Abstract
We describe a 60 year old man who developed respiratory insufficiency after treatment with 2 rounds of nivolumab monotherapy. Imaging revealed subtle ground glass infiltrates which progressed to diffuse opacities and consolidation. The patient was treated with high dose corticosteroids, empiric antimicrobial therapy and infliximab. Bronchoscopy with lavage revealed negative cultures and progressive bloody aliquots of fluid consistent with diffuse alveolar hemorrhage. The patient succumbed to respiratory failure. An autopsy study confirmed extensive alveolar hemorrhage. Our reports highlights clinical and diagnostic findings with immunotherapy-induced pneumonitis.
Keywords: Drug-induced pneumonitis, Diffuse alveolar hemorrhage, Immune checkpoint inhibitor, Immunotherapy, Cancer
1. Case
A 60 year old man with metastatic bladder cancer presented with dyspnea and dry cough, 8 days after cycle 1 of nivolumab. His past medical history was unremarkable aside from a 20 pack-year smoking history. Admitting blood work demonstrated a stable hemoglobin of 9.9 g/dL with a normal white blood cell count (7.6 K/μL) and platelet count (269 K/μL). Other laboratory investigations, including coagulation profile, chemistry, liver tests, blood urea nitrogen, and creatinine were within normal limits. The patient was not taking any anti-platelet or anticoagulant therapy. Computed tomography (CT) of the chest revealed new subtle bilateral ground glass opacities (Fig. 1A–D). He was treated for presumed COPD exacerbation with bronchodilators and antibiotics, and he was discharged home. Six days following cycle 2 of nivolumab, the patient was hospitalized for severe, progressive dyspnea and dry cough. At presentation, he denied fevers or chills. CT chest showed interval progression of ground glass infiltrates and consolidation (Fig. 1E and F). Nivolumab was held. Despite high dose corticosteroids, empiric antibiotics and infliximab, the patient required intubation for progressive hypoxemia respiratory failure (Fig. 1G). Bronchoscopic examination with bronchoalveolar lavage (BAL) was performed and suggested diffuse alveolar hemorrhage (DAH). Cultures, cytology and serologic studies, including cytoplasmic anti-neutrophil cytoplasmic antibody (C-ANCA), perinuclear anti-neutrophil cytoplasmic antibody (P-ANCA), anti-nuclear antibody (ANA), anti-double strand DNA antibody, and anti-glomerular basement membrane antibody were all negative. The patient succumbed to refractory respiratory failure. Autopsy studies (Fig. 2) confirmed florid bland DAH without other lung pathology.
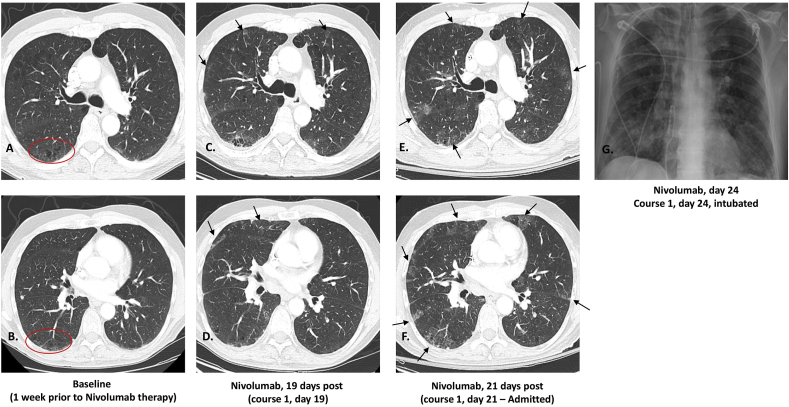
Fig. 1.
Computed tomography (CT) of the chest imaging of the upper and lower chest at baseline (A–B) shows subtle emphysematous changes (circle). Five days after cycle 1 of nivolumab (C–D), bilateral, predominantly subpleural ground glass opacities are seen, and no pulmonary embolism was detected. Symptoms and degree of hypoxia appeared disproportionate to the relatively subtle CT findings at day 5 post-nivolumab therapy. At cycle 2, day 11 post-nivolumab, the ground glass opacities are more prominent (E–F). A portable chest radiograph following intubation shows diffuse bilateral airspace disease (G).
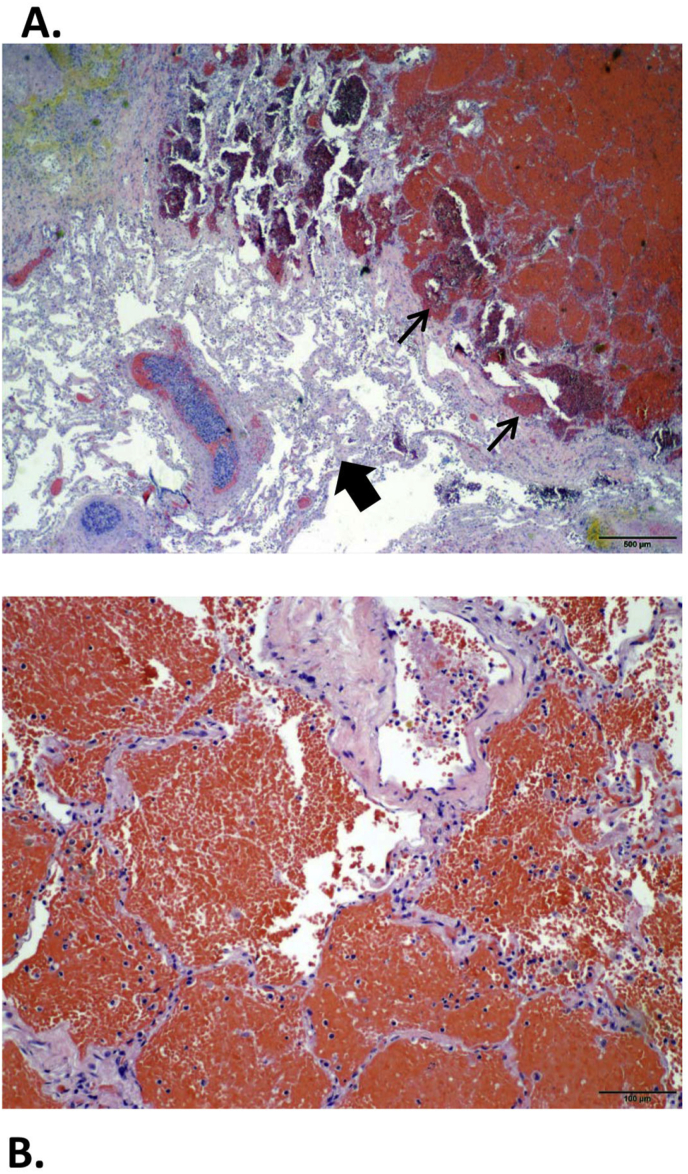
Fig. 2.
Autopsy studies confirmed extensive alveolar hemorrhage (A, thin arrows reveal red blood cells filling alveoli) with minimal inflammatory reaction within the lung parenchyma (A, thick arrows demonstrate relatively unaffected lung parenchyma). Blood extended into the airways (B, higher power view of red blood cells filling alveolar spaces) and lung, forming bilateral central airway clots and markedly consolidated and hemorrhagic lung parenchyma. No endobronchial sources for bleeding and no pulmonary emboli were found. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Discussion
The most frequent histopathologic manifestations of pneumonitis associated with the PD-1 inhibitor are organizing pneumonia (OP) and nonspecific interstitial pneumonitis (NSIP) [1,2]. We present a case of fatal DAH following nivolumab therapy, a PD-1-targeted immune checkpoint inhibitor. Patients with DAH typically present with symptoms of cough and dyspnea, which may rapidly evolve over a few days to a week, as in our patient. Hemoptysis is absent in up to 1/3 of patients, and thus cannot be used to exclude the diagnosis [3]. Low grade fever and anemia are also nonspecific findings. The diagnosis of drug-induced DAH is strongly suggested by the temporal relationship between drug exposure and symptom development, coupled with patchy diffuse areas of ground glass attenuation and consolidation on chest CT. These common CT findings of DAH are nonspecific, but distinct from the migratory patchy, alveolar opacities distributed over the lung periphery in association with OP and the ill-defined ground glass attenuations and reticulonodular opacities with peripheral and lower lobe distribution that define NSIP. In our patient, competing diagnoses, such as infection and capillaritis were excluded, based on negative cultures and serologies, minimal inflammatory infiltrates within the lung parenchyma and the absence of perivascular inflammatory infiltrates at necropsy.
Only 2 other descriptions of DAH associated with PD-1 axis inhibition are reported in the literature. Ikeda and colleagues described one patient who developed DAH in association with pseudoprogression 3 months after initiation of nivolumab for metastatic melanoma. In this case, DAH rapidly improved with corticosteroid therapy [4]. In another report, following 8 cycles of nivolumab therapy, fatal DAH developed in association with Goodpasture's syndrome, which was confirmed at autopsy [5]. DAH occurred in our patient in the absence of any associated bleeding disorders or inflammatory peritumoral reaction within the lung. The pathogenic mechanisms involved in DAH-related immune checkpoint inhibitors have not been fully elucidated, however damage to endothelial cells within small blood vessels of the lung caused by cytotoxic T lymphocytes has been postulated. Recent evidence suggests that immune activation by PD-1 and PD-L1 blockade may impair coagulation homeostasis and disrupt the balance between clotting and bleeding. This imbalance in the coagulation-fibrinolysis system may not only promote hypercoagulability and thromboembolic events but also trigger downstream disorders, such as local consumptive coagulopathy and tissue damage and lead to bleeding complications [6].
Our case represents the third reported case DAH attributed to PD-1 inhibition and highlights the fact that this association may occur in the absence of other lung pathology or bleeding disorders. Drug-induced DAH following anticancer pharmacotherapies has been rarely reported following cytotoxic chemotherapies such as gemtuzumab, all-trans retinoic acid and the molecular targeted agent, rituximab. In this setting, DAH typically occurs in in the context of diffuse alveolar damage (DAD) [[7], [8], [9], [10], [11]]. No significant pathologic foci of DAD were seen at autopsy in our case, rendering this diagnosis as an underlying trigger for DAH unlikely. We conclude that nivolumab-related DAH was the proximate cause of fatal lung injury in our patient. Early recognition of signs and symptoms of immunotherapy-associated pneumonitis is crucial, as increased morbidity and mortality are associated with delays in treatment.
3. Conclusion
DAH is a rare complication of the anti-PD-1 inhibitor, nivolumab and may occur in the absence of other bleeding diatheses and disorders. Signs and symptoms of DAH may be subtle at initial presentation but rapidly evolve to respiratory failure and death. Early recognition is critical to improving the outcome of this potentially fatal complication.
Funding
This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant (CA016672).
Author contributions
VRS and SAF developed the conception and design of the study and drafted the article. VRS, SAF, SKM, and LH facilitated acquisition of radiological and pathological data. All authors (VRS, SAF, SKM, LH) provided critical revision of intellectual content and final approval of the version to be published.
Declaration of competing interest
The author declares that no conflicts of interest exist.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101131.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Naidoo J., Page D.B., Li B.T., Connell L.C., Schindler K., Lacouture M.E., Postow M.A., Wolchok J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tirumani S.H., Ramaiya N.H., Keraliya A., Bailey N.D., Ott P.A., Hodi F.S., Nishino M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3:1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamora M.R., Warner M.L., Tuder R., Schwarz M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltim.) 1997;76:192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda T., Yamaguchi H., Dotsu Y., Taniguchi H., Gyoutoku H., Senju H., Sakamoto N., Iwanaga S., Kuwatsuka Y., Fukuda M., Mukae H. Diffuse alveolar hemorrhage with pseudoprogression during nivolumab therapy in a patient with malignant melanoma. Thorac Cancer. 2018;9:1522–1524. doi: 10.1111/1759-7714.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi N., Tsuji K., Tamiya H., Shinohara T., Kuroda N., Takeuchi E. Goodpasture's disease in a patient with advanced lung cancer treated with nivolumab: an autopsy case report. Lung Canc. 2018;122:22–24. doi: 10.1016/j.lungcan.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Sato R., Imamura K., Sakata S., Ikeda T., Horio Y., Iyama S., Akaike K., Hamada S., Jodai T., Nakashima K., Ishizuka S., Sato N., Saruwatari K., Saeki S., Tomita Y., Sakagami T. Disorder of coagulation-fibrinolysis system: an emerging toxicity of anti-PD-1/PD-L1 monoclonal antibodies. J. Clin. Med. 2019;8 doi: 10.3390/jcm8060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vulsteke C., Dierickx D., Verbeken E., Wolter P., Thomas J., Schoffski P. Rituximab-induced fatal interstitial pneumonitis: case report. Leuk. Lymphoma. 2010;51:546–548. doi: 10.3109/10428190903518303. [DOI] [PubMed] [Google Scholar]
- 8.Lataifeh A.R., Nusair A. Fatal pulmonary toxicity due to carfilzomib (Kyprolis) J. Oncol. Pharm. Pract. 2016;22:720–724. doi: 10.1177/1078155215588630. [DOI] [PubMed] [Google Scholar]
- 9.Fyfe A.J., McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213–215. doi: 10.4997/JRCPE.2010.306. [DOI] [PubMed] [Google Scholar]
- 10.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 11.Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir. Res. 2012;13:39. doi: 10.1186/1465-9921-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.