubMed PMID: 23747565; PubMed Central PMCID: PMCPMC4109693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevissen T.E.T., Komander D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017;86:159–192. doi: 10.1146/annurev-biochem-061516-044916. Epub 2017/05/13. doi: 10.1146/annurev-biochem-061516-044916. PubMed PMID: 28498721. [DOI] [PubMed] [Google Scholar]
- Michelle C., Vourc’h P., Mignon L., Andres C.R. What was the set of ubiquitin and ubiquitin-like conjugating enzymes in the eukaryote common ancestor? J. Mol. Evol. 2009;68(6):616–628. doi: 10.1007/s00239-009-9225-6. Epub 2009/05/20. doi: 10.1007/s00239-009-9225-6. PubMed PMID: 19452197; PubMed Central PMCID: PMCPMC2691932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.R., Boutell C., Keppler M., Densham R., Weekes D., Alamshah A. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009;462(7275):886–890. doi: 10.1038/nature08593. Epub 2009/12/18. doi: 10.1038/nature08593. PubMed PMID: 20016594. [DOI] [PubMed] [Google Scholar]
- Mossman K.L., Saffran H.A., Smiley J.R. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 2000;74(4):2052–2056. doi: 10.1128/jvi.74.4.2052-2056.2000. Epub 2000/01/22. doi: 10.1128/jvi.74.4.2052-2056.2000. PubMed PMID: 10644380; PubMed Central PMCID: PMCPMC111685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa H.H., Thompson T.W., Kushnir A.S., Haenchen S.D., Bayless A.M., Hilliard J.G. Herpes simplex virus 1 ICP0 phosphorylation site mutants are attenuated for viral replication and impaired for explant-induced reactivation. J. Virol. 2011;85(23):12631–12637. doi: 10.1128/JVI.05661-11. Epub 2011/09/23. doi: 10.1128/JVI.05661-11. PubMed PMID: 21937654; PubMed Central PMCID: PMCPMC3209388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa H.H., Thompson T.W., Davido D.J. N-terminal phosphorylation sites of herpes simplex virus 1 ICP0 differentially regulate its activities and enhance viral replication. J. Virol. 2013;87(4):2109–2119. doi: 10.1128/JVI.02588-12. Epub 2012/12/12. doi: 10.1128/JVI.02588-12. PubMed PMID: 23221554; PubMed Central PMCID: PMCPMC3571471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen M.A., Ciufo D.M., Hayward G.S. Mapping of intracellular localization domains and evidence for colocalization interactions between the IE110 and IE175 nuclear transactivator proteins of herpes simplex virus. J. Virol. 1994;68(5):3250–3266. doi: 10.1128/jvi.68.5.3250-3266.1994. Epub 1994/05/01. PubMed PMID: 8151787; PubMed Central PMCID: PMCPMC236816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S., Matunis M.J., Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17(1):61–70. doi: 10.1093/emboj/17.1.61. Epub 1998/02/28. doi: 10.1093/emboj/17.1.61. PubMed PMID: 9427741; PubMed Central PMCID: PMCPMC1170358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel C.H., Albrecht N., Milovic-Holm K., Mariyanna L., Keyser B., Abel B. Herpes simplex virus immediate-early protein ICP0 is targeted by SIAH-1 for proteasomal degradation. J. Virol. 2011;85(15):7644–7657. doi: 10.1128/JVI.02207-10. Epub 2011/06/03. doi: 10.1128/JVI.02207-10. PubMed PMID: 21632771; PubMed Central PMCID: PMCPMC3147933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D., Tahiliani P., Kumar A., Chandu D. The ubiquitin-proteasome system. J. Biosci. (Rajshari) 2006;31(1):137–155. doi: 10.1007/BF02705243. Epub 2006/04/06. doi: 10.1007/bf02705243. PubMed PMID: 16595883. [DOI] [PubMed] [Google Scholar]
- Newhart A., Rafalska-Metcalf I.U., Yang T., Negorev D.G., Janicki S.M. Single-cell analysis of Daxx and ATRX-dependent transcriptional repression. J. Cell. Sci. 2012;125(Pt 22):5489–5501. doi: 10.1242/jcs.110148. Epub 2012/09/15. doi: 10.1242/jcs.110148. PubMed PMID: 22976303; PubMed Central PMCID: PMCPMC3561858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll M.P., Proenca J.T., Efstathiou S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012;36(3):684–705. doi: 10.1111/j.1574-6976.2011.00320.x. Epub 2011/12/14. doi: 10.1111/j.1574-6976.2011.00320.x. PubMed PMID: 22150699; PubMed Central PMCID: PMCPMC3492847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F., Tsuchiya H. The emerging complexity of ubiquitin architecture. J. Biochem. 2017;161(2):125–133. doi: 10.1093/jb/mvw088. Epub 2016/12/25. doi: 10.1093/jb/mvw088. PubMed PMID: 28011818. [DOI] [PubMed] [Google Scholar]
- Orzalli M.H., DeLuca N.A., Knipe D.M. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Nat. Acad. Sci. U. S. A. 2012;109(44):E3008–17. doi: 10.1073/pnas.1211302109. Epub 2012/10/03. doi: 10.1073/pnas.1211302109. PubMed PMID: 23027953; PubMed Central PMCID: PMCPMC3497734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzalli M.H., Broekema N.M., Diner B.A., Hancks D.C., Elde N.C., Cristea I.M. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc. Nat. Acad. Sci. U. S. A. 2015;112(14):E1773–81. doi: 10.1073/pnas.1424637112. Epub 2015/04/02. doi: 10.1073/pnas.1424637112. PubMed PMID: 25831530; PubMed Central PMCID: PMCPMC4394261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzalli M.H., Broekema N.M., Knipe D.M. Relative contributions of herpes simplex virus 1 ICP0 and vhs to loss of cellular IFI16 vary in different human cell types. J. Virol. 2016;90(18):8351–8359. doi: 10.1128/JVI.00939-16. Epub 2016/07/15. doi: 10.1128/JVI.00939-16. PubMed PMID: 27412599; PubMed Central PMCID: PMCPMC5008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J., Lees-Miller S.P., Everett R.D. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 1999;73(1):650–657. doi: 10.1128/jvi.73.1.650-657.1999. Epub 1998/12/16. PubMed PMID: 9847370; PubMed Central PMCID: PMCPMC103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfoh R., Lacdao I.K., Georges A.A., Capar A., Zheng H., Frappier L. Crystal structure of USP7 ubiquitin-like domains with an ICP0 peptide reveals a novel mechanism used by viral and cellular proteins to target USP7. PLoS Pathog. 2015;11(6):e1004950. doi: 10.1371/journal.ppat.1004950. Epub 2015/06/06. doi: 10.1371/journal.ppat.1004950. PubMed PMID: 26046769; PubMed Central PMCID: PMCPMC4457826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placek B.J., Huang J., Kent J.R., Dorsey J., Rice L., Fraser N.W. The histone variant H3.3 regulates gene expression during lytic infection with herpes simplex virus type 1. J. Virol. 2009;83(3):1416–1421. doi: 10.1128/JVI.01276-08. Epub 2008/11/14. doi: 10.1128/JVI.01276-08. PubMed PMID: 19004946; PubMed Central PMCID: PMCPMC2620911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C., Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366(6467):818–822. doi: 10.1126/science.aax3769. Epub 2019/11/16. doi: 10.1126/science.aax3769. PubMed PMID: 31727826. [DOI] [PubMed] [Google Scholar]
- Pourchet A., Modrek A.S., Placantonakis D.G., Mohr I., Wilson A.C. Modeling HSV-1 latency in human embryonic stem cell-derived neurons. Pathogens. 2017;6(2) doi: 10.3390/pathogens6020024. Epub 2017/06/09. doi: 10.3390/pathogens6020024. PubMed PMID: 28594343; PubMed Central PMCID: PMCPMC5488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozhidaeva A.K., Mohni K.N., Dhe-Paganon S., Arrowsmith C.H., Weller S.K., Korzhnev D.M. Structural characterization of interaction between human ubiquitin-specific protease 7 and immediate-early protein ICP0 of herpes simplex Virus-1. J. Biol. Chem. 2015;290(38):22907–22918. doi: 10.1074/jbc.M115.664805. Epub 2015/08/01. doi: 10.1074/jbc.M115.664805. PubMed PMID: 26224631; PubMed Central PMCID: PMCPMC4645603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W.R., Schaffer P.A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 1987;61(3):829–839. doi: 10.1128/jvi.61.3.829-839.1987. Epub 1987/03/01. PubMed PMID: 3027408; PubMed Central PMCID: PMCPMC254026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kato A., Arii J., Koyanagi N., Kozuka-Hata H., Oyama M. Ubiquitin-specific protease 9X in host cells interacts with herpes simplex virus 1 ICP0. J. Vet. Med. Sci. 2016;78(3):405–410. doi: 10.1292/jvms.15-0598. Epub 2015/11/26. doi: 10.1292/jvms.15-0598. PubMed PMID: 26596467; PubMed Central PMCID: PMCPMC4829507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Blyszcz T., Gonzalez-Prieto R., Vertegaal A.C.O., Goldberg A.L. Inhibiting ubiquitination causes an accumulation of SUMOylated newly synthesized nuclear proteins at PML bodies. J. Biol. Chem. 2019;294(42):15218–15234. doi: 10.1074/jbc.RA119.009147. Epub 2019/07/10. doi: 10.1074/jbc.RA119.009147. PubMed PMID: 31285264; PubMed Central PMCID: PMCPMC6802522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T.H., Lin H.K., Scaglioni P.P., Yung T.M., Pandolfi P.P. The mechanisms of PML-nuclear body formation. Mol. Cell. 2006;24(3):331–339. doi: 10.1016/j.molcel.2006.09.013. Epub 2006/11/04. doi: 10.1016/j.molcel.2006.09.013. PubMed PMID: 17081985; PubMed Central PMCID: PMCPMC1978182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirata N., Kudoh A., Daikoku T., Tatsumi Y., Fujita M., Kiyono T. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J. Biol. Chem. 2005;280(34):30336–30341. doi: 10.1074/jbc.M500976200. Epub 2005/06/21. doi: 10.1074/jbc.M500976200. PubMed PMID: 15964848. [DOI] [PubMed] [Google Scholar]
- Sloan E., Tatham M.H., Groslambert M., Glass M., Orr A., Hay R.T. Analysis of the SUMO2 proteome during HSV-1 infection. PLoS Pathog. 2015;11(7):e1005059. doi: 10.1371/journal.ppat.1005059. Epub 2015/07/23. doi: 10.1371/journal.ppat.1005059. PubMed PMID: 26200910; PubMed Central PMCID: PMCPMC4511656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E., Orr A., Everett R.D. MORC3, a component of PML nuclear bodies, has a role in restricting herpes simplex virus 1 and human cytomegalovirus. J. Virol. 2016;90(19):8621–8633. doi: 10.1128/JVI.00621-16. Epub 2016/07/22. doi: 10.1128/JVI.00621-16. PubMed PMID: 27440897; PubMed Central PMCID: PMCPMC5021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Weller S.K. HSV-I and the cellular DNA damage response. Future Virol. 2015;10(4):383–397. doi: 10.2217/fvl.15.18. Epub 2015/07/28. doi: 10.2217/fvl.15.18. PubMed PMID: 26213561; PubMed Central PMCID: PMCPMC4508672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel M., Chan B., Brinkmann M.M. Coevolution pays off: herpesviruses have the license to escape the DNA sensing pathway. Med. Microbiol. Immunol. 2019;208(3–4):495–512. doi: 10.1007/s00430-019-00582-0. Epub 2019/02/26. doi: 10.1007/s00430-019-00582-0. PubMed PMID: 30805724. [DOI] [PubMed] [Google Scholar]
- Stow N.D., Stow E.C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 1986;67(Pt 12):2571–2585. doi: 10.1099/0022-1317-67-12-2571. Epub 1986/12/01. doi: 10.1099/0022-1317-67-12-2571. PubMed PMID: 3025339. [DOI] [PubMed] [Google Scholar]
- Suzich J.B., Cliffe A.R. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology. 2018;522:81–91. doi: 10.1016/j.virol.2018.07.011. Epub 2018/07/18. doi: 10.1016/j.virol.2018.07.011. PubMed PMID: 30014861; PubMed Central PMCID: PMCPMC6092753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellman N.M., Triezenberg S.J. Herpes simplex virus establishment, maintenance, and reactivation: in vitro modeling of latency. Pathogens. 2017;6(3) doi: 10.3390/pathogens6030028. Epub 2017/06/24. doi: 10.3390/pathogens6030028. PubMed PMID: 28644417; PubMed Central PMCID: PMCPMC5617985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellman N.M., Botting C., Madaj Z., Triezenberg S.J. An immortalized human dorsal root ganglion cell line provides a novel context to study herpes simplex virus 1 latency and reactivation. J. Virol. 2017;91(12) doi: 10.1128/JVI.00080-17. Epub 2017/04/14. doi: 10.1128/JVI.00080-17. PubMed PMID: 28404842; PubMed Central PMCID: PMCPMC5446634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.L., Sawtell N.M. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J. Virol. 2006;80(22):10919–10930. doi: 10.1128/JVI.01253-06. Epub 2006/09/01. doi: 10.1128/JVI.01253-06. PubMed PMID: 16943285; PubMed Central PMCID: PMCPMC1642178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.L., Preston C.M., Sawtell N.M. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5(3):e1000352. doi: 10.1371/journal.ppat.1000352. Epub 2009/03/28. doi: 10.1371/journal.ppat.1000352. PubMed PMID: 19325890; PubMed Central PMCID: PMCPMC2654966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeshi N.D., Mertins P., Svinkina T., Carr S.A. Large-scale identification of ubiquitination sites by mass spectrometry. Nat. Protoc. 2013;8(10):1950–1960. doi: 10.1038/nprot.2013.120. Epub 2013/09/21. doi: 10.1038/nprot.2013.120. PubMed PMID: 24051958; PubMed Central PMCID: PMCPMC4725055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L., Almine J.F. Camouflage and interception: how pathogens evade detection by intracellular nucleic acid sensors. Immunology. 2019;156(3):217–227. doi: 10.1111/imm.13030. Epub 2018/12/01. doi: 10.1111/imm.13030. PubMed PMID: 30499584; PubMed Central PMCID: PMCPMC6376273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme E., Laukens K., Dang T.H., Van Ostade X. A manually curated network of the PML nuclear body interactome reveals an important role for PML-NBs in SUMOylation dynamics. Int. J. Biol. Sci. 2010;6(1):51–67. doi: 10.7150/ijbs.6.51. Epub 2010/01/21. doi: 10.7150/ijbs.6.51. PubMed PMID: 20087442; PubMed Central PMCID: PMCPMC2808052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lint A.L., Murawski M.R., Goodbody R.E., Severa M., Fitzgerald K.A., Finberg R.W. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 2010;84(20):10802–10811. doi: 10.1128/JVI.00063-10. Epub 2010/08/06. doi: 10.1128/JVI.00063-10. PubMed PMID: 20686034; PubMed Central PMCID: PMCPMC2950559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni E., Gatherer D., Tong L., Everett R.D., Boutell C. Functional characterization of residues required for the herpes simplex virus 1 E3 ubiquitin ligase ICP0 to interact with the cellular E2 ubiquitin-conjugating enzyme UBE2D1 (UbcH5a) J. Virol. 2012;86(11):6323–6333. doi: 10.1128/JVI.07210-11. Epub 2012/03/23. doi: 10.1128/JVI.07210-11. PubMed PMID: 22438555; PubMed Central PMCID: PMCPMC3372195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang K., Li J., Zheng C. Herpes simplex virus 1 ubiquitin-specific protease UL36 inhibits beta interferon production by deubiquitinating TRAF3. J. Virol. 2013;87(21):11851–11860. doi: 10.1128/JVI.01211-13. Epub 2013/08/30. doi: 10.1128/JVI.01211-13. PubMed PMID: 23986588; PubMed Central PMCID: PMCPMC3807349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D.E., Weller S.K. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 2004;78(9):4783–4796. doi: 10.1128/JVI.78.9.4783-4796.2004. Epub 2004/04/14. doi: 10.1128/jvi.78.9.4783-4796.2004. PubMed PMID: 15078960; PubMed Central PMCID: PMCPMC387708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.C., Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012;20(12):604–611. doi: 10.1016/j.tim.2012.08.005. Epub 2012/09/12. doi: 10.1016/j.tim.2012.08.005. PubMed PMID: 22963857; PubMed Central PMCID: PMCPMC3989139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Paige J.S., Jaffrey S.R. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat. Biotechnol. 2010;28(8):868–873. doi: 10.1038/nbt.1654. Epub 2010/07/20. doi: 10.1038/nbt.1654. PubMed PMID: 20639865; PubMed Central PMCID: PMCPMC2946519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F., Schaffer P.A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 1995;69(10):6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. Epub 1995/10/01. PubMed PMID: 7666525; PubMed Central PMCID: PMCPMC189522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang K., Wang S., Zheng C. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. J. Virol. 2013;87(23):12935–12948. doi: 10.1128/JVI.01952-13. Epub 2013/09/27. doi: 10.1128/JVI.01952-13. PubMed PMID: 24067962; PubMed Central PMCID: PMCPMC3838126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Muller S., Ronchetti S., Freemont P.S., Dejean A., Pandolfi P.P. Role of SUMO-1-modified PML in nuclear body formation. Blood. 2000;95(9):2748–2752. Epub 2000/04/26. PubMed PMID: 10779416. [PubMed] [Google Scholar]