Abstract
Background
Psoriasis is a pro-inflammatory disease with unknown etiology, that is characterized by skin inflammation and keratinocytes hyperproliferation. Specific inhibition of inflammation has shown positive effects avoiding the progression of the psoriatic lesions in different animal models of the disease, turning this strategy as a remarkable therapeutic alternative.
Objective
To screen the effectiveness of a novel IFN-α/β signalling inhibitor in the development reduction of skin lesions in IMQ and TPA mice models of psoriasis.
Methods
We used a Phage-peptide library for the screening of a peptide with inhibitory effects on the development of psoriasis-like lesions in mice. To evaluate the in vivo effect of the phage-peptides (Phpep3D) and the derived peptide (Pep3D), we administered Phpep3D or Pep3D intradermally in mice with imiquimod (IMQ)-induced psoriasis and 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced psoriasis. We scored the lesions, and we determined the number of neutrophils and the production of some pro-inflammatory cytokines in the lesions.
Results
In this work, we describe how the Ph3pepD and Pep3D reduced skin thickness, redness, and acanthosis despite the presence of the psoriasis inducers, IMQ or TPA. We also found that Pep3D reduced the number of GR1+ infiltrated cells and decreased the production of IL-17A and TNFα in the psoriatic skin of mice. In-silico, docking analysis showed that Pep3D may interact with the interferon-alpha receptor, but further analyses should be performed to uncover the mechanism of action of this peptide.
Conclusion
Our results suggest that Pep3D could be used as a new treatment for psoriasis.
Keywords: Biological sciences, Immunology, Inflammation, Health sciences, IFNα, IFNAR1, Peptide, Phage-peptide, Imiquimod, Mice models of psoriasis
Biological sciences; Immunology; Inflammation; Health sciences; IFNα; IFNAR1; Peptide; Phage-peptide; Imiquimod; Mice models of psoriasis.
1. Introduction
The etiology of psoriasis remains unknown, but the lack of control in the skin inflammation, or the dysregulation in the proliferation of keratinocytes accompanied by angiogenesis, may trigger the vicious cycle of inflammation and cellular proliferation that ultimately results in psoriatic lesions formation [1, 2, 3].
In 2000 Hida et al. showed that the excessive interferon alpha/beta (IFN-α/β) signaling due to the genetic deletion of IRF-2 (interferon regulatory factor 2) is one of the primary causes for the development of psoriasis-like skin lesion in mice. Furthermore, they showed that IRF-2−/− knockout mice do not develop psoriasis-like lesions when they are also IFNAR1−/−, an observation suggesting the central role of IFNα in the development of psoriasis [4]. Afterwards, this hypothesis was supported in 2005 when Nestle et al. demonstrated that the blockade of IFNAR1 inhibited the conversion of healthy skin into psoriatic injured skin in the AGR−/- xenograft model [5]. Lande et al. (2007), and Ganguly et al. (2009) reported that DNA and RNA can make complexes with LL37, activating human dendritic cells (DCs) through toll like receptor-7 or -8 (TLR-7, -8), which in turn induce IFNα production. Interestingly these DNA- and RNA-LL37 complexes were reported present in psoriatic skin [6, 7]. Based on these findings, it was hypothesized that the beginning of psoriasis may be triggered by antimicrobial peptides like LL-37, and by DNA or RNA released after cell damage, forming complexes that induce plasmacytoid dendritic cells (pDCs) to the production of IFNα [5, 6, 7, 8]. In turn, this cytokine activates the DCs that migrate to the lymph nodes and produce IL-12 and IL-23, causing the differentiation of lymphocytes to the Th1 and Th17 profiles, respectively. These lymphocytes return to the skin and produce inflammatory cytokines, including IL-17, with a role in the induction of keratinocytes proliferation [3].
In 2009 van der Fits reported a mice model of psoriasis induced by imiquimod (IMQ), a TLR7 and TLR8 ligand that generates skin lesions mediated by IL-23/IL-17 axis that resembles human psoriasis [9]. Even though, controversial results were reported by Walter et al. and Wohn et al. in 2013 where they reported that IMQ commercial presentation (Aldara) can induce acanthosis in a TLR-7 independent fashion and that the IFNAR1−/− mice developed psoriasis as well as WT mice [10, 11]. Despite these controversial reports, the IMQ mice model is still one of the most extensively used models to describe the physiopathology of human-like psoriasis. In opposition to Walter and Wohn, Ueyama et al. (2014) and Gui et al. (2016) published data supporting the fact that TLR-7 and IFNAR1 have a crucial role in the evolution of psoriasis, because TLR7−/− and IFNAR1−/− mice do not develop psoriasis induced by IMQ [12, 13].
Yao et. al. (2009) made comparisons between psoriatic and healthy skin and found that from all the signalling pathways altered, the IFNα/β signalling pathway was the most dominant and significantly up regulated. Components of the pathway, such as IFNαR1, IFNαR2, STAT1, IRF1, MPL, ISG15, and IFI6, were all significantly overexpressed in the psoriatic skin in comparison with non-lesioned skin of psoriatic patients [14]. This results supported Hida's report [4].
Another evidence of the closed association between IFNα and psoriasis was described in the use of the IFNα recombinant protein (rIFNα) as an anti-tumor therapy. In 1986 Quesada and Gutterman reported that patients with metastatic renal-cell carcinoma treated with rIFNα showed exacerbation or the onset of psoriasis [15], and since then, similar cases have been reported [16, 17].
Based on what we described above, we decided to look for a therapeutic target to regulate IFNα pathway. In this work, we used a Phage-display library and anti-human IFNα polyclonal antibodies for the screening of phage-peptides (Phpep) with anti-psoriatic effects. We found a cyclic peptide that diminished the severity of IMQ-induced skin lesions, in part, by the reduction of the infiltrated skin granulocytes GR1+, and also by the decrement in the production of inflammatory cytokines in the skin.
2. Material and methods
2.1. Ethics approval and consent to participate
All experimental procedures, including the treatment with psoriasis inductors, administration of phages or peptides, and death of animals, were approved and performed according to the standards of the Ethics Committee for Animal Use of the Instituto Politécnico Nacional/Escuela Nacional de Ciencias Biológicas (IPN/ENCB), that follows the EU Directive 2010/63/EU animal care for animals. The registration and approval of the protocol is under the file number ENCB/CEI/036/2018, CONBIOETICA09CEI03720130520.
2.2. Selection of phage-peptides, biopanning
Based on the fact that antibodies interact with the most exposed amino acids in a tertiary structure of a protein, and that these sites are as well potential sites of interaction with the receptors, in our system we used polyclonal anti-IFNα antibodies to emulate IFNAR1 as coated target to select phage-peptides with potential IFNα-antagonistic activity. Commercially available polyclonal anti-IFNα antibodies were used in preference to monoclonal antibodies to increase the sequence variability of selected phage-peptide clones.
We used 1μg/100μL of rabbit polyclonal antibody anti-human IFNα1 (Abcam, Cambridge, UK) in coupling buffer (0.1M de NaHCO3, pH 8.6). With this solution one 96-well plate was incubated overnight at 4 °C and washed 3 times with TBS-tween 0.1%. The blocking buffer (200 μL of 3% skim milk in TBS) was then added and incubated at room temperature for 1h. Next, 100 μL solution with 1 × 1011 phages of the Ph.D.™-7 Phage Display Peptide Library (New England Biolabs, Beverly, MA, USA) were added into the antibodies-coated well and incubated 1 h at room temperature. Then, the well was washed 8 times with 300 μL of TBS-Tween 0.1% and the bounded phage-peptides (Phpep) were eluted with 100 μL of elution buffer (0.2 M Glycine-HCl pH 2.2) and neutralized with 15 μL of 1 M Tris-HCl pH 9.1 buffer. One microliter of the elution were used for the quantification of the Phpep by counting plaque forming units (PFU) in LB agar. The rest of the Phpep were amplified infecting E. coli strain ER2738, purified using polyethylene glycol (PEG), and then the Phpep were quantified to determine the PFUs.
The Phpep selection, elution and quantification, described above is considered as one round of biopanning. For the second and third biopanning rounds we used 1 × 109 phages/100μL. After the third biopanning, we plated Phpep in LB for random Phpep selection.
2.3. Induction of psoriasis like-lesions in mice
All the experiments were performed after the protocol received approval by the Ethics Committee for Animal Use of the Instituto Politécnico Nacional/Escuela Nacional de Ciencias Biológicas (IPN/ENCB). All efforts were done to minimize the number of animals used and their suffering.
For IMQ-induced psoriasis, female BALB/c mice of 6–8 weeks old were shaved at their lower back, and except for the healthy group, all animals were treated topically on the back with 62.5 mg of IMQ cream (5%) (Aldara; 3M Pharmaceuticals) for six days or 12 mg of the IMQ cream on one ear [9].
For psoriasis TPA-induced model, female BALB/c mice of 6–8 weeks received 6 topical applications of 50 μg/mL TPA (Sigma- Aldrich, St. Louis, US) dissolved in acetone on shaved lower backs or in one ear [18].
As control groups we worked with healthy mice, mice treated with acetone (TPA vehicle) and psoriatic mice (induced with IMQ or TPA) treated with betamethasone (βMet). Each group consisted of 3 mice. In the ear model we measured the ear thickness using a digimatic thickness gauge (Mitutoyo, Illinois, US). All measurements were performed by the same person.
2.4. Analysis of the anti-psoriatic activity of selected phage-peptides
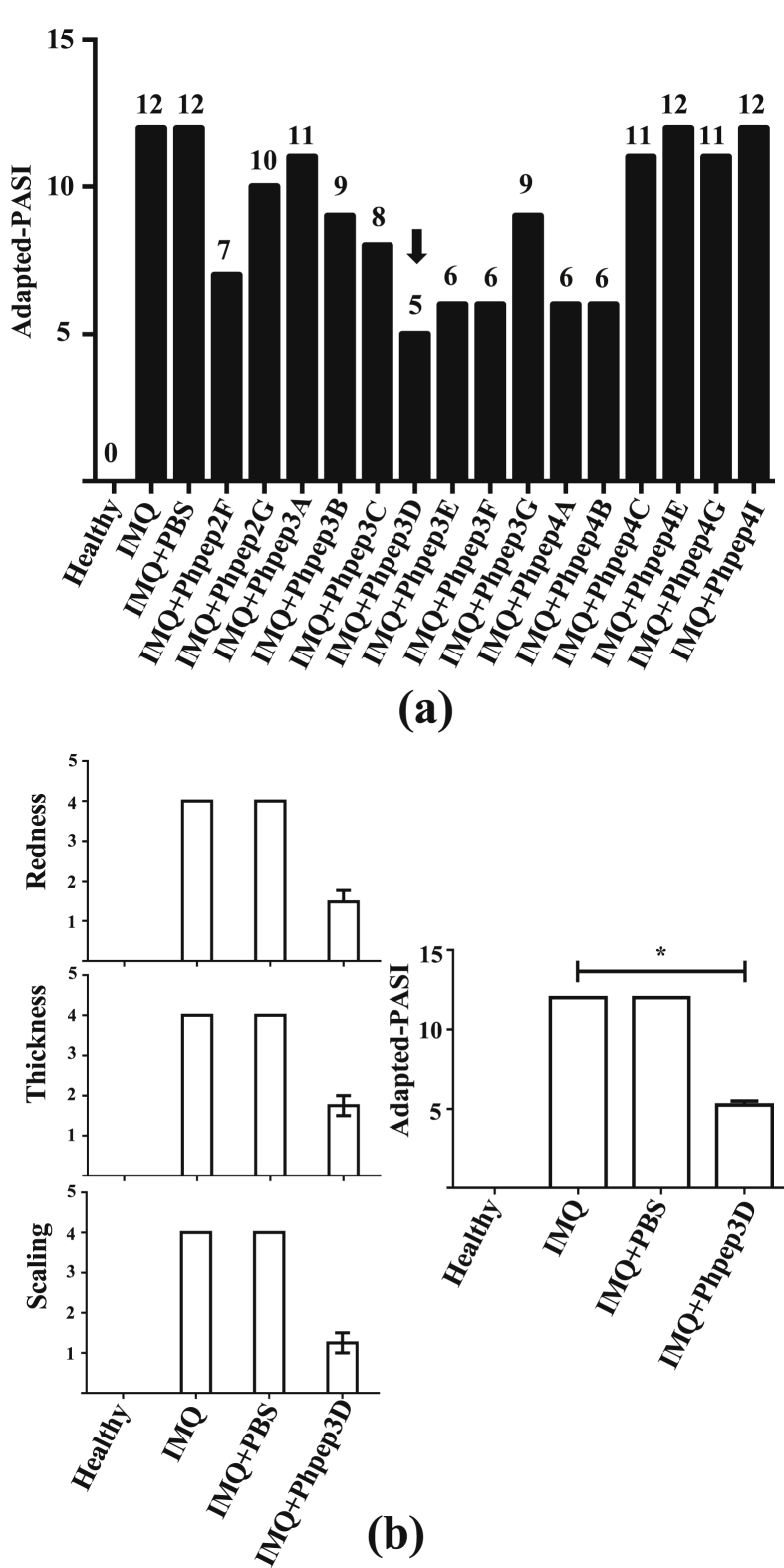
As a first fast screening of selected Phpep with anti-psoriatic activity, we used one mice per Phpep selected clone. 1 × 109 Phpep were administered intradermally in the back of mice on days 1, 2, 3 and 4 of IMQ administration protocol. At the end of the treatment, the redness, scaling and thickness were evaluated to score the “adapted-PASI”. The adapted-PASI assessed the severity of the induced murine psoriasis erythema, desquamation and induration; these severity parameters are measured on a 0–4 scale (from none to the maximum damage). The sum of these 4 values is the value of the adapted-PASI. An adapted-PASI value of 12, indicates a maximum severe injury.
To corroborate the first screening results, we selected the clone with the highest anti-psoriatic effect (Phpep3D) and repeated the assay using this time 4 mice and we evaluated again the adapted-PASI. Besides, at day 7 the treated skin was collected for histological evaluation.
2.5. In vitro determination of Phpep3D blocking IFNα activity
To evaluate the blocking effect of Phpep3D over IFNα activity, we used the HEK-Blue™-IFN system. In brief, 50000 HEK-Blue-IFN cells were cultured in 96 well plates with 180 μL of DMEM supplemented with 10% of FBS, 100 mg/mL zeocin, 30 g/mL blasticidin and 100 g/mL normocin. The cells were pre-incubated with 20 μL of Phpep3D or PhpepIrre at 37 °C in a 5% CO2 atmosphere for 1h. Then the cells were washed with DMEM and incubated with DMEM containing 400 pg IFNα. The next day 20 μL of supernatants were transferred to an ELISA 96 wells plate and 180 μL de QUANTI-Blue (5-bromo-4-chloro-3-indolyl phosphate analog and nitro blue tetrazolium) were added to develop the activity of the Secreted Embryonic Alkaline Phosphatase (SEAP) and the optical density was determined at 655 nm. A dose-response curve was done to designate the optimal IFNα concentration to be used in the blocking assay.
2.6. Sequencing of selected paghe-peptide clones
DNA sequence from selected clones was obtained using the primer 5′-CCCTCATAGTTAGCGTAACG-3’. The synthetic peptide Pep3D was purchased to Peptide 2.0 (Chantilly, VA, USA) as a cyclic peptide.
2.7. Evaluation of anti-psoriatic effect of peptide Pep3D in IMQ and TPA murine models of psoriasis
IMQ- and TPA-induced psoriasis mice models were treated with 2 or 20 ng of Pep3D. Pep3D was administered intradermally in the back or in the ear of a group of three mice on days 1, 2, 3 and 4 of the lesion induction protocol. A control group of 3 mice was included using 6 ng of βMet cream. For the analysis of infiltrated granulocytes (GR1+) the treated ears were digested with 60 U/mL of collagenase type I (Life technologies, Grand Island, NY, USA), 153.5 μg/mL of liberase TL (Roche Diagnostics, Mannheim, Germany) and 6.5 ng/mL of DNase I (Roche Diagnostics, Mannheim, Germany) for 2 h at 37 °C. To identify the GR1+ population we use a mix of PE-Cy5-anti-CD45 (monoclonal rat against mouse; Biolegend, San Diego, CA, USA) and Alexa Flour 488-anti-GR1 (monoclonal rat anti-mouse; Biolegend, San Diego, CA, USA) antibodies. In the other hand, a different portion of digested ears was subjected to three cycles of freezing/thawing to break cells and to determine cytokines in the supernatant of lyzed samples. We measured inflammatory cytokines using Th1/Th2/Th17 CBA Cytokine Kit (BD Bioscience, San Jose; CA, USA). Cells and cytokines were analyzed using a FACSAria III cell sorter (BD Bioscience).
2.8. In-silico analysis of the peptide Pep3D
As the clone of phage-peptide 3D (Phpep 3D) was the one with the most evident anti-psoriatic activity, the tridimensional structure of its derived peptide (Pep3D) was modeled in-silico.
First, a homology modeling strategy was followed to obtain a raw 3D structural model using as template a cyclic peptide (accession code: 5VAV) downloaded from the Protein Data Bank (PDB). The raw tridimensional model was built with Modeller version 9.15 [19] generating 20 models and selecting that with the lowest Discrete Optimized Protein Energy (DOPE) energy function. In order to obtain a more structurally stable and reliable structure of the peptide, an Equilibrium Molecular Dynamics (EMD) in explicit solvent was conducted as follows. The peptides were placed in a water box of dimensions 10 Å × 10 Å x 10 Å in the presence of NaCl to neutralize charges. The system was prepared in Visual Molecular Dynamics (VMD) version 1.9.3 and the full-atom Point Spread Function (PSF) and PDB models were built using CHARMM22 force field [20]. The EMD was run in NAMD2 with a constant temperature of 310 K and a total simulation time of 4 ns (2 millions of steps). To analyze the structural behavior of the peptides under the Molecular Dynamics, the Root-mean-square deviation (RMSD) trajectories were extracted to identify the zone of structural-conformation equilibrium (if reached). A representative structure from this zone was taken to perform a docking analysis with this peptide, the IFNα and also with the IFNα receptor. For this analysis, the IFNα subunits were downloaded from the PDB (accession code subunit: 3S98, subunit2: 3S9D). All IFNα and peptide PDB models were validated in the MolProbity server to add missing hydrogens atoms and correct atom nomenclature if needed [21]. Validated IFNα and peptide models were analyzed in the PatchDock server to calculate potential protein-peptide complexes, allowing poses within an RMSD of at most 4 Å [22, 23]. The 10 best results were closer examined to extract Score, Area and Atomic Contact Energy (ACE) parameters.
2.9. Statistical analysis
The comparison of the PASI between the imiquimod group and the treated groups was analyzed by the Kruskal-Wallis ANOVA test. For the comparation of infiltrating cells and cytokines quantification the results are presented as the mean ± SEM and differences between samples were evaluated using Dunnett's multiple comparison tests, after one-way ANOVA, comparing the treated groups against the IMQ group. All the analysis were carried out using GraphPad Prism (GraphPad, San Diego, CA, USA). P ≤ 0.05 was considered significant.
3. Results
3.1. Selection of phage-peptides according to the anti-psoriatic effect
To identify sequences of peptides with anti-psoriatic activity, we used the phage display technology using as target anti-human IFNα polyclonal antibodies. Three rounds of Phpep selection (biopanning) were performed. Phpep titer of the first biopanning was 1.3 × 104 PFU/100 μL, in the second 6.1 × 105 PFU/100μL and in the third 1.6 × 107 PFU/100μL, implying that the specific phage enrichment was adequate. We avoided a forth biopanning as in our experience from here is common to get several whild (white) clones with no peptide-cassette. After 3 biopannings, a total of 150 plaques were randomly selected, and from these, 138 clones of phage-peptides (Phpep) contained an exogenous peptide sequence. The PCR amplified fragments were sequenced, and only 15 different peptide sequences were found (Table 1). We assessed the anti-psoriatic activity of the 15 different sequences Phpep clones employing the IMQ murine model and scoring the adapted-PASI aspects. This first screening was done using only one mouse per Phpep clone (15 mice). As shown in Figure 1a, the clones Phpep 2F, 3D, 3E, 3F, 4A, and 4B had the highest effects avoiding the development of lesions, and in the case of the phage-peptide clone 3D (Phpep 3D), it reduced the score of the disease in more than 50% (adapted-PASI = 5). To confirm the Phpep3D anti-psoriatic effect we repeated the assay using this time 4 mice with imiquimod-induced psoriasis, and we obtained consistent and reproducible results, about redness, thickness and skin scaling in the 4 treated mice (Figures 1b, 2a and 2b).
Table 1.
Amino acid sequence that displayed the eluted Phpep's after three biopanning selection rounds. Fifteen different sequences were found on the 138 clones sequenced.
| ID | Sequence | Peptide Frequency (%) |
|---|---|---|
| Phpep2F | A-C-T-A-A-V-S-N-W-C-G-G-G | 11 |
| Phpep2G | A-C-T-V-R-T-S-A-D-C-G-G-G | 9 |
| Phpep3A | A-C-P-T-W-A-W-K-W-C-G-G-G | 1 |
| Phpep3B | A-C-V-Q-M-P-A-S-H-C-G-G-G | 8 |
| Phpep3C | A-C-D-W-F-T-P-H-R-C-G-G-G | 7 |
| Phpep3D | A-C-I-G-N-S-N-T-L-C-G-G-G | 9 |
| Phpep3E | A-C-I-G-N-S-N-E-L-C-G-G-G | 7 |
| Phpep3F | A-C-L-K-L-G-E-K-W-C-G-G-G | 9 |
| Phpep3G | A-C-E-F-S-K-F-R-S-C-G-G-G | 11 |
| Phpep4A | A-C-E-W-Y-S-P-H-S-C-G-G-G | 5 |
| Phpep4B | A-C-P-L-N-Y-H-W-I-C-G-G-G | 7 |
| Phpep4C | A-C-P-P-A-F-A-W-V-C-G-G-G | 1 |
| Phpep4E | A-C-A-W-Y-S-P-L-S-C-G-G-G | 2 |
| Phpep4G | A-C-I-G-P-H-V-I-L-C-G-G-G | 9 |
| Phpep4I | A-C-S-W-Q-I-G-G-N-C-G-G-G | 4 |
| PhpepIrre | A-C-E-L-F-N-W-V-I-C-G-G-G | -- |
Figure 1.
Selection of the phage-peptides according to the anti-psoriatic effect. Fifteen phage-peptides (Phpep) clones carrying different peptide sequences were selected and administered intradermally (109 UFP) in mice treated with IMQ as psoriasis inductor (1 mice per Phpep clone). We evaluated: (a) Score of adapted-PASI including redness (score 0–4), scaling (score 0–4), and thickness (score 0–4); n = 1. Global adapted-PASI score 0–12.(b) Repetition of assay over clone Phpep3D. Score of redness, thickness and scaling of mice are represented individually and globally as adapted-PASI; n = 4. ∗P < 0.05 (Kruskal-Wallis ANOVA test).
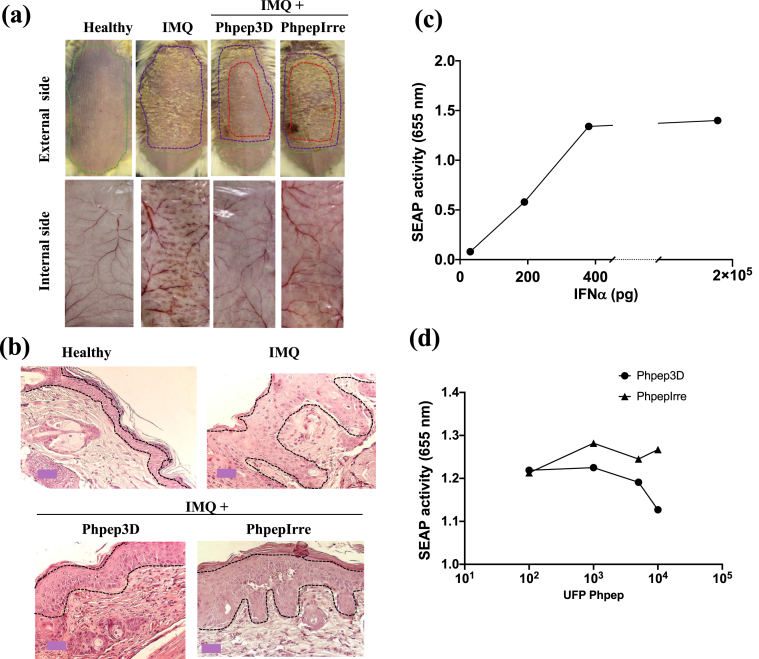
Figure 2.
Anti-psoriatic effect of Phpep3D assayed in the IMQ-induced psoriasis murine model, and blocking assay of IFNα activity. (a) 1 × 109 Phpep were administered intradermally in the back of mice on days 1, 2, 3 and 4 of the IMQ administration protocol. PhpepIrre was used as negative control. The dotted purple lines correspond to the area of the skin treated with IMQ; red dotted lines show the skin area treated with Phpep3D or the PhpepIrre; green dotted lines show the not-treated skin areas. Psoriatic lesions are shown from the external side of the skin (scaling) and from the internal side of the skin (angiogenesis). Representative results from 4 mice used on every group of treatment. (b) Histochemistry analysis of skins treated with Phpep3D or PhpepIrre. The dotted lines in black enclose to the epidermis area. (c) IFNα activity evaluated in vitro in the HEK-BlueTM-IFNα system. The figure shows the optical density of the secreted embryonic alkaline phosphatase (SEAP) activity produced when HEK-Blue-IFN cells were treated at different concentrations of IFNα. (d) HEK-Blue-IFN cells were first treated with different concentrations of Phpep3D or PhpepIrre and then with 400 pg of IFNα.
In Figure 2a, we exhibit representative results of 4 repetitions of the Phpep3D anti-psoriatic effects causing significant reduction in the severity of the lesion compared to the lack of effect in mice treated with irrelevant Phpep (PhpepIrre). In Figure 2a, the mice's back areas were delimited according to the treatment; the topical treatment with IMQ was applied in the whole area of the back (purple-dotted line), except for a discrete area (green-dotted line) used as “negative control” in the skin of the same animal. When the phage-peptide was intradermally applied, it was injected in four points shaping a 1cm2 square in the center of the back (red-dotted line). Results of additional repetitions of this experiment are shown in supplementary figure 1.
The angiogenesis is another biological process impaired in psoriatic lesions. In the skin treated with IMQ and Phpep3D the angiogenesis clearly decreased in comparison to the the skin of mice only treated with IMQ. Again, the use of the PhpepIrre had no effect (Figure 2a internal side of the skin). The skin histology analysis of the Phpep3D-treated mice revealed a significant reduction of hyperkeratosis and acanthosis in comparison to the mice only treated with IMQ (Figure 2b).
To analyze if the Phpep3D interfered with the interaction between IFNα and its receptor, we used the commercial system HEK-Blue-IFNα™. We determined that 400 pg μg/mL of TNFα was the optimal concentration to induce the activity of the IFNαR1 in this system (Figure 2C), and we found that Phpep3D blocked the IFNα activity getting lower levels of SEAP in HEK-Blue-IFNα™ cells treated with the Phpep3D + IFNα rather than in cells treated with the PhpepIrre + IFNα (Figure 2d).
3.2. Pep3D controls the development of psoriatic lesions in the murine models of psoriasis
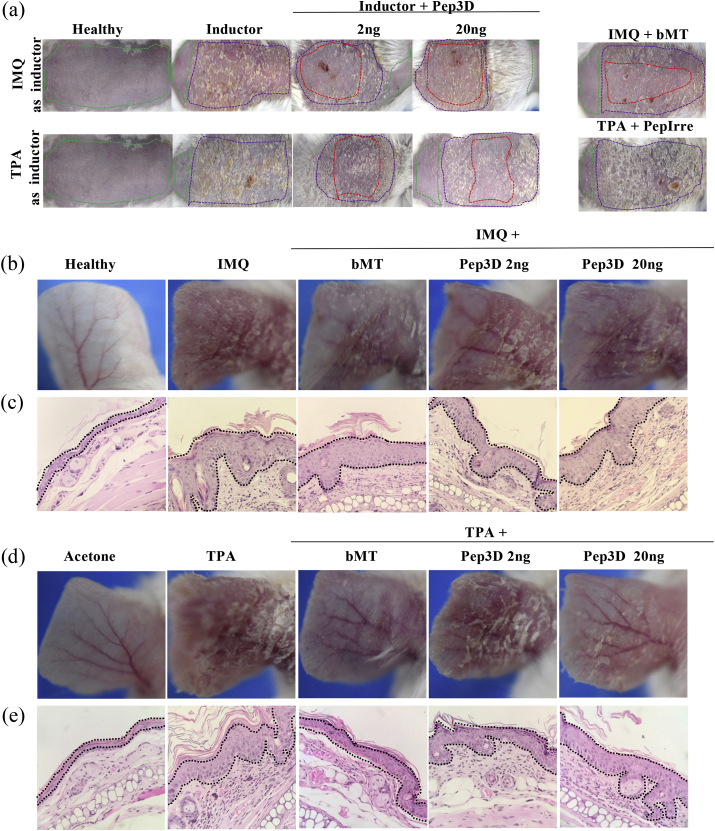
Based on the positive effects arresting the development of psoriatic lesions and on the blocking effect of Phpep3D in the interaction IFNα/IFNR, we synthesized the correspondent displayed peptide (Pep3D). We used again the IMQ and TPA murine models to assess the activity of Pep3D in psoriatic mice with doses of 2 or 20 ng of the peptide. The administration was similar as the previously done with the phages.
According to our results, both concentrations of Pep3D showed protective effect in the psoriatic model induced by IMQ. More severe lesions were developed when TPA was used and as consequence the effect of the peptide was only observed at the concentration of 20 ng (Figure 3a). As expected, the irrelevant peptide showed no protective effect in both models (not shown). The dotted lines in Figure 3a surround to treated areas in the back of mice.
Figure 3.
Anti-psoriatic effect of Pep3D assayed in psoriasis murine models. (a) The Pep3D assayed at 2 and 20 ng in the murine psoriasis models induced with IMQ or TPA in the back of the animals. An irrelevant peptide (PepIrre) and betamethasone (bMT) were used as controls. The dotted lines in color correspond to: area of the skin treated with the inductor of psoriasis (purple line), treated with Pep3D or PepIrre (red line), and not-treated skin (green line). The anti-psoriatic effect of Pep3D was also assayed in the ear of mice induced with IMQ (b) and TPA (d) (3 mice per group of treatment). Histochemistry analysis of the ears treated with Pep3D in IMQ model (c) and TPA model (e). Epidermis area is delimited with black dotted lines. The anti-inflammatory βMT, and acetone as solvent of TPA, were used as controls.
Similar results were observed when the disease was induced in the ear of the animals, where those treated with the Pep3D showed inhibition of inflammation (Figures 3b and 3d). The lesions induced by IMQ were lesser using the Pep3D than those with no peptide treatment (Figure 3b). Histological analysis showed that Pep3D also reduced the thickening of the skin and inhibited the formation of acanthosis when compared with the lesions induced with IMQ without Pep3D treatment (Figure 3c). In the TPA-induced model assayed in the ear, again the psoriatic lesions were more severe than those induced with IMQ, therefore the protective effect of Pep3D was only seen using 20ng (Figure 3d). We also compared the effect of Pep3D to the effect of βMet as the treatment of reference, and we observed that the psoriatic lesions diminished in both treatments in a similar grade (Figure 3d). About the histology in this model, we found the thickness and the acanthosis diminished in the same proportion in the ears treated with Pep3D and with βMet (Figure 3e).
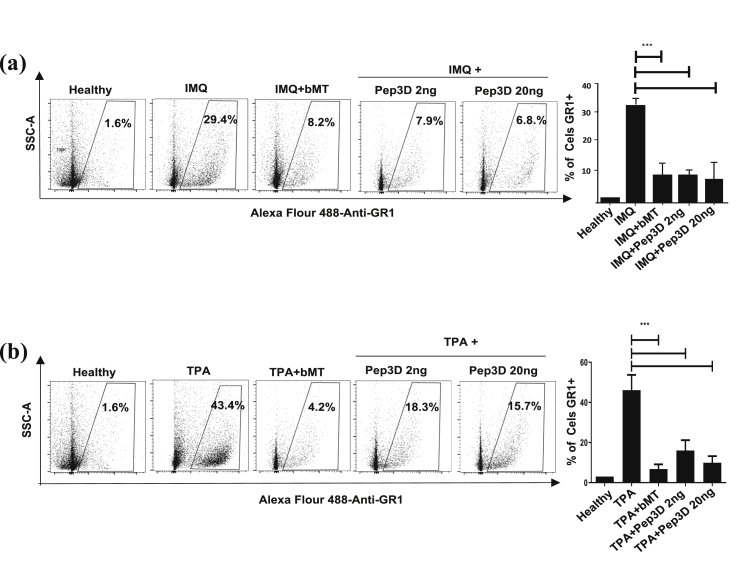
3.3. Pep3D inhibits infiltration of granulocytes GR1+ in the skin of mice with psoriasis induced with IMQ and TPA
We found that the skin of mice with psoriasis increased 18-fold and 27-fold the GR1+ cells population, in reference to healthy skin, when lesions were induced with IMQ or TPA, respectively. Figure 4 shows that the treatment with Pep3D inhibited the increase in the infiltration of GR1+ cells in the psoriatic lesions induced with IMQ (p < 0.05) just as TPA did (p < 0.05). Similar inhibition was observed in the control group treated with βMet (n = 3 mice, p < 0.05).
Figure 4.
Pep3D decreased the infiltrate of neutrophils in the skin of mice with psoriasis induced by IMQ and TPA. The anti-psoriatic effect of Pep3D was assayed in the ear of mice treated with IMQ (a) or TPA (b) to induce psoriasis. The skin of the ears was enzymatically digested, and the number of neutrophils was determined by flow cytometry using Alexa Flour 488-anti-GR-1. The graphic represents the mean of 3 independent experiments done ±SEM. ∗∗∗P < 0.0001. (Dunnett's multiple comparison tests, after one-way ANOVA).
3.4. Pep3D inhibits the production of cytokines IL-17 and TNFα induced in IMQ and TPA murine models
IL-17 and TNFα are cytokines strongly related to the development and maintenance of psoriasis, for this reason we determined the presence of both after the treatment with Pep3D. It is well known that the IMQ and TPA are both inductors of IL-17 and TNFα in the skin of treated mice and we also got these results in the mice treated with the inductors (Figures 5a and 5b), and interestingly the treatment with Pep3D significantly diminished the production of IL-17 (p < 0.05) but not of TNFα in the skin of mice treated with IMQ. In contrast, in the TPA model Pep3D decreased significantly (p < 0.05) the production of TNFα but not of IL-17 (Figure 5). These differences could be related to the molecular mechanism activated by each inducer for the generation of the disease.
Figure 5.
Pep3D inhibited the high production of IL-17 and TNFα induced by IMQ and TPA in the psoriasis murine models. The cytokines were quantitated from the supernatants of the lysed skin by flow cytometry. The Pep3D was assayed in the IMQ-induced (a) and in the TPA-induced psoriasis models (b). The graphics represent the mean of 3 independent experiments done ±SEM. ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.0001. (Dunnett's multiple comparison tests, after one-way ANOVA).
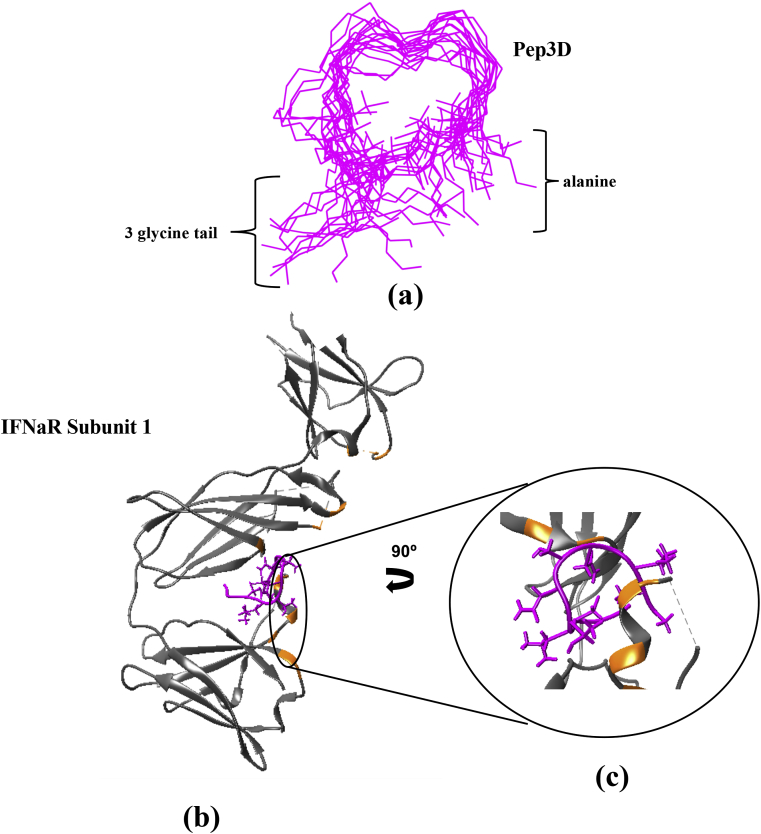
3.5. In-silico analysis of peptide Pep3D
As Pep3D was effective stopping the development of psoriatic lesions, we modeled its theoretical tridimensional structure. Pep3D contained 13 aminoacids; the first aminoacid is an alanine, the 2nd and the 10th are cysteines that bend the structure forming a loop, and the last 3 aminoacids are glycines that shape a tail in the structure. The amino acids conforming the loop are aliphatic, which provides some flexibility to the peptide. For the analysis of the structure, a raw non-optimized structure of the Pep3D was calculated by homology modeling. The EMD analysis showed that the conformational structure reached equilibrium and is rigid as non-important RMSD variations were observed. In Figure 6a we overlaid 22 models of Pep3D where can be seen that the loop structure is the most stable part of the molecule. With the optimized structure we evaluated if it could interact with the IFNα or with the IFNAR1, and we found that Pep3D is able to bind specifically to a zone between the sub-dominiums 2 and 3 of IFNΑR1 where the hot spots of the receptor are located (Figure 6b). This interaction is mediated by hydrogen bonds (energy 4–15 kcal/mol) and occurred between the ser158, tyr163 and lys240 from the IFNAR1 and the Pep3D. In this same area also occur 749 Van der Waals interactions between tyr138, tyr157, ans242, pro243, asn155, lys240, ser158, glu294, ile156, lys161, val116, arg241, leu239, ala237, lys296, arg159, leu277 and glu109 from the IFNAR1 and the Pep3D. Figure 6c shows a 90° turn sight where is visible that Pep3D fits the IFNΑR1 cave, and we suggest that this interaction could confer the antagonist activity of Pep3D. Despite Pep3D did not show identity to the lineal sequence of the cytokine, the docking analysis showed bonding between the spatial conformations of Pep3D and IFNαR1 that support the blocking effects of Pep3D over IFNαR1 mentioned along the work.
Figure 6.
Docking between IFNAR subunit 1 and Pep3D. (a) Superposition of 22 dynamic models of PepD3 after RMSD analysis. (b) The Pep3D (pink) interact with some hot spots (orange) of IFNAR1 where the IFN is recognized. (c) 90°-turn view of the interaction site.
4. Discussion
Recently, the use of biopharmaceuticals for the treatment of diverse diseases has increased. In the case of psoriasis, the most common molecules used as treatment are the humanized monoclonal antibodies that block the activity of inflammatory cytokines. Some of these antibodies inactivate TNFα or its receptor (Etanercept, Infliximab, Adalimumab, Certolizumab pegol), IL-17 or its receptor (Secukinumab, Ixekizumab, Brodalumab) and IL-12/IL-23 (Ustekinumab, Guselkumab, Tildrakizumab) [24]. However, there are some other signaling pathways involved in the pathogenesis of psoriasis that can also be used as target.
Controversial discussion about the role that IFNα plays in the etiology of the psoriasis is in debate, however several reports support that the impediment of the IFNα/β signaling pathway shows positive effects protecting against the development of psoriasis in mice and humans [4, 5, 6, 7, 8, 12, 13, 14] in comparison to reports with opposite results [10, 11]. Notwithstanding this controversy, more research is required to prove one or the other hypothesis. Here we report a peptide obtained from phage-display technology using anti-IFNα antibodies as the target. The phage-peptide named Phpep3D blocked in vitro the interaction of IFNα-IFNAR1, and the derived peptide (Pep3D) showed affinity to hot spots of IFNAR1 in an analysis in-silico, and even more, Pep3D had protective effects against the development of psoriatic lesions as shown in two different animal psoriasis models. Pep3D is a peptide with low molecular weight (1.16 KDa) that could drive to a better tissue penetration in comparison with monoclonal antibodies due to its size, therefore Pep3D could be a good option to treat psoriasis in humans. Analysis of the Pep3D linear sequence showed homology to proteins not related to that of IFNα, but not to any other inflammatory cytokine or psoriasis-related proteins, an explainable finding as we used anti-IFNα antibodies as anchor to mimic the cytokine receptor. It is probable that this strategy favored the selection of spatial conformational rather than sequential peptides able to interact with the cytokine receptor.
Different monoclonal antibodies have been developed to block the activity of IFNα or its receptor: Sifalimumab (medi-545), Rontalizumab, and AGS 009 are antibodies that bind to IFNα, meanwhile Anifrolumab (medi-546) recognizes the IFNα receptor [25]. These antibodies where first used for the treatment of systemic lupus erythematosus (SLE), as IFNα have an essential role in this pathology, however, only Anifrolumab has achieved the phase IIIb and is expected to begin its commercialization in the short time [26, 27]. Nevertheless, only Sifalimumab has been tested for the treatment of psoriasis, but there was no difference in the PASI of patients treated with the antibody in comparison to those that received placebo. The authors suggested that the absence of effect could be due to the involvement of other type I interferons in the development of psoriatic chronic lesions [28]. In concordance with this, Zhang et. al. showed that the main type I interferon produced by keratinocytes in the psoriatic lesions is interferon β, and therefore the therapeutic strategy must be directed to block the receptor instead to the cytokine [29].
The model of psoriasis induced by IMQ could represent a model where innate immunity is responsible for the development of the lesions, because IMQ, as a TLR7 ligand, induces in pDC high production of IFNα that could trigger the development of psoriasis [12]. On account of the controversy about whether TLR7 and IFNAR1 are necessary or not for the generation of psoriasis [10], we decided also to test the effect of Pep3D in the murine model of psoriasis induced by TPA. This model represents the disease induced by the cells of adaptive immunity (in the chronic phase of the pathology), because TPA is a T lymphocytes activator that leads to the high production of IFNγ and IL-17A, causing the arrival of cells of the innate immunity which are the primary producers of IFNα [18, 30]. In both murine models Pep3D diminished the development of psoriatic lesions. Based on the results we suggest that this peptide could have beneficial effects on the treatment of chronic psoriasis, but more information is needed.
Recently, Conrad et. al. reported that between 2-5% of the patients with chronic inflammation treated with anti-TNFα antibodies, exhibited the development or exacerbation of psoriatic lesions. When the authors analyzed the mechanisms involved, they identified that these lesions were related to the IFNα produced by cells of innate immunity, because the absence of the TNFα induce immature pDCs to produce IFNα predominantly [31]. This demonstrates again the importance of IFNα in psoriasis and the necessity to obtain new biopharmaceuticals that block the activity of this cytokine, as is the case of Pep3D.
We also demonstrated that the treatment with Pep3D inhibited significantly the infiltration of GR1+ cells (mainly neutrophils) in the skin of the mice treated with IMQ and TPA. Furthermore, when the cytokines were measured in the Pep3D-treated skins, IL-17 did not increase in the IMQ-induced psoriasis model, and TNFα did not increase in the TPA-induced psoriasis model. Our results are in concordance to those reported by Gui et al. in 2016 [13]; they also found an important inhibition in the infiltration of neutrophils and in the amount of inflammatory cytokines when they used IFNAR1−/− mice treated with IMQ and in WT mice treated with IMQ and halofuginone. Halofuginone down regulates IFNAR1 expression by the induction of its ubiquitination and degradation by the proteasome [13]. Our results suggest that Pep3D blocks the activity of IFNAR1, avoiding the recruitment of neutrophils, and as consequence diminishing the presence of IL-17 and TNFα in the psoriatic skin. Actually, antibodies anti-IL17 and anti-TNFα are used for the treatment of psoriasis.
On the other hand, as Pep3D might be affecting the interaction IFNα/IFNRA1 this peptide could also interfere in the keratinocytes proliferation, because IFNα induces the expression of the IL-22 receptor in these cells, favoring keratinocytes proliferation [32]. However, there could be some other routes were Pep3D could be interfering as consequence of the IFNα/IFNRA1 inhibition.
As Pep3D inhibited the arrival of neutrophils to the IMQ-treated skin, we suggest that as consequence the proteases released by neutrophils for the maturation of the IL-36 family cytokines are also diminished. The IL-36 family cytokines have been directly associated to psoriasis [33]. The decrease in the number of neutrophils recruited into the psoriatic lesions, could also affect the presence of NETs in the skin. Majewski et al described that NETs activate the dDCs to produce more IFNα, amplifying the epidermal inflammation [34]. Interestingly, Aga et. al. reported that IFNα also has an anti-apoptotic effect over neutrophils, prolonging their half-life in the lesions [35].
The in-silico analysis showed that Pep3D theoretically binds to IFNΑR1 in a region that interferes with the recognition of its natural ligand, suggesting this is the cause of its antagonistic effect. Our results showed that the interaction of Pep3D with IFNAR1 is due to its conformational structure, since the linear sequence of Pep3D showed no similitudes with IFNα. The phage-peptide library used in this work display peptides of 7 amino acids with a three-dimensional structure forming loops due to the 2 cysteine that flank the heptapeptide. Pep3D could also be acting like the small molecules that are used to inhibit the activity of enzymes or ligand-receptor interactions, which are of low molecular weight and interact biochemically with their target molecules, showing no identity to the linear sequence of the natural ligand.
In conclusion, Pep3D has a potential to be used as a therapeutic to treat psoriasis, as it blocks the function of IFNR, inhibits the infiltration of granulocytes and reduces the production of IL-17 in the skin of two mice models of psoriasis.
Declarations
Author contribution statement
L. ZAPI-COLÍN, G. GUTIÉRREZ-GONZÁLEZ and C. CEDILLO-PELÁEZ: Performed the experiments; Analyzed and interpreted the data.
S. RODRÍGUEZ-MARTÍNEZ and M.E. CANCINO-DIAZ: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
J.C. CANCINO-DIAZ: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
A. MÉNDEZ-TENORIO: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
S.M. PÉREZ-TAPIA: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
F. GÓMEZ-CHÁVEZ: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Secretaría de Investigación y Posgrado (SIP) - Instituto Politécnico Nacional. M.E. Cancino-Diaz received the funding SIP-20200589; S. Rodríguez-Martínez received the funding SIP-20200588; F. Gómez-Chávez received the funding SIP-20200200.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank all the staff of the Animal Care Facilities of the “Escuela Superior de Medicina of Instituto Politécnico Nacional”, and especially to M. en C. Ma. Eugenia Aguilar Nájera who provided the mice for this study. We also want to thank Gabriela Mellado Sánchez, Edith González González and Ignacio Mejía Calvo for their support in the in vitro determination of IFNα activity assay.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Anti-psoriatic effect of Phpep3D assayed in the IMQ-induced psoriasis murine model. The experiment was repeated 4 times, but in figure 2 we only show representative results. In this figure we show the other 3 results. 1 × 109 Phpep3D were administered intradermally in the back of mice on days 1, 2, 3 and 4 of the IMQ administration protocol. An irrelevant phage-peptide (PhpepIrre) was used as negative control. The dotted purple lines correspond to the area of the skin treated with IMQ; red dotted lines show the skin area treated with Phpep3D or the PhpepIrre. Green dotted lines show the not-treated skin areas.
References
- 1.Nograles K.E., Davidovici B., Krueger J.G. NIH Public Access; 2010. New insights in the immunologic basis of psoriasis. in Seminars in cutaneous medicine and surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grine L. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26(1):25–33. doi: 10.1016/j.cytogfr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Lowes M.A., Suárez-Fariñas M., Krueger J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014;32(1):227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hida S. CD8+ T cell–mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-α/β signaling. Immunity. 2000;13(5):643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 5.Nestle F.O. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lande R. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 7.Ganguly D. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206(9):1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albanesi C. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 2009;206(1):249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Fits L. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 Axis. J. Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 10.Walter A. Aldara activates TLR7-independent immune defence. Nat. Commun. 2013;4(1) doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 11.Wohn C. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(26):10723–10728. doi: 10.1073/pnas.1307569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueyama A. Mechanism of pathogenesis of imiquimod-induced skin inflammation in the mouse: a role for interferon-alpha in dendritic cell activation by imiquimod. J. Dermatol. 2014;41(2):135–143. doi: 10.1111/1346-8138.12367. [DOI] [PubMed] [Google Scholar]
- 13.Gui J. Therapeutic elimination of the type 1 interferon receptor for treating psoriatic skin inflammation. J. Invest. Dermatol. 2016;136(10):1990–2002. doi: 10.1016/j.jid.2016.06.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y. Correction: type I interferon: potential therapeutic target for psoriasis? PloS One. 2009;4(3) doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quesada J., Gutterman J. Psoriasis and alpha-interferon. Lancet. 1986;327(8496):1466–1468. doi: 10.1016/s0140-6736(86)91502-3. [DOI] [PubMed] [Google Scholar]
- 16.Pauluzzi P. Psoriasis exacerbation induced by interferon-alpha. Report of two cases. Acta Derm. Venereol. 1993;73(5):395. doi: 10.2340/0001555573395. [DOI] [PubMed] [Google Scholar]
- 17.Funk J. Psoriasis induced by interferon-α. Br. J. Dermatol. 1991;125(5):463–465. doi: 10.1111/j.1365-2133.1991.tb14774.x. [DOI] [PubMed] [Google Scholar]
- 18.Hvid H. TPA induction leads to a Th17-like response in transgenic K14/VEGF mice: a novel in vivo screening model of psoriasis. Int. Immunol. 2008;20(8):1097–1106. doi: 10.1093/intimm/dxn068. [DOI] [PubMed] [Google Scholar]
- 19.Webb B., Sali A. 2016. Comparative Protein Structure Modeling Using MODELLER, in Current Protocols in Protein Science; pp. 2.9.1–2.9.37. [DOI] [PubMed] [Google Scholar]
- 20.Phillips J.C. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis I.W. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. (Web Server) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneidman-Duhovny D. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–W367. doi: 10.1093/nar/gki481. (Web Server) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duhovny D., Nussinov R., Wolfson H.J. 2002. Efficient unbound docking of rigid molecules, in algorithms in bioinformatics; pp. 185–200. [Google Scholar]
- 24.Rønholt K., Iversen L. Old and new biological therapies for psoriasis. Int. J. Mol. Sci. 2017;18(11) doi: 10.3390/ijms18112297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathian A. Targeting interferons in systemic lupus erythematosus: current and future prospects. Drugs. 2015;75(8):835–846. doi: 10.1007/s40265-015-0394-x. [DOI] [PubMed] [Google Scholar]
- 26.Furie R. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis & Rheumatology. 2017;69(2):376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riggs J.M. Characterisation of anifrolumab, a fully human anti-interferon receptor antagonist antibody for the treatment of systemic lupus erythematosus. Lupus Science & Medicine. 2018;5(1) doi: 10.1136/lupus-2018-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissonnette R. A randomized, double-blind, placebo-controlled, phase I study of MEDI-545, an anti–interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. J. Am. Acad. Dermatol. 2010;62(3):427–436. doi: 10.1016/j.jaad.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L.-j. Antimicrobial peptide LL37 and MAVS signaling drive interferon-β production by epidermal keratinocytes during skin injury. Immunity. 2016;45(1):119–130. doi: 10.1016/j.immuni.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alford J.G. Temporal infiltration of leukocyte subsets into mouse skin inflamed with phorbol ester. Agents Actions. 1992;37(3-4):260–267. doi: 10.1007/BF02028118. [DOI] [PubMed] [Google Scholar]
- 31.Conrad C. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-017-02466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tohyama M. IFN-α enhances IL-22 receptor expression in keratinocytes: a possible role in the development of psoriasis. J. Invest. Dermatol. 2012;132(7):1933–1935. doi: 10.1038/jid.2011.468. [DOI] [PubMed] [Google Scholar]
- 33.Henry C.M. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 2016;14(4):708–722. doi: 10.1016/j.celrep.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 34.Majewski P. Inhibitors of serine proteases in regulating the production and function of neutrophil extracellular traps. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aga E. Type-1 interferons prolong the lifespan of neutrophils by interfering with members of the apoptotic cascade. Cytokine. 2018;112:21–26. doi: 10.1016/j.cyto.2018.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-psoriatic effect of Phpep3D assayed in the IMQ-induced psoriasis murine model. The experiment was repeated 4 times, but in figure 2 we only show representative results. In this figure we show the other 3 results. 1 × 109 Phpep3D were administered intradermally in the back of mice on days 1, 2, 3 and 4 of the IMQ administration protocol. An irrelevant phage-peptide (PhpepIrre) was used as negative control. The dotted purple lines correspond to the area of the skin treated with IMQ; red dotted lines show the skin area treated with Phpep3D or the PhpepIrre. Green dotted lines show the not-treated skin areas.