Abstract
Objectives
The effects of 2-methacryloyloxyethyl phosphorylcholine (MPC)-polymer on the adherence of microorganisms such as non-Candida albicans Candida (NCAC) and methicillin-resistant Staphylococcus aureus (MRSA), frequently detected in oral infections in immunocompromised and/or elderly people, to denture resin material, are still unclear. Here, we report the effects of MPC-polymer on the adherence of C. albicans, NCAC, and MRSA to acrylic denture resin.
Methods
Sixteen strains of C. albicans, seven strains of C. glabrata, two strains of C. tropicalis, one strain of C. parapsilosis, and six strains of MRSA were used. We cultured the fungal/bacterial strains and examined the cell growth and adherence of fungi/bacteria to mucin-coated acrylic denture resin plates (ADRP) with or without MPC-polymer coating, by scanning electron microscopy. The cell surface hydrophobicity of the fungal/bacterial strains was measured by the adsorption to hydrocarbons.
Results
MPC-polymer did not affect the growth of all strains of Candida species and MRSA, but significantly suppressed adherence to ADRP in most strains of C. albicans and all strains of NCAC and MRSA. A significant positive correlation was found between cell hydrophobicity and the reduction rates of microbial adherence to ADRP treated with 5% of MPC-polymer.
Conclusions
MPC-polymer treatment for acrylic resin material suppresses the adherence of C. albicans, NCAC and MRSA via their hydrophilicity interaction.
Clinical significance
The application of MPC-polymer for denture hygiene is potent to prevent oral candidiasis, denture stomatitis and opportunistic infection, caused by Candida species and MRSA, via suppressing the adherence of those fungus/bacteria.
Keywords: Microbiology, Medical microbiology, Dentistry, Dental materials, Oral medicine, Prosthetic dentistry, 2-Methacryloyloxyethyl phosphorylcholine, Denture, Candida, MRSA, Hydrophilicity
Microbiology; Medical microbiology; Dentistry; Dental materials; Oral medicine; Prosthetic dentistry; 2-Methacryloyloxyethyl phosphorylcholine; Denture; Candida; MRSA; Hydrophilicity.
1. Introduction
The number of elderly people in the world has been increasing due to the significant improvements in health and medical care [1, 2]. It is predicted that the elderly will comprise over 25% of the total population by 2025 in Japan [3]. However, the number of elderly individuals with frailty due to cognitive impairment and cerebrovascular disorder is also on the rise. Research has shown that lowering the daily activities of the elderly will worsen their oral health rapidly, resulting in a high prevalence of edentulism and denture wearing [2]. Dentures may act as a reservoir for respiratory and systemic opportunistic pathogens, and as an ecological niche for antibiotic-resistant bacteria to thrive in [4, 5]. To prevent these problems, denture hygiene formulas with reliable cleaning effects and safety are needed.
Candida species are frequently implicated in mixed bacteria-fungal infections and form biofilms on the surfaces of indwelling medical devices [6]. In oral region, Candida species cause denture stomatitis through direct adherence to denture surfaces [7], and, caries, root caries and periodontitis of abutment teeth because Candida co-aggregate with other bacteria occurring their infectious diseases [8]. Candida albicans, a diploid, polymorphic opportunistic fungus that can exist as yeast, hyphal, and pseudohyphal forms [9], is a predominant fungus that causes a variety of oral infections such as denture stomatitis in immunocompromised and elderly populations. It has been reported that C. albicans represents over 80% of the fungal isolates from all forms of human candidiasis and remains the most common pathogen [10]. Non-C. albicans Candida (NCAC), however, such as Candida glabrata, Candida tropicalis, and Candida parapsilosis, have also been frequently isolated [10, 11]. NCAC are responsible for higher counts in denture stomatitis patients [12]. C. glabrata is most frequently co-isolated with C. albicans in mixed species oral infections [13], and is reported to establish oropharyngeal candidiasis by binding to C. albicans hyphae [14]. C. glabrata has become intermediately resistant to all azole class antifungals, and about 15–20% of the strains develop resistance during therapy and antifungal prophylaxis with fluconazole [11, 15]. Neutrophil extracellular traps (NETs) are networks of extracellular fibers composed of modified chromatin decorated with specific cytoplasmic and granular proteins that entrap and eliminate bacteria, fungi, parasites, and viruses, are one of the infection defense mechanisms of neutrophils. Recently, it was reported that C. glabrata in some clinical isolates did not show NETs formation, which may be correlated with the high mortality rates in infected patients [16]. C. tropicalis is capable of adhesion with C. albicans and a dual culture biofilm of C. albicans with C. tropicalis offers a growth advantage for C. tropicalis [17]. C. tropicalis also has reduced susceptibility to azoles [11]. In denture biofilms, not only Candida spp. but also Streptococcus spp., Staphylococcus spp., gram-positive rods, lactobacilli, Propionibacterium spp., Veillonella spp., and gram-negative rods are isolated [18, 19]. Opportunistic microorganisms such as Staphylococcus aureus and methicillin-resistant S. aureus (MRSA), are frequently isolated from the mouth of elderly people [20, 21, 22] and have been reported as a cause of denture stomatitis [23]. S. aureus has been reported to co-aggregate with C. albicans [24]. Therefore, it is necessary to consider not only C. albicans but also NCAC and opportunistic bacteria as targets for denture hygiene control.
Recenty various kinds of biomaterials have been studied in vitro and in animal models to develop novel strategy for dental treatment, including bone regeneration, implant and denture [25]. It has been shown that the biological properties MPC-polymer, which is characterized by a phospholipid polar group that mimics a biomembrane, is completely harmless to humans; effective in reducing protein adsorption and bacterial adhesion, and inhibiting cell attachment [26, 27, 28]. We previously reported that MPC-polymer application significantly inhibited both the adherence and biofilm formation of Streptococcus mutans on saliva-coated hydroxyapatite, and streptococcal adherence to oral epithelial cells; and reduced the adherence of Fusobacterium nucleatum to streptococcal biofilms in vitro [29]. We also reported that mouth-rinsing with 5% of MPC-polymer inhibited the increase of oral bacterial numbers in a clinical trial [30]. F. nucleatum is known to play a central role as a physical bridge to mediate the co-aggregation of bacterial cells and promote an anaerobic microenvironment. The inhibition of this co-aggregation by MPC-polymer coating suggests that it is a potent measure to prevent dental plaque formation and subsequent dental caries and periodontal disease [30].
Ikeya et al. reported that complete dentures with photoreactive monomer bearing a phenylazide group, 2-methacryloyloxyethyl-4-azidobenzoate (PMBPAz) significantly inhibited bacterial plaque accumulation in vivo as confirmed by methylene blue staining technique [31]. Graft polymerization of MPC on an acrylic resin denture significantly reduced the adherence of C. albicans [32]. However, the effects of MPC-polymer on the adherence of microorganisms remain unclear, especially in NCAC and MRSA that are frequently detected in the immunocompromised and elderly populations.
In the present study, we demonstrated that MPC-polymer treatment for acrylic resin material suppressed the adherence of C. albicans, NCAC and MRSA via their hydrophilicity interaction. Application of MPC-polymer for denture hygiene is a promising strategy to prevent denture stomatitis and related infections caused by these microbes.
2. Materials and methods
2.1. Bacterial strains, culture media, and culture conditions
The fungal/bacterial strains used are shown in Table 1. Sabouraud dextrose medium composed of 10 g/L peptone and 40 g/L glucose, and Trypticase soy broth (TSB, Becton Dickinson, Sparks, MD, USA) were used for Candida species and MRSA, respectively. All Candida species were cultured aerobically at 37 °C for 48 h. MRSA was cultured aerobically with shaking at 37 °C for 18 h.
Table 1.
Bacterial strain and their source used in this study.
| Fungal/Bacterial species | Strain | Source |
|---|---|---|
| Candida albicans | JCM1542, JCM2085 | Type strains |
| CAD1, KG5, KG8, KG12, KG13, TCa8, TCa9, TCa10, TCa12, TCa14, TCa15, TCa16, TG13, TU3 | Clinical isolates | |
| C. glabrata | JCM3761 | Type strain |
| 4-332, 4–337, 4–389, 4–401, 4–422, 4-438 | Clinical isolates | |
| C. tropicalis | JCM1541 | Type strain |
| TCa5 | Clinical isolate | |
| C. parapsilosis | KG7 | Clinical isolate |
| Staphylococcus aureus (MRSA) | COL, T-1, T-15, T-26, T-31, T-36 | Clinical isolates |
2.2. Preparation of acrylic denture resin plates (ADRPs) and MPC-polymers
Heat cured acrylic denture resins (ACRON®, GC Corporation, Tokyo, Japan) were used for this study. Acrylic denture resin plates (ADRPs) were prepared in thin plates of 10 mm × 10 mm × 1 mm (thickness) dimensions, in accordance with ISO guidelines (ISO/DIS 1567: 1997). Polymerized ADRPs were sanded using waterproof sandpapers (#240, #600), and polished using formulated pastes containing rouge.
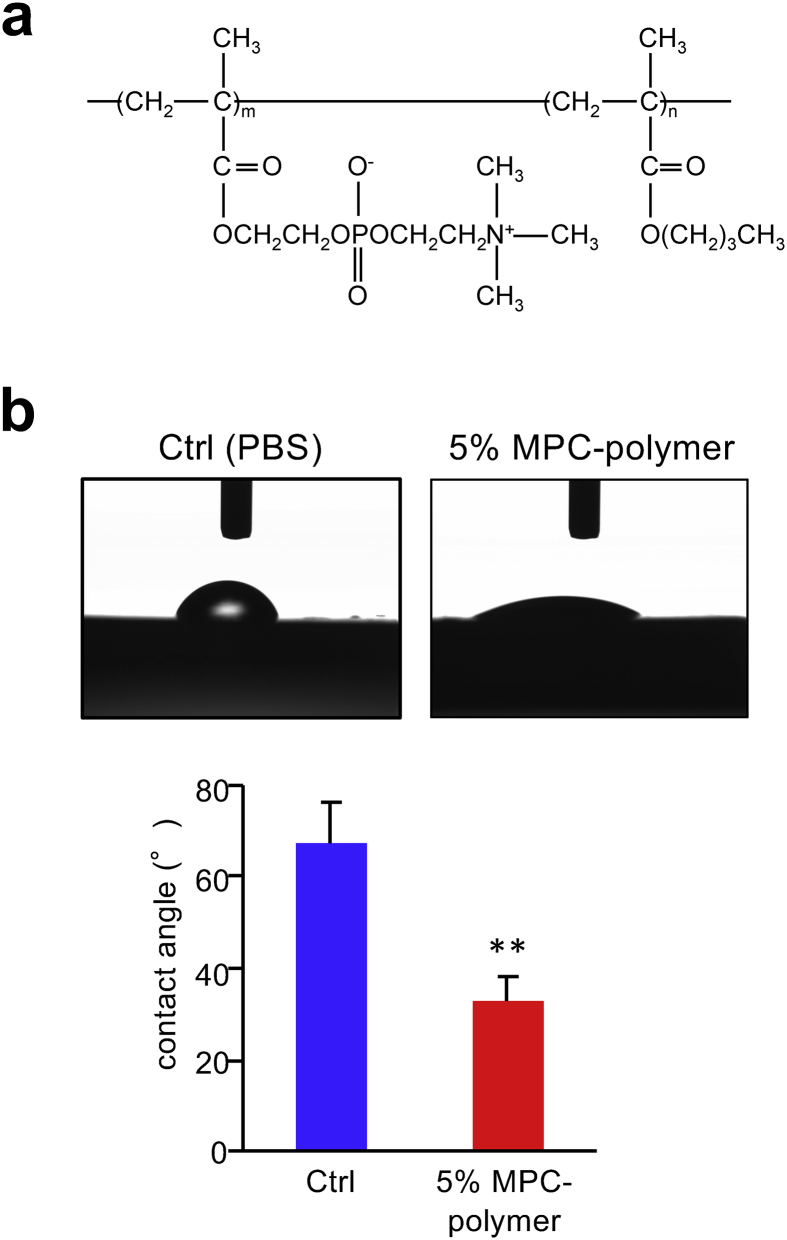
The biosafety of the MPC-polymer (Figure 1a) (Lipidure-PMB®; NOF Corp., Tokyo, Japan) was approved by the Food and Drug Administration (FDA) (510(k)-K000975). Soluble maximum concentration of MPC-polymer in water is 5%. Phosphate buffered saline (PBS, 0.01 M, pH 7.4) was used as the control in this study as already reported in our previous clinical trials and in vitro experiments [30, 33] and then MPC-polymer was also diluted with PBS.
Figure 1.
(a) Chemical structure of MPC-polymer. (b) Hydrophilicity of MPC-polymer-coated ADRP. We used the 10 mm × 10 mm × 1 mm (thickness) ADRP treated with 5% of MPC-polymer or PBS (control; Ctrl). Graph shows the contact angle of the 5% of MPC-polymer or PBS-coated ADRP surfaces measured by a contact angle meter. Bars represent the mean ± SD of three measurements. ∗∗P < 0.01.
2.3. Contact angle measurement
To confirm the wettability which is defined as the tendency of one fluid to spread on or adhere to a solid surface in the presence of other immiscible fluids, we performed the contact angle measurement in 5% MPC-polymer. ADRP was soaked in 0.5 mg/mL mucin (Sigma-Aldrich, USA) solution for 10 min in a 24-well plate and treated with 5% MPC-polymer or PBS as a control for 1 min. All excess of MPC-polymer or PBS was removed using lint-free wipers. One-microliter of PBS was then dropped onto the ADRP, and the contact angle was measured using a contact angle meter (DropMaster500, Kyowa Interface Science, Saitama, Japan). At least three biological replicates were performed for this experiment.
2.4. Growth inhibition of Candida species and MRSA by MPC-polymer
C. albicans JCM2085, C. glabrata JCM3761, C. tropicalis JCM1541, and MRSA COL were used. Powdery MPC-polymer was adjusted to 5% using Sabouraud dextrose medium or TSB. Approximately 1 × 106 colony forming unit (CFU) of fungal/bacterial cells were added to 3 mL of medium containing 5% of MPC-polymer. Growth of each fungal/bacterial culture was measured by OD600 at the indicated time-points.
2.5. Microbial adherence assay and scanning electron microscopy
Fungal/bacterial strains used in the microbial adherence assay are shown in Table 1. When denture is worn in the oral cavity, saliva will adhere almost instantly; moreover, Candida species bind to salivary mucins, which are proteins secreted by the salivary glands [34, 35]. We used the mucin-coated ADRP based on these viewpoints. ADRP was soaked in 0.5 mg/mL mucin solution for 10 min in a 24-well plate and treated for 1 min using MPC-polymer or PBS as a control. ADRP was placed into 1 mL fungal/bacterial suspension (1 × 107 CFU), and then incubated at room temperature for 90 min. To remove non-adherent cells, ADRP was washed twice with PBS, and cells adhered on the ADRP surface were collected in PBS treated with 0.25% trypsin. Recovered cells were serially diluted, and plated on Sabouraud dextrose or TSB agar plates, and incubated at 37 °C for 24 h. Colonies grown on the plates were counted, and the rate of adherence reduction by 5% MPC-polymer treatment was calculated as follows:
| Rate of adherence reduction (%) = 100 - (Number of CFUs with 5% MPC-polymer treatment/Number of CFUs with PBS treatment × 100) |
ADRPs adhered to C. albicans JCM2085, C. glabrata JCM3761, C. tropicalis JCM1541, and MRSA COL strains were dried to the critical point, coated with gold, and examined by scanning electron microscopy (SEM) (Miniscope TM-1000; Hitachi, Tokyo, Japan). At least three biological replicates were performed for each experiment were performed and representative images were shown in the Figure.
2.6. Hydrocarbon adsorption assay
For adsorption to hydrocarbon assay, fungal/bacterial cells shown in Table 1 were harvested by centrifugation at 12,000 rpm for 3 min after incubation and resuspended in phosphate urea magnesium sulfate (PUM) buffer (22.2 g of K2HPO4•3H2O, 7.26 g of KH2PO4, 1.8 g of urea, 0.2 g of MgSO4•7H2O, and 1 L of distilled water, pH 7.1) to an optical density (OD) of 0.5 at a wavelength of 660 nm. Cell-surface hydrophobicity was estimated using a modification of the hydrocarbon adsorption technique described by Rosenberg et al. [36]. An aliquot of 0.5 mL hexadecane (Sigma-Aldrich) was added to 3 mL of the microbial suspension in a test tube. The test tube was vigorously agitated for 2 min using a vortex mixer and then allowed to stand for 10 min at 37 °C. The turbidity of the aqueous phase was measured with a spectrophotometer, and the hydrophobicity was expressed as the percentage reduction of the initial turbidity of the aqueous suspension. At least three biological replicates were performed for this experiment.
2.7. Statistical analysis
Normal distributions in each data were confirmed by Shapiro-Wilk test. For contact angle measurement and microbial adherence assay, unpaired Student's t-test and paired Students' t-test, which is a common parametric test for comparing two groups with normal distributions, were used. Correlation between the hydrophobicity of fungus/bacteria and the microbial adherence to ADRP treated with MPC-polymer was assessed using Spearman's rank correlation coefficient. All statistical analyses were performed using SPSS version 24.0 (SPSS Japan Inc., Tokyo, Japan). Differences were considered significant when the probability values were less than 5% (P < 0.05).
3. Results
3.1. Effect of MPC-polymer coating on the hydrophobicity of ADRP
We determined the hydrophobicity of MPC-polymer coated-ADRP. The contact angle of mucin coated-ADRP treated with 5% of MPC-polymer (32.8 ± 5.4°) was significantly smaller than that of the control ADRP treated with PBS (67.4 ± 9.0°) (P < 0.01) (Figure 1b). This result indicates that MPC-polymer treatment changed the acrylic resin surface to be more hydrophilic and high wettability.
3.2. Effects of MPC-polymer on cell growth and adherence of C. albicans and NCAC to ADRP
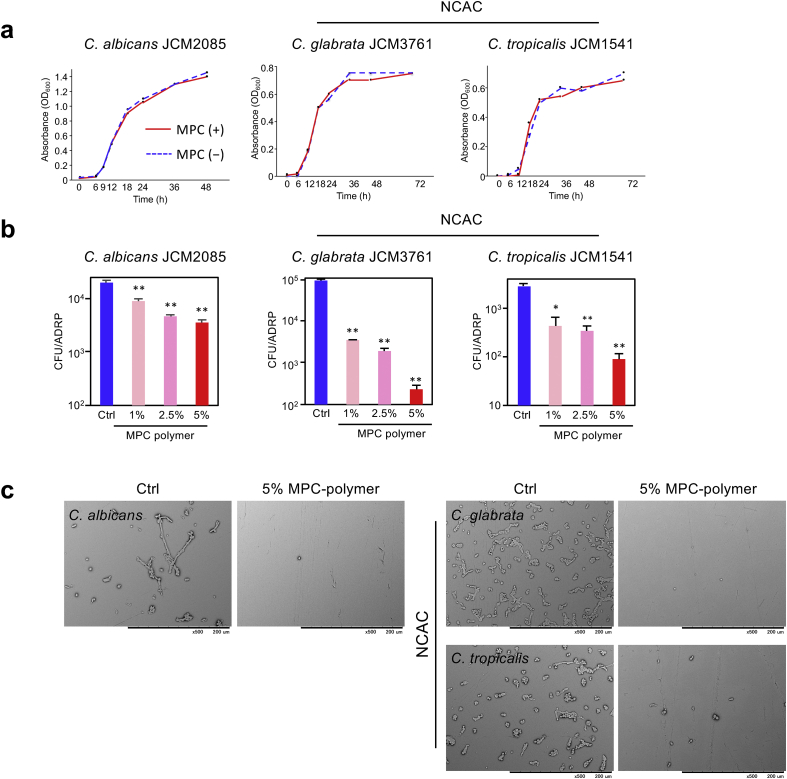
We examined the effect of 5% MPC-polymer on the growth of C. albicans JCM2085 and NCAC. In all strains, MPC-polymer did not have any inhibitory effect on cell growth (Figure 2a).
Figure 2.
Effects of MPC-polymer on cell growth and adherence of C. albicans and NCAC to ADRP. (a) C. albicans JCM2085, C. glabrata JCM3761, and C. tropicalis JCM1541 are used. 1 × 106 CFU of fungal cells were added into 3 mL of the medium containing 5% of MPC-polymer. Growth of each fungal culture was monitored as turbidity by the measurement of OD600 at the indicated time-points. (b) The adherence of fungal cells was determined by microbial adherence assay. Approximately 1 × 107 CFU of fungal cells was placed on ADRP and incubated for 90 min at room temperature. Graph shows the number of adherent cells on the ADRP surface after incubation with PBS (control; Ctrl), 1%, 2.5%, and 5% of MPC-polymer, respectively. Bars represent mean ± SD of three measurements. ∗P < 0.05 and ∗∗P < 0.01. (c) The adherent fungal cells on the ADRP surface after incubation with PBS and 5% of MPC-polymer were observed by SEM.
We examined the effect of MPC-polymer treatment on the adhesion of C. albicans JCM2085 and NCAC to mucin-coated ADRP. In all fungal strains, the number of cells adhered to ADRP treated with MPC-polymer was significantly lower than that in the control, in a concentration-dependent manner (Figure 2b). We also examined the adherent fungal cells on the ADRP by SEM. The number of fungi on the ADRP treated with 5% MPC-polymer was remarkably lower than that of the control (Figure 2c).
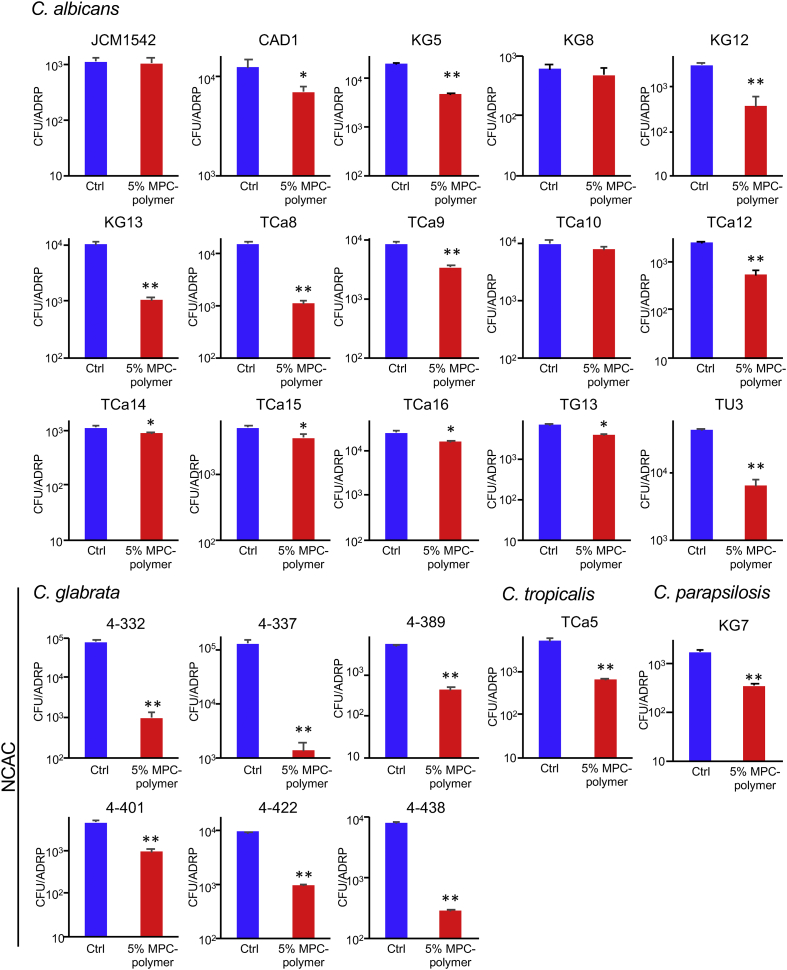
To confirm the inhibitory effect of MPC-polymer on fungal adherence, we used several fungal strains, including 15 strains of C. albicans, six strains of C. glabrata, one strain of C. tropicalis, and one strain of C. parapsilosis (Figure 3). In 12 out of the 15 C. albicans strains, treatment with 5% MPC-polymer significantly suppressed adherence to ADRP. It is also of interest that the 5% MPC-polymer treatment remarkably suppressed the adherence of all NCAC to ADRP (Figure 3).
Figure 3.
Effects of MPC-polymer coating on the adherence of various species of C. albicans and NCAC. We used several fungal strains, including 15 strains of C. albicans, six strains of C. glabrata, one strain of C. tropicalis, and one strain of C. parapsilosis. The adherence of fungal cells was determined by the same method as in Figure 2b. Graph shows the number of adherent cells on the surface of ADRP treated with PBS or 5% of MPC-polymer. Bars represent the mean ± SD of three measurements. ∗P < 0.05 and ∗∗P < 0.01.
3.3. Effects of MPC-polymer on cell growth and adherence of MRSA to ADRP
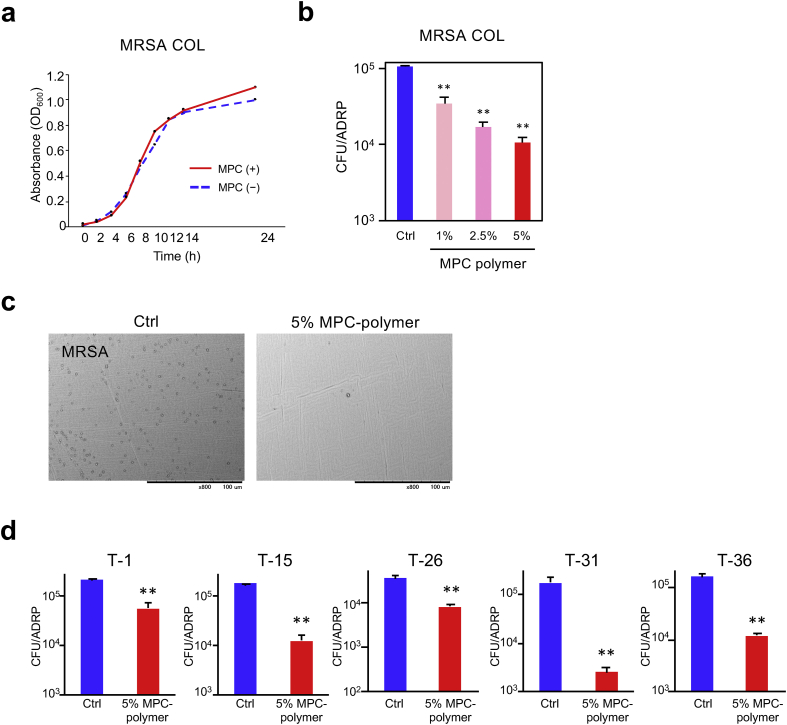
S. aureus is prevalent in the oral cavity and oropharynx in the elderly and immunocompromised hosts [21, 22]. S. aureus can change to MRSA through horizontal gene transfer and natural selection, conferring multiple drug resistance to beta-lactam antibiotics [37]. MRSA is a cause of several difficult-to-treat infections in humans. Therefore, we investigated the effect of MPC-polymer on MRSA cell growth and adherence to ADRP treated with MPC-polymer. Results show that 5% of MPC-polymer did not influence the cell growth of MRSA COL strain (Figure 4a). In the microbial adherence assay, the number of adherent MRSA COL strain to ADRP treated with MPC-polymer was significantly lower in a concentration-dependent manner (Figure 4b). We also confirmed the inhibitory effect by SEM (Figure 4c). For other strains of MRSA, 5% of MPC-polymer treatment significantly suppressed bacterial adherence to ADRP (Figure 4d).
Figure 4.
Effects of MPC-polymer on MRSA (a) 1 × 106 CFU of bacterial cells were added into 3 mL of the medium containing 5% of MPC-polymer. Bacterial culture growth was monitored by OD600 at the indicated time-points. (b) The adherence of bacterial cells was determined using the same method as that shown in Figure 2b. The graph shows the number of adherent cells on the surface of ADRP treated with PBS (control; Ctrl), 1%, 2.5%, or 5% of MPC-polymer, respectively. Bars represent the mean ± SD of three measurements. ∗∗P < 0.01. (c) Adherent bacterial cells on the surface of ADRP treated with PBS or 5% of MPC-polymer were observed by SEM. (d) The adherence of MRSA was determined by the same method as in Figure 2b. Graph shows the number of adherent cells on the surface of ADRP treated with PBS or 5% MPC-polymer. Bars represent the mean ± SD of three measurements. ∗∗P < 0.01.
3.4. Correlation between cell surface hydrophobicity of fungi/bacteria and adherence to the MPC-polymer-treated ADRP
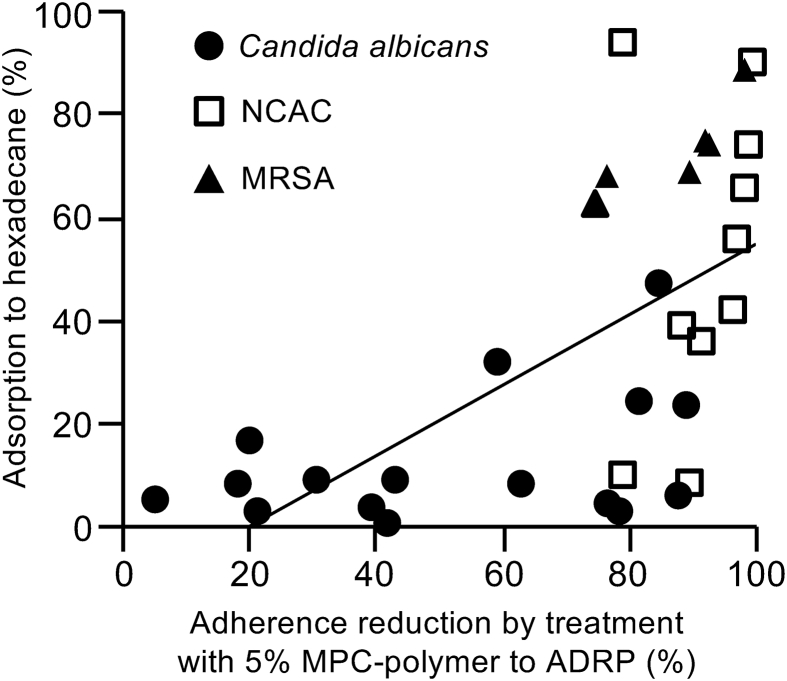
Since the 5% of MPC-polymer did not influence the cell growth of all fungal/bacterial strains (Figures 2a and 4a), the suppressive effect of MPC-polymer on the adherence of fungal/bacterial strains to the acrylic resin material is not due to cell growth inhibition. We found that MPC-polymer possesses high wettability, i.e. MPC-polymer increases surface hydrophilicity of ADRP (Figure 1b). To elucidate the mechanism of MPC-polymer on the suppression of fungal/bacterial adherence to ADRP, we measured the hydrophobicity of fungi and bacteria as listed in Table 1. Higher rate of adsorption to hexadecane indicates more higher hydrophobicity. Rate of adsorption to hexadecane in 16 strains of C. albicans was 0–52.7%, NCAC including seven strains of C. glabrata, two strains of C. tropicalis, and one strain of C. parapsilosis were 7.6–88.8%, 37.8–55.0%, and 93.2%, respectively. Six strains of MRSA adsorbed hexadecane between 62.0–88.4% (Table 2). Figure 5 shows the correlation between the hydrophobicity of fungi/bacteria and the rate of bacterial adherence reduction by ADRP treated with 5% of MPC-polymer. The hydrophobicity of the fungi/bacteria was significantly correlated with the change in adherence to ADRP (r = 0.67, P < 0.01). These results suggest that ADRP treated with MPC-polymer suppresses the adherence of fungus/bacteria which are higher cell-surface hydrophobicity.
Table 2.
Cell surface hydrophobicity of Candida species and MRSA.
| Strain | Adsorption to hydrocarbon (S.D.) (%) | |
|---|---|---|
|
Candida albicans | ||
| JCM1542 | 5.09 | (2.52) |
| JCM2085 | 23.49 | (7.90) |
| CAD1 | 8.95 | (5.64) |
| KG5 | 3.75 | (0.36) |
| KG8 | 15.94 | (4.88) |
| KG12 | 52.69 | (17.95) |
| KG13 | 5.72 | (0.23) |
| TCa8 | 23.03 | (6.67) |
| TCa9 | 8.13 | (8.04) |
| TCa10 | 31.36 | (12.55) |
| TCa12 | 2.45 | (2.13) |
| TCa14 | 2.38 | (2.07) |
| TCa15 | 7.88 | (1.71) |
| TCa16 | 8.63 | (0.89) |
| TG13 | 3.54 | (0.44) |
| TU3 |
0.00 |
(0.00) |
| NCAC | ||
| JCM3761 | 88.76 | (7.91) |
| 4–332 | 64.44 | (6.29) |
| 4–337 | 72.68 | (19.78) |
| 4–389 | 34.82 | (3.79) |
| 4–401 | 8.93 | (2.53) |
| 4–422 | 7.62 | (0.10) |
| 4–438 | 40.72 | (2.25) |
| JCM1541 | 55.00 | (17.35) |
| TCa5 | 37.78 | (7.13) |
| KG7 |
93.15 |
(5.59) |
| MRSA | ||
| COL | 68.67 | (2.56) |
| T-1 | 61.97 | (6.65) |
| T-15 | 73.93 | (9.28) |
| T-26 | 67.43 | (12.18) |
| T-31 | 88.37 | (2.67) |
| T-36 | 74.33 | (8.01) |
Figure 5.
Correlation between hydrophobicity of Candida species and MRSA and their adherence to MPC-polymer-treated ADRP. We calculated the rate of adherence reduction by 5% of MPC-polymer treatment. Graph illustrates the rate of hexadecane adsorption and the rate of fungal/bacterial adherence reduction by ADRP treated with 5% of MPC-polymer. The correlation coefficient (r) was 0.67 (P < 0.01).
4. Discussion
Dentures can act as a reservoir for pathogens that could provoke life threatening aspiration pneumonia, which is one of the major causes of poor quality of life in the elderly. Reduction of fungal/bacterial load, especially Candida species and MRSA, from denture surfaces can prevent oral infections including denture stomatitis, and various systemic diseases such as aspiration pneumonia. Adherence of pathogens to host cells and medical devices is an essential step in the establishment of pathogenic infections. Therefore, inhibition of microbial adherence has been considered an effective strategy for the prevention of infectious diseases caused by pathogenic microbes.
This is the first report describing the effect of MPC-polymer on the adherence to acrylic denture resin material using clinical strains of C. albicans, NCAC, and MRSA. Among NCAC, C. glabrata is most frequently co-isolated with C. albicans and is thought to be a co-infecting pathogen with C. albicans [13, 14], that can cause more severe symptoms and are more difficult to treat [38, 39]. C. glabrata was reported to have a two-fold greater degree to adhere to acrylic denture surfaces compared to C. albicans in vitro [40]. This is in concordance with our study that a higher number of C. glabrata adhered to ADRP compared to C. albicans (Figures 2b and 2c). It is also noteworthy that the adherence of C. glabrata to ADRP was inhibited much stronger than that of C. albicans by MPC-polymer (Figures 2b and 3). These results suggest that MPC-polymer can efficiently suppress co-infection by C. albicans and C. glabrata.
The main reservoir of S. aureus is thought to be the anterior nares, but this bacterium is often isolated from the oral cavity and oropharynx in the elderly and immunocompromised hosts [21, 22, 41]. S. aureus is also reported as a common oral microfloral resident in denture plaque [20]. In Japan, MRSA was isolated from 10% of randomly selected denture-wearing patients [42]. It has also been reported that oral S. aureus/MRSA was significantly associated with hospital acquired pneumonia (HAP) incidences, suggesting that S. aureus/MRSA is a microbiological risk factor for HAP [43]. Here, we have demonstrated that MPC-polymer coating remarkably suppressed the adherence of MRSA to the surface of acrylic denture resin plates (Figure 4), suggesting that MPC-polymer can be useful for preventing HAP in the denture-wearing elderly in hospitals or nursing homes.
We found a significant positive correlation between cell-surface hydrophobicity and the fungal/bacterial adherence reduction rates in ADRP treated with 5% of MPC-polymer (Figure 5). Minagi et al. reported that when the contact angle of denture base resin materials is increased, the number of adherent C. tropicalis cells observed also increased, but not the cells of C. albicans; suggesting that fungal adherence to denture materials is related to the hydrophobic interactions [44]. In addition to MPC-polymer provides high wettability to the surface of ADRP (Figure 1b), hydrophobic interactions may play an important role, at least in part, in the adherence of these microorganisms to ADRP.
The reduction rates of fungal adherence to ADRP treated with MPC-polymer were higher in certain strains of C. albicans (JCM2085, KG5, KG12, KG13, and TCa12), even though their cell-surface hydrophobicity was rather low (hydrophillicity). Multiple mechanisms of adherence have been proposed. The molecular interactions involved in the adherence are classified into non-specific (electrostatic and hydrophobic interactions) and ligand–receptor specific interaction. The specific interactions, enhanced by morphogenetic transition from budding yeast to hyphae, are governed by multifunctional adhesins, which are mannoproteins localized on the fungal surface [45]. Several studies have established the role of some fungal proteins in adherence to host tissues. Among them, a hyphal wall protein (Hwp1) and two agglutinin-like proteins (Als1 and Ala1) have been suggested to be involved in adherence to epithelial and endothelial cells [46]. The EAP1 gene in C. albicans, which encodes a glycosylphosphatidylinositol-anchored, glucan-cross-linked cell wall protein, is required for adhesion and biofilm formation to the epithelial cells and polystyrene [47]. In C. albicans on silicone biomaterial, the expression of EAP1 and Hwp1 was significantly upregulated during the adhesion phase [48]. Moreover, the expression of LIP6, which encodes one of C. albicans lipases, was enhanced in germ tubes adhering to polystyrene petri dishes [49]. The lipolytic activity could increase hydrophobic interactions through the release of fatty acids [50]. Further investigation on the involvement of these genes in the MPC-polymer coating effect on the adherence of Candida to acrylic resin material is required.
There is a variety of denture cleaning methods, such as mechanical, chemical, or a combination of both, to remove the denture biofilm. A mechanical method is effective to remove C. albicans, C. glabrata, and C. tropicalis from acrylic resin surfaces [51]. It has been reported that soaking in peroxide solutions was effective in reducing C. albicans from denture bases; however, soaking should be combined with brushing to control fungal growth more effectively [52]. The MPC-polymer itself does not destroy the denture biofilm, but inhibits the adherence of microorganisms by changing the wettability of the surface. MPC-polymer, hence, can be used after complete removal of the initial denture biofilm and to prevent recurrent biofilm formation. It is important that MPC-polymer has been approved by the FDA and its various coating applications such as contact lenses and cosmetics are underway [53]. Taking into account these findings and facts, MPC-polymer can be useful for the prevention of oral infections in denture-wearing elderly and immunocompromised patients.
5. Conclusion
MPC-polymer coating of acrylic denture resin material is a potent method for suppressing denture biofilm formation by suppressing the adherence of pathogenic microbes, including C. albicans, NCAC, and MRSA that commonly cause denture stomatitis and related infections.
Declarations
Author contribution statement
Natsumi Fujiwara, Keiji Murakami: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kaya Yoshida, Yasusei Kudo, Kazumi Ozaki, Katsuhiko Hirota: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Shunsuke Sakurai: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Hideki Fujii, Maiko Suzuki, Yoichiro Miyake: Analyzed and interpreted the data; Wrote the paper.
Hiromichi Yumoto: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the NOF Corporation, Japan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Natsumi Fujiwara, Email: NFUJIWARA@augusta.edu, nfujiwara@tokushima-u.ac.jp.
Hiromichi Yumoto, Email: yumoto@tokushima-u.ac.jp.
References
- 1.Oeppen J., Vaupel J.W. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 2.Petersen P.E., Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2005;33(2):81–92. doi: 10.1111/j.1600-0528.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 3.Senpuku H., Sogame A., Inoshita E., Tsuha Y., Miyazaki H., Hanada N. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology. 2003;49(5):301–309. doi: 10.1159/000071711. [DOI] [PubMed] [Google Scholar]
- 4.Sumi Y., Miura H., Sunakawa M., Michiwaki Y., Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology. 2002;19(1):25–29. doi: 10.1111/j.1741-2358.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith A.J., Brewer A., Kirkpatrick P., Jackson M.S., Young J., Watson S., Thakker B. Staphylococcal species in the oral cavity from patients in a regional burns unit. J. Hosp. Infect. 2003;55(3):184–189. doi: 10.1016/j.jhin.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Hirota K., Yumoto H., Sapaar B., Matsuo T., Ichikawa T., Miyake Y. Pathogenic factors in Candida biofilm-related infectious diseases. J. Appl. Microbiol. 2017;122(2):321–330. doi: 10.1111/jam.13330. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima T., Ikawa S., Kitano K., Maeda N. A proposal of remedies for oral diseases caused by Candida: a mini review. Front. Microbiol. 2018;9:1522. doi: 10.3389/fmicb.2018.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikawa H., Hamada T., Yamamoto T. Denture plaque--past and recent concerns. J. Dent. 1998;26(4):299–304. doi: 10.1016/s0300-5712(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 9.Gow N.A., Brown A.J., Odds F.C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 2002;5(4):366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 10.Silva S., Negri M., Henriques M., Oliveira R., Williams D.W., Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012;36(2):288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 11.Xiao M., Fan X., Chen S.C., Wang H., Sun Z.Y., Liao K., Chen S.L., Yan Y., Kang M., Hu Z.D., Chu Y.Z., Hu T.S., Ni Y.X., Zou G.L., Kong F., Xu Y.C. Antifungal susceptibilities of Candida glabrata species complex, Candida krusei, Candida parapsilosis species complex and Candida tropicalis causing invasive candidiasis in China: 3 year national surveillance. J. Antimicrob. Chemother. 2015;70(3):802–810. doi: 10.1093/jac/dku460. [DOI] [PubMed] [Google Scholar]
- 12.Valentini F., Luz M.S., Boscato N., Pereira-Cenci T. Biofilm formation on denture liners in a randomised controlled in situ trial. J. Dent. 2013;41(5):420–427. doi: 10.1016/j.jdent.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Redding S.W., Kirkpatrick W.R., Coco B.J., Sadkowski L., Fothergill A.W., Rinaldi M.G., Eng T.Y., Patterson T.F. Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. J. Clin. Microbiol. 2002;40(5):1879–1881. doi: 10.1128/JCM.40.5.1879-1881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tati S., Davidow P., McCall A., Hwang-Wong E., Rojas I.G., Cormack B., Edgerton M. Candida glabrata binding to Candida albicans hyphae enables its development in oropharyngeal candidiasis. PLoS Pathog. 2016;12(3) doi: 10.1371/journal.ppat.1005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos-Garcia L., Jimenez-Valdes R.J., Hernandez-Bello R., Palma-Nicolas J., Gonzalez G.M., Sanchez-Gonzalez A. Candida albicans and non-albicans isolates from bloodstream have different capacities to induce neutrophil extracellular traps. J Fungi. 2019;5(2) doi: 10.3390/jof5020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathirana R.U., McCall A.D., Norris H.L., Edgerton M. Filamentous non-albicans Candida species adhere to Candida albicans and benefit from dual biofilm growth. Front. Microbiol. 2019;10:1188. doi: 10.3389/fmicb.2019.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulthwaite L., Verran J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007;64(4):180–189. doi: 10.1080/09674845.2007.11732784. [DOI] [PubMed] [Google Scholar]
- 19.Shi B., Wu T., McLean J., Edlund A., Young Y., He X., Lv H., Zhou X., Shi W., Li H., Lux R. The denture-associated oral microbiome in health and stomatitis. mSphere. 2016;1(6) doi: 10.1128/mSphere.00215-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiwabara T., Yoshijima Y., Hongama S., Nagao K., Hirota K., Ichikawa T. Denture plaque microflora in geriatric inpatients and maxillary defect patients. Prosthodont. Res. Pract. 2007;6(3):153–159. [Google Scholar]
- 21.Tada A., Senpuku H., Motozawa Y., Yoshihara A., Hanada N., Tanzawa H. Association between commensal bacteria and opportunistic pathogens in the dental plaque of elderly individuals. Clin. Microbiol. Infect. 2006;12(8):776–781. doi: 10.1111/j.1469-0691.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita K., Ohara M., Kojima T., Nishimura R., Ogawa T., Hino T., Okada M., Toratani S., Kamata N., Sugai M., Sugiyama M. Prevalence of drug-resistant opportunistic microorganisms in oral cavity after treatment for oral cancer. J. Oral Sci. 2013;55(2):145–155. doi: 10.2334/josnusd.55.145. [DOI] [PubMed] [Google Scholar]
- 23.Garbacz K., Kwapisz E., Wierzbowska M. Denture stomatitis associated with small-colony variants of Staphylococcus aureus: a case report. BMC Oral Health. 2019;19(1):219. doi: 10.1186/s12903-019-0910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harriott M.M., Noverr M.C. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19(11):557–563. doi: 10.1016/j.tim.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurobane T., Shiwaku Y., Anada T., Hamai R., Tsuchiya K., Baba K., Iikubo M., Takahashi T., Suzuki O. Angiogenesis involvement by octacalcium phosphate-gelatin composite-driven bone regeneration in rat calvaria critical-sized defect. Acta Biomater. 2019;88:514–526. doi: 10.1016/j.actbio.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara K., Fukumoto K., Iwasaki Y., Nakabayashi N. Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 2. Protein adsorption and platelet adhesion. Biomaterials. 1999;20(17):1553–1559. doi: 10.1016/s0142-9612(98)00206-3. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki Y., Aiba Y., Morimoto N., Nakabayashi N., Ishihara K. Semi-interpenetrating polymer networks composed of biocompatible phospholipid polymer and segmented polyurethane. J. Biomed. Mater. Res. 2000;52(4):701–708. doi: 10.1002/1097-4636(20001215)52:4<701::aid-jbm15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Lewis A.L., Cumming Z.L., Goreish H.H., Kirkwood L.C., Tolhurst L.A., Stratford P.W. Crosslinkable coatings from phosphorylcholine-based polymers. Biomaterials. 2001;22(2):99–111. doi: 10.1016/s0142-9612(00)00083-1. [DOI] [PubMed] [Google Scholar]
- 29.Hirota K., Yumoto H., Miyamoto K., Yamamoto N., Murakami K., Hoshino Y., Matsuo T., Miyake Y. MPC-polymer reduces adherence and biofilm formation by oral bacteria. J. Dent. Res. 2011;90(7):900–905. doi: 10.1177/0022034511402996. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara N., Yumoto H., Miyamoto K., Hirota K., Nakae H., Tanaka S., Murakami K., Kudo Y., Ozaki K., Miyake Y. 2-Methacryloyloxyethyl phosphorylcholine (MPC)-polymer suppresses an increase of oral bacteria: a single-blind, crossover clinical trial. Clin. Oral Invest. 2019;23(2):739–746. doi: 10.1007/s00784-018-2490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeya K., Iwasa F., Inoue Y., Fukunishi M., Takahashi N., Ishihara K., Baba K. Inhibition of denture plaque deposition on complete dentures by 2-methacryloyloxyethyl phosphorylcholine polymer coating: a clinical study. J. Prosthet. Dent. 2018;119(1):67–74. doi: 10.1016/j.prosdent.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Turkcan I., Nalbant A.D., Bat E., Akca G. Examination of 2-methacryloyloxyethyl phosphorylcholine polymer coated acrylic resin denture base material: surface characteristics and Candida albicans adhesion. J. Mater. Sci. Mater. Med. 2018;29(7):107. doi: 10.1007/s10856-018-6116-7. [DOI] [PubMed] [Google Scholar]
- 33.Yumoto H., Hirota K., Hirao K., Miyazaki T., Yamamoto N., Miyamoto K., Murakami K., Fujiwara N., Matsuo T., Miyake Y. Anti-inflammatory and protective effects of 2-methacryloyloxyethyl phosphorylcholine polymer on oral epithelial cells. J. Biomed. Mater. Res. 2015;103(2):555–563. doi: 10.1002/jbm.a.35201. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman M.P., Haidaris C.G. Analysis of Candida albicans adhesion to salivary mucin. Infect. Immun. 1993;61(5):1940–1949. doi: 10.1128/iai.61.5.1940-1949.1993. https://www.ncbi.nlm.nih.gov/pubmed/8478083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikawa H., Sadamori S., Hamada T., Okuda K. Factors involved in the adherence of Candida albicans and Candida tropicalis to protein-adsorbed surfaces. An in vitro study using immobilized protein. Mycopathologia. 1992;118(3):139–145. doi: 10.1007/BF00437146. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg M., Gutnick D., Rosenberg E. Adherence of bacteria to hydrocarbons - a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980;9(1):29–33. [Google Scholar]
- 37.Chambers H.F., Deleo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redding S.W., Kirkpatrick W.R., Dib O., Fothergill A.W., Rinaldi M.G., Patterson T.F. The epidemiology of non-albicans Candida in oropharyngeal candidiasis in HIV patients. Spec. Care Dent. 2000;20(5):178–181. doi: 10.1111/j.1754-4505.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 39.Redding S.W., Zellars R.C., Kirkpatrick W.R., McAtee R.K., Caceres M.A., Fothergill A.W., Lopez-Ribot J.L., Bailey C.W., Rinaldi M.G., Patterson T.F. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J. Clin. Microbiol. 1999;37(12):3896–3900. doi: 10.1128/jcm.37.12.3896-3900.1999. https://jcm.asm.org/content/37/12/3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo G., Samaranayake L.P. Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans. APMIS. 2002;110(9):601–610. doi: 10.1034/j.1600-0463.2002.1100902.x. [DOI] [PubMed] [Google Scholar]
- 41.Hirota K., Yoneyama T., Sakamoto M., Miyamoto H., Kurihara M., Kayama S., Murakami K., Yumoto H., Matsuo T., Miyake Y. High prevalence of Pseudomonas aeruginosa from oropharyngeal biofilm in patients with cerebrovascular infarction and dysphagia. Chest. 2010;138(1):237–238. doi: 10.1378/chest.10-0240. [DOI] [PubMed] [Google Scholar]
- 42.Tawara Y., Honma K., Naito Y. Methicillin-resistant Staphylococcus aureus and Candida albicans on denture surfaces. Bull. Tokyo Dent. Coll. 1996;37(3):119–128. [PubMed] [Google Scholar]
- 43.Ewan V.C., Sails A.D., Walls A.W., Rushton S., Newton J.L. Dental and microbiological risk factors for hospital-acquired pneumonia in non-ventilated older patients. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0123622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect. Immun. 1985;47(1):11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tronchin G., Poulain D., Vernes A. Cytochemical and ultrastructural studies of Candida albicans. III. Evidence for modifications of the cell wall coat during adherence to human buccal epithelial cells. Arch. Microbiol. 1984;139(2-3):221–224. doi: 10.1007/BF00402004. [DOI] [PubMed] [Google Scholar]
- 46.Sundstrom P. Adhesion in Candida spp. Cell Microbiol. 2002;4(8):461–469. doi: 10.1046/j.1462-5822.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 47.Li F., Svarovsky M.J., Karlsson A.J., Wagner J.P., Marchillo K., Oshel P., Andes D., Palecek S.P. Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukaryot. Cell. 2007;6(6):931–939. doi: 10.1128/EC.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samaranayake Y.H., Cheung B.P., Yau J.Y., Yeung S.K., Samaranayake L.P. Human serum promotes Candida albicans biofilm growth and virulence gene expression on silicone biomaterial. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0062902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchais V., Kempf M., Licznar P., Lefrancois C., Bouchara J.P., Robert R., Cottin J. DNA array analysis of Candida albicans gene expression in response to adherence to polystyrene. FEMS Microbiol. Lett. 2005;245(1):25–32. doi: 10.1016/j.femsle.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Stehr F., Felk A., Gacser A., Kretschmar M., Mahnss B., Neuber K., Hube B., Schafer W. Expression analysis of the Candida albicans lipase gene family during experimental infections and in patient samples. FEMS Yeast Res. 2004;4(4-5):401–408. doi: 10.1016/S1567-1356(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 51.Paranhos H.F., Silva-Lovato C.H., de Souza R.F., Cruz P.C., de Freitas-Pontes K.M., Watanabe E., Ito I.Y. Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin. J. Prosthodont. 2009;18(5):427–431. doi: 10.1111/j.1532-849X.2009.00450.x. [DOI] [PubMed] [Google Scholar]
- 52.Lin J.J., Cameron S.M., Runyan D.A., Craft D.W. Disinfection of denture base acrylic resin. J. Prosthet. Dent. 1999;81(2):202–206. doi: 10.1016/s0022-3913(99)70249-0. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu T., Goda T., Minoura N., Takai M., Ishihara K. Super-hydrophilic silicone hydrogels with interpenetrating poly(2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials. 2010;31(12):3274–3280. doi: 10.1016/j.biomaterials.2010.01.026. [DOI] [PubMed] [Google Scholar]