Abstract
BACKGROUND:
Gait speed is an important outcome that relates to mobility, function, and mortality, and is altered in people with Parkinson’s disease (PwPD). However, changes in gait speed may not reflect changes in other important aspects of gait.
OBJECTIVE:
To characterize which outcomes change concomitantly with walking speed in PwPD. This information can inform the choice of outcome variables for characterizing and tracking gait performance in this population.
METHODS:
67 PwPD and 40 neurotypical adults completed 2-minute overground walking bouts at comfortable and fast self-selected speeds. Eight inertial sensors were used to characterize gait and turning. We identified a subset of participants (38 per group) where the PD participant’s “fast” walk was similar speed to neurotypical participants “comfortable” walk, facilitating an across-group gait comparison controlling for gait speed.
RESULTS:
Walking at fast gait speed compared to comfortable lead to significant changes in stride length, cadence, and stride time variability, but not in steps to turn, trunk ROM, and trunk and lumbar stability in PwPD. Sub-group analyses showed that despite walking at a similar speed as neurotypical adults, PwPD exhibit altered turning outcomes, lumbar stability, and stride length/cadence.
CONCLUSIONS:
Gait speed is a critical outcome for characterizing mobility. However, in PwPD, several important outcomes do not exhibit a uniform relationship with gait speed, and remain altered compared to neurotypical adults despite “normalizing” walking speed. Given the complex relationship between gait speed and other gait quality measures, care should be taken when choosing outcome measures to characterize the breadth of gait abnormality in PwPD.
Keywords: Parkinson’s disease, gait, speed, kinematics
INTRODUCTION
Gait speed is reduced in people with Parkinson’s disease (PwPD) [1, 2], and is a commonly measured to track progression through rehabilitation interventions in this group. However, despite the importance of gait speed as a measure of general function, changes in this outcome may not reflect other, more subtle gait or turning deficits. For example, whether increasing gait speed in PD reflects improvements of quality of gait (trunk stability, gait regularity, etc.), is poorly characterized [3, 4]. Understanding which gait outcomes do and do not improve at higher walking speed in PwPD can inform which outcomes to test in the clinic to fully capture gait ability.
Gait speed can be adjusted through changes in temporal (e.g. cadence) or spatial (e.g. step length) outcomes. Interestingly, the way in which PwPD achieve increased walking speed differs from neurotypical adults (NTA). For example, to increase gait velocity, PwPD exhibit a disproportionate increase in cadence, rather than step length, compared to NTA [5, 6]. Further, when matched for walking speed, PwPD exhibited lower total work [7] compared to healthy adults, due primarily to lower work at the hip [8].
The effect of faster gait on other outcomes, such as variability or trunk control, in PwPD is less consistent. For example, Frenkel-Toledo & colleagues demonstrated that with increasing walking speed, variability of stride time (but not swing time), was reduced in PwPD [4]. This is somewhat inconsistent with data from healthy adults, which suggest a relatively consistent decrease in variability with increasing gait speed [9]. Moreover, little is known about the movement and stability of the trunk with increasing gait speed. Maintenance of trunk stability is a critical aspect of effective walking, is altered in PwPD [10] and may be related to falls [11]. However the impact of gait speed on trunk control in PwPD is poorly characterized, and may be inconsistent across levels of disease severity.
In addition to straight ahead gait, the relationship between gait speed and changing direction while walking (turning) have not been adequately characterized. Turning is more vulnerable to functional impairments compared to straight ahead gait since turning requires better inter-limb coordination, more multisensory integration, more coupling between posture and gait and modification of locomotor patterns [12]. Difficulties turning while walking are especially common among PwPD, and negatively affect functional independence [13]. However, it is unclear whether increased gait speed is associated with improved turning performance, as it has been recently reported that fast turning, but not slow turning, led to instability in PwPD compared to NTA [14].
Therefore, the purpose of this study was to determine the impact of walking speed on gait and turning outcomes in PwPD and NTA. Specifically, we determined which spatiotemporal gait parameters 1) were altered in PwPD and NTA when walking at fast versus comfortable walking speeds, and 2) remain altered in PwPD compared to NTA controlling for gait speed (i.e. when gait speed was matched across a subset of both groups). Understanding the relationship between gait speed and other gait parameters can inform clinicians on which locomotor outcomes may not be fully captured by gait speed assessments, and which gait parameters should be corrected for changes in gait speed.
MATERIALS & METHODS
Participants:
Data were collected from 67 PwPD and 40 NTA. All participants with PD were clinically diagnosed with idiopathic PD by a neurologist (Brain Bank Criteria [15]). All tests were conducted in the practical OFF levodopa state, after withholding anti-parkinsonian medication for ≥12 h. NTA with similar age, height, and weight were recruited from the community. Inclusion criteria for both groups were (1) between 50 and 90 years old, (2) neurologist-diagnosed PD (for PD group), and (3) ability to stand and walk unassisted. Exclusion criteria for both groups were: (1) any peripheral or central nervous system or musculoskeletal disorders that interfere with gait or balance, (2) Montreal Cognitive Assessment score <18, and (3) inability to follow instructions. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Oregon Health & Science University, OHSU (#4131) and the OHSU/VAPORHCS joint IRB (#8979). These participants were part of a larger interventional study (Clinical Trials NCT02231073 and NCT02236286), of which we used the baseline session of the study.
To identify alterations in gait characteristics in PwPD that are independent of gait speed, PwPD were matched to NTA by gait speed on an individual basis. Gait speed was the only outcome on which participants were matched. Individuals with PD were ranked on the basis of their gait speed in the fast walking condition, while healthy individuals were ranked based on the comfortable gait speed. All individuals with PD with a fast gait speed lower than the lowest comfortable gait speed of the NTA were excluded (n = 29]. Then, pairs of individuals with PD (fast speed) and NTA (comfortable speed) were made. Specifically, we searching for the closest gait speed of a neurotypical control to an individual with PD, allowing a maximum difference in gait speed < 0.05 m.s−1. The difference in gait speed in the matched pairs ranged between −0.04 (e.g. higher gait speed in PwPD than in NTA) and 0.02 m.s−1. Although some PD participants did walk at a comparable speed as healthy controls in the comfortable speed condition, this pairing of PD-fast and NTA-comfortable gait speeds facilitated the largest number of paired walking speeds and thus the largest sample for comparison.
Protocol:
PwPD were clinically rated by a trained examiner on the Motor Section (III) of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [16]. For the walking tasks, each participant was outfitted with eight inertial sensors (APDM, Inc., Portland, OR, USA), worn on the sternum, lumbar spine, bilaterally on the wrists, shins, and feet. Each inertial sensor recorded tri-axial accelerations, angular velocities, and magnetic field at 128 Hz. Here, we focused on two walking trials: i) a self-paced 2-minute trial walking trial, and ii) as fast as possible 2-minute walking trial while maintaining safety (no running or jogging). In both conditions, participants were instructed to walk back and forth continuously between two lines 25 feet (7.62 m) apart with 180 degree turns at the ends of the walkway.
Outcome variables:
Mobility Lab software V2 (APDM, Inc.) was used to calculate the following outcome variables during the portion of steady state walking for both trials: walking speed, stride length, cadence, stride and swing time, arm range of motion (ROM), trunk ROM in the AP and ML directions, step and swing time variability (coefficient of variation), and double support time (as percentage of gait cycle). We excluded trials with less than 30 strides to be able to calculate valid variability measures [17]. During the 2-minute walks, turns were separately analyzed and the average turning velocity and number of steps were reported. Finally, the maximum finite-time Lyapunov exponent (LyE) was estimated during walking, after removing turns, for both trunk and lumbar motion to characterize trunk stability during gait [18]. LyE quantifies the rate of divergence of nearby state-space trajectories and conceptually, describes the rate at which variability is controlled. Larger values of LyE represent faster divergence, and therefore slower (e.g., worse) control. We included LyE in this analysis because it 1) is strongly associated with retrospective and prospective falls [11], 2) serves as a valid measure of gait stability [19], and 3) is distinct from other measures of variability. LyE was estimated using the three-dimensional accelerations for both the sternum and lumbar sensor location. For each bout of walking between turns, the accelerations were time normalized to 130 data points per stride and embedded in a 9-dimensional state-space using the accelerations and their twice-time delayed copies with a fixed time delay of 0.25*the average stride time [18, 20]. The distance between neighboring trajectories was tracked, the LyE was estimated from the slope of the log mean divergence curve between 0 and 0.5 strides [19], with a larger LyE values indicating less stability [21]. The final estimate of LyE was obtained by taking the average LyE across straight bouts of gait.
Statistical analyses:
Paired and independent sample t-tests were utilized to assess participant demographics and to determine the effect of speed on walking variables. Independent sample t-tests were used to determine which gait characteristics were significantly different in PwPD and controls while walking at similar walking speeds. Shapiro-Wilk tests were used to identify normality of data, and non-parametric assessments used as necessary. Cohen’s d statistics were calculated to quantify the effect size for across and within group analyzes. Previously described guidelines [22] are also reported to provide context regarding magnitude of effect size (low=0.2; medium=0.5; high=0.8). Given the large number of comparisons run, α level was set to 0.001 for all analyses.
RESULTS
Demographics are shown in table 1 for all participants (PwPD and NTA), as well as the subset of matched participants (discussed further below).
Table 1:
Participant demographics for full cohort and speed-matched cohorts.
| Full Cohort | Speed-matched Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD (n=67) | NTA (n=40) | PD (n=38) | NTA (n=38) | |||||||
| mean | SD | mean | sd | p-value | mean | SD | mean | sd | p-value | |
| Age (years) | 69.80 | 7.54 | 69.69 | 7.56 | 0.946 | 69.41 | 7.495 | 69.80 | 7.612 | 0.821 |
| Height (cm) | 175.5 | 9.7 | 172.3 | 10.1 | 0.119 | 175.2 | 9.3 | 172.0 | 10.2 | 0.148 |
| MDS-UPDRS III | 38.52 | 12.13 | -- | -- | -- | 35.89 | 9.33 | -- | -- | -- |
| H&Y stage | n | (%) | -- | -- | -- | n | (%) | -- | -- | -- |
| 1 | 0 | 0 | -- | -- | -- | 0 | 0 | -- | -- | -- |
| 2 | 58 | 86.6 | -- | -- | -- | 35 | 92.1 | -- | -- | -- |
| 3 | 6 | 9.0 | -- | -- | -- | 3 | 7.9 | -- | -- | -- |
| 4 | 3 | 4.5 | -- | -- | -- | 0 | 0 | -- | -- | -- |
MDS-UPDRS: Movement Disorders Society- Unified Parkinson’s Disease Rating Scale; H&Y: Hoehn & Yahr
Impact of gait speed on spatio-temporal outcomes (Objective 1; all participants)
The first objective of this manuscript was to characterize changes in spatio-temporal gait outcomes at comfortable and fast walking speeds across both groups. These data are shown in Table 2. For all participants and both groups (N, PwPD = 67; N, NTA = 40), walking speed in the “fast” condition, was significantly higher than at the “comfortable” walking speed. Further, we observed that during fast compared to comfortable walking, both groups exhibited larger stride length, cadence, swing time, arm range of motion (ROM), turn velocity, and trunk ROM in the ML direction. Double support time was smaller in both groups, and stride time variability reduced in PwPD at fast walking speed compared to comfortable. Trunk ROM in the AP direction, LyE of trunk or lumbar movements, and the number of steps during turns were not statistically different during fast compared to comfortable walking trials. Figure 1 visually illustrates the effect size of walking at fast vs. comfortable gait speed on outcomes in PwPD and NTA controls.
Table 2:
Gait outcomes in neurotypical adults (NTA) and people with PD at comfortable and fast walking speeds. Data are reported for all participants of both groups [N(PD)=67; N(NTA)=40] unless otherwise noted.
| 95% Confidence Interval of the Difference | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean Comfortable | Mean Fast | Mean Change | Lower | Upper | p-value | Effect size (Cohen’s d) | ||
| Speed (m/s) | NTA | 1.20 | 1.50 | 0.31 | 0.26 | 0.35 | <0.001 | 2.38 |
| PD | 0.99 | 1.21 | 0.23 | 0.20 | 0.26 | <0.001 | 1.84 | |
| Stride Length (m) | NTA | 1.27 | 1.39 | 0.12 | 0.09 | 0.14 | <0.001 | 1.59 |
| PD | 1.04 | 1.15 | 0.11 | 0.09 | 0.13 | <0.001 | 1.64 | |
| Cadence (steps/min) | NTA | 112.23 | 129.15 | 16.92 | 14.23 | 19.61 | <0.001 | 2.01 |
| PD | 112.20 | 124.21 | 12.02 | 10.20 | 13.84 | <0.001 | 1.61 | |
| Swing Time (% stride) | NTA | 39.44 | 41.66 | 2.22 | 1.86 | 2.59 | <0.001 | 1.93 |
| PD | 38.38 | 40.09 | 1.71 | 1.44 | 1.99 | <0.001 | 1.52 | |
| Arm RoM (deg) | NTA | 47.58 | 65.18 | 17.60 | 14.16 | 21.04 | <0.001 | 1.64 |
| PD | 28.78 | 35.83 | 7.05 | 5.27 | 8.83 | <0.001 | 0.97 | |
| Turn Velocity (deg/s) | NTA | 184.16 | 217.60 | 33.44 | 27.31 | 39.57 | <0.001 | 1.74 |
| PD | 137.22 | 156.44 | 19.22 | 15.42 | 23.02 | <0.001 | 1.23 | |
| Steps to turn (n) | NTA | 3.74 | 3.91 | 0.17 | 0.02 | 0.32 | 0.030 | 0.36 |
| PD | 4.66 | 4.68 | 0.02 | −0.14 | 0.18 | 0.802 | 0.03 | |
| Trunk AP (deg) | NTA | 8.32 | 9.16 | 0.84 | 0.19 | 1.49 | 0.012 | 0.41 |
| PD | 7.18 | 7.55 | 0.38 | 0.04 | 0.72 | 0.030 | 0.27 | |
| Trunk ML (deg) | NTA | 5.16 | 6.00 | 0.83 | 0.53 | 1.14 | <0.001 | 0.88 |
| PD | 4.28 | 4.93 | 0.66 | 0.46 | 0.85 | <0.001 | 0.84 | |
| Stride Time Variability (a.u) | NTA | 0.04 | 0.03 | −0.004 | −0.01 | 0.00 | 0.230 | 0.19 |
| PD | 0.06 | 0.05 | −0.01 | −0.02 | −0.01 | <0.001 | 0.51 | |
| Swing Time Variability (a.u) | NTA | 0.02 | 0.02 | 0.0002 | 0.00 | 0.00 | 0.881 | 0.02 |
| PD | 0.03 | 0.03 | −0.003 | −0.01 | 0.00 | 0.003 | 0.37 | |
| LyE- Trunk (a.u) | NTA* | 0.31 | 0.32 | 0.01 | −0.02 | 0.04 | 0.445 | 0.16 |
| PD** | 0.36 | 0.37 | 0.004 | −0.01 | 0.02 | 0.659 | 0.06 | |
| LyE- Lumbar (a.u) | NTA* | 0.41 | 0.41 | 0.01 | −0.03 | 0.04 | 0.715 | 0.07 |
| PD** | 0.46 | 0.48 | 0.02 | 0.00 | 0.04 | 0.055 | 0.25 | |
| Double Support Time (% stride) | NTA | 10.51 | 8.43 | −2.07 | −2.52 | −1.63 | <0.001 | 1.48 |
| PD | 11.78 | 10.15 | −1.64 | −1.91 | −1.36 | <0.001 | 1.46 | |
n=25;
n=61;
LyE: Lyapunov Exponent; a.u.: Arbitrary Units
Bolded values represent significant impact of walking speed
Figure 1:
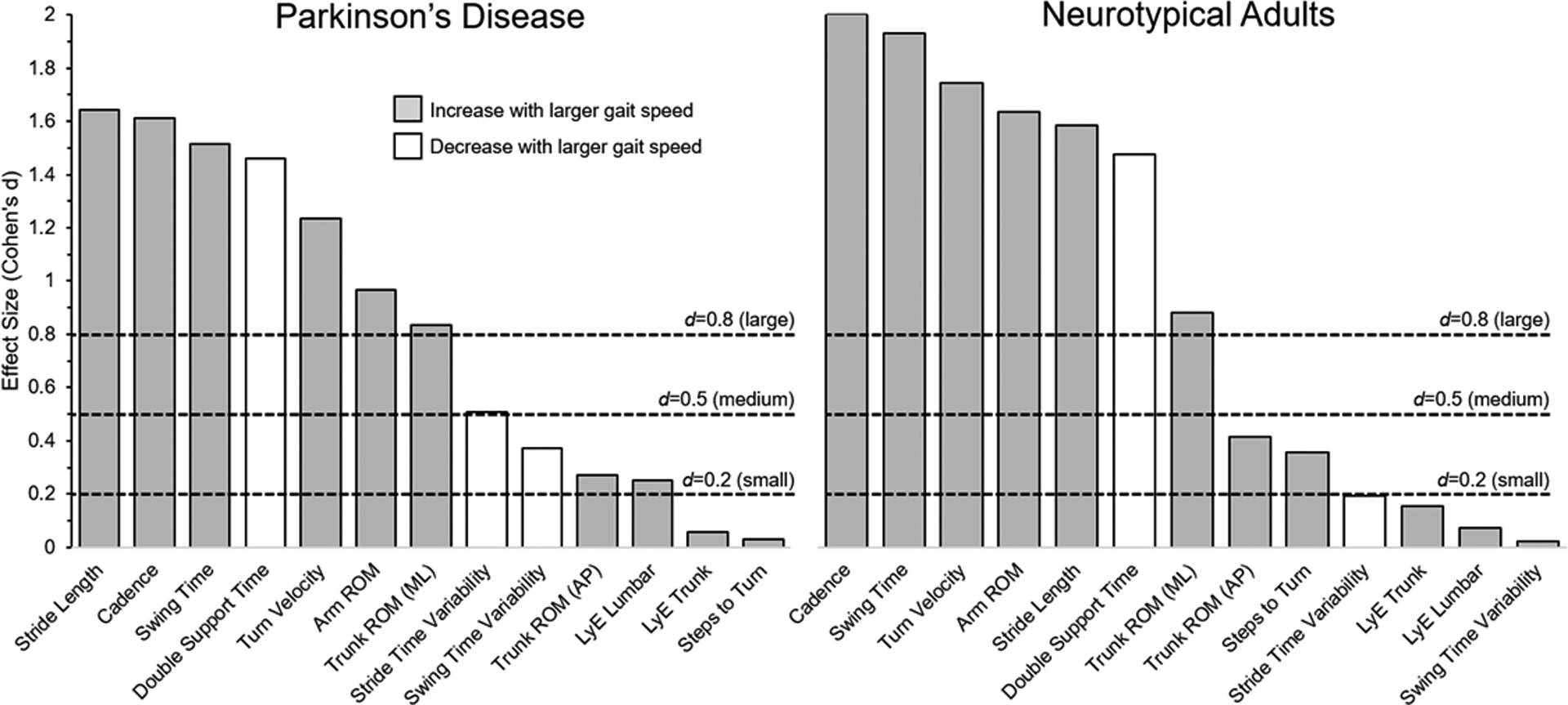
Effect of increasing gait speed (Cohen’s d) on gait kinematic outcomes in people with PD (left panel) and neurotypical adults (right panel). Horizontal dotted lines represent “large”, “medium” and “small” effect sizes (Cohen J., 1988).
Matching NTA and PwPD for gait speed (Objective 2; subset of participants)
The second objective of this manuscript was to characterize differences in gait of people with PD compared to NTA when they walk at similar gait speeds. For the matched subset of PwPD and NTA, despite walking at a similar speed, some gait outcomes remained statistically significantly different in PwPD compared to NTA (table 3, figure 2). In particular, while walking at a similar speed to NTA, PwPD exhibited statistically (p<0.001) smaller stride length and turn velocity, as well as increased cadence, steps to turn. PD also exhibited larger (i.e. worse) stability at the lumbar segment, measured as lumbar LyE. Swing time, trunk ROM (AP and ML), step and swing time variability, and double support time were not different in PD and NTA when walking at the same speed.
Table 3:
Gait kinematics for the subset of PD who, at their fast walking speed, exhibited similar speed as NTA walking at a comfortable speed.
| 95% Confidence Interval of the Difference | |||||||
|---|---|---|---|---|---|---|---|
| NTA Comfortable (n=38) | PwPD Fast (n=38) | Mean difference | Lower | Upper | p-value | Cohen’s d effect size | |
| Speed (m/s) | 1.21 | 1.21 | 0.002 | −0.07 | 0.08 | 0.961 | 0.01 |
| Stride Length (m) | 1.27 | 1.16 | −0.11 | −0.16 | −0.05 | <0.001 | 0.91 |
| Cadence (steps/min) | 112.94 | 124.08 | 11.14 | 6.18 | 16.09 | <0.001 | 1.04 |
| Swing Time (% stride) | 39.54 | 40.02 | 0.48 | −0.33 | 1.29 | 0.240 | 0.28 |
| Arm RoM (deg) | 48.11 | 41.79 | −12.66 | −20.32 | −4.99 | 0.002 | 0.76 |
| Turn Velocity (deg/s) | 184.83 | 157.63 | −27.20 | −40.73 | −13.67 | <0.001 | 0.93 |
| Steps to turn (n) | 3.76 | 4.54 | 0.78 | 0.48 | 1.08 | <0.001 | 1.20 |
| Trunk AP (deg) | 8.35 | 7.62 | -0.73 | −1.87 | 0.41 | 0.205 | 0.30 |
| Trunk ML (deg) | 5.14 | 4.63 | -0.51 | −1.22 | 0.20 | 0.156 | 0.33 |
| Step time Variability (a.u) | 0.04 | 0.05 | 0.010 | 0.00 | 0.02 | 0.014 | 0.58 |
| Swing time Variability (a.u) | 0.02 | 0.02 | 0.002 | 0.00 | 0.01 | 0.344 | 0.22 |
| LyE- Lumbar (a.u) | 0.40* | 0.51** | 0.10 | 0.06 | 0.14 | <0.001 | 1.24 |
| LyE- Trunk (a.u) | 0.32* | 0.38** | 0.06 | 0.02 | 0.10 | 0.007 | 0.68 |
| Double Support Time (% stride) | 10.40 | 10.00 | −0.39 | −1.33 | 0.55 | 0.409 | 0.19 |
n=32;
n=37;
Bolded values represent significant across-group differences at the p<0.001 level. LyE: Lyapunov Exponent; a.u.: Arbitrary Units
Figure 2:
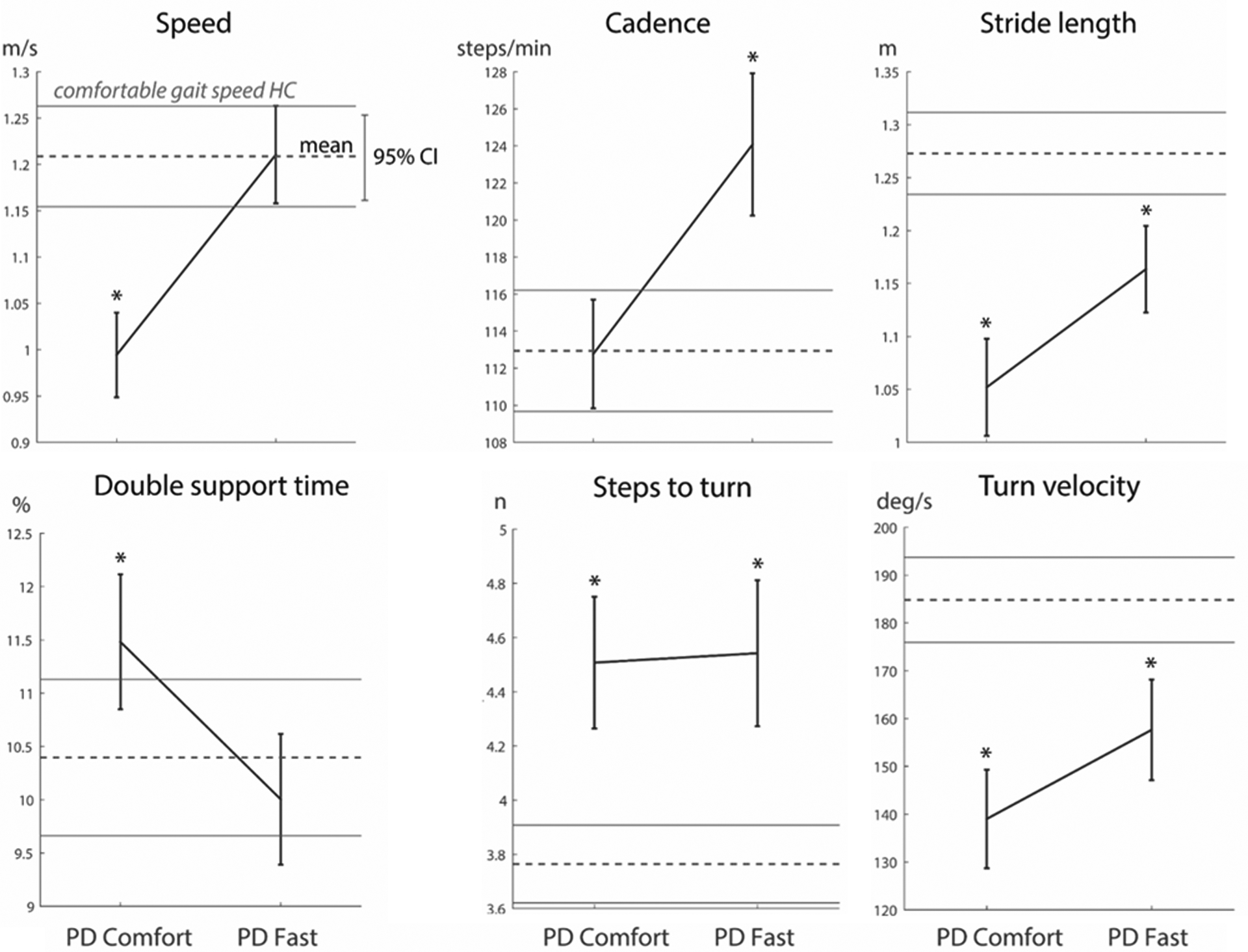
Visualization of selected spatio-temporal gait outcome changes in PD during comfortable and fast speeds (solid line), referenced to comfortable walking speed in neurotypical adults; dotted line, lines above & below represent 1 SD). *represents significant difference between control comfortable speed at p<0.001 level.
DISCUSSION
In this manuscript, we characterize the impact of gait speed on quality of gait and turning in PwPD. Specifically, our two primary objectives were to 1) characterize spatio-temporal outcomes at comfortable and fast walking speeds in PwPD and NTA, and 2) determine which gait outcomes remained different between PwPD and NTA while controlling for gait speed. Similar to previous results [23, 24] we observed that walking at a fast gait speed (compared to comfortable) had a large effect (Cohen’s d >0.8) on a number of spatio-temporal outcomes in PwPD including stride length, cadence, and stride time variability. We extend previous work to show that other important outcomes of mobility, including steps to turn, AP trunk ROM, and stability (LyE) were not significantly impacted (i.e. Cohen’s d effect size =<0.27) by gait speed in PwPD. Further, when PwPD walked at speeds similar to NTA, numerous gait outcomes remained different in PwPD compared to their NTA peers, including stride length & cadence, turn outcomes, and lumbar LyE. Together, these findings demonstrate the complex relationship between gait outcomes, and underscore the importance of capturing multiple outcome variables beyond gait speed when assessing quality of gait in PwPD.
Previous work suggests that gait velocity deficits in PwPD are primarily the result of reduced stride length, rather than cadence [2, 6, 24]. Results from our cohort of participants are consistent with these findings as, at comfortable walking speeds, individuals with PD exhibited reduced walking speed and stride length, but similar cadence, compared to NTA (Fig 2). Walking with smaller rather than slower steps has been attributed to an underscaling of movement via cortico-thalamo-basal ganglia circuitry [2]. Interestingly, in the current study when PwPD walked at a speed similar to that of NTA, they increased both cadence and stride length. However, given the initial deficit in stride length (but not cadence), the increases in step length and cadence resulted in a cadence much larger, and a stride length that remained low in PwPD compared to NTA (Fig. 2).
Despite the consistent relationship between walking speed and some spatio-kinematic outcomes noted above, we observed that other important gait outcomes (e.g. gait variability, AP trunk movement, LyE, turning kinematics) were not uniformly impacted by gait speed (table 2). The lack of an effect of gait speed on LyE and gait variability is not fully consistent with previous work. For example, LyE has previously been shown to be impacted by walking speed [25]. However, previous reports precisely manipulated gait speed by varying treadmill speeds. Here, participants were able to self-select a faster pace. No constraints were placed on the subjects, and they were free to vary stride-to-stride speed. We suspect the disparity in the relation between LyE and gait speed may be due to the difference in treadmill versus overground walking [26]. Future work will systematically explore this relationship. It is notable that, due to technical challenges, LyE was calculated on a subset of the control (25/40) and PD (61/67) participants. As such, these conclusions should be interpreted with caution.
Previous work has also shown PwPD [4, 23], and NTA [25, 27] to reduce gait variability when walking at higher speeds. We observed this effect in the PD, but not NTA. The discrepancy between our and previous results may be for several reasons. First, the most pronounced changes in variability across walking speed is typically observed to be between “slow” and “comfortable” speeds, often with small or no change from “comfortable” to “fast” [25], as we measured in the current study. Second, the lack of reduction in gait variability in the current study may have been due to a floor effect. Stride time variability below 5% stems from standard deviations less than ~5 ms (at mean stride time of ~1 s). Precision of deviations in temporal measures is limited by the sample frequency, which was 128 Hz in our data collection (e.g. ~8 ms sample duration). This highlights the possibility that to detect small (i.e. <0.01) changes in variability, sensors with sampling rates higher than 128 Hz may be warranted. Finally, most previous reports measured gait variability while walking on a treadmill. As discussed above, treadmill walking (compared to overground in the current study) can have substantial impacts on the structure of kinematic profiles, and thus may have contributed to different findings across studies.
The second aim of this study sought to understand how gait differed between PwPD and NTA while controlling for speed. We observed that, despite walking at the same speed as NTA, a number of important gait characteristics remained different in PwPD (e.g. reduced stride length, arm ROM, turn velocity; increased cadence, steps to turn, LyE). These findings are consistent with previously reported difficulty of PwPD to scale-up movement amplitude with increasing speed [5]. In particular, although participants were not instructed explicitly to increase turning speed (only to increase gait speed), turning outcomes were worse in PwPD compared to NT in this condition. This potentially reflects the increased balance challenges posed by turning above and beyond straight-line walking [28]. It also underscores the possibility that despite the ability to increase walking speed, PwPD exhibit turning-specific changes that may necessitate focused, task-specific training [29]). This is especially important given the increased likelihood of hip related injuries during falls during turning. Together, these data suggest that there is considerable variability in the degree to which gait speed impacts gait outcomes, and highlights several gait outcomes that are not improved in PwPD despite higher gait velocity.
Finally it is notable that when controlling for gait speed, swing time variability, double support time, and trunk ROM were not significantly different than NTA. These outcomes are important measures of gait stability, and have been previously observed to be altered in PwPD [30, 31]. Interestingly, the lack across-group differences when controlling for gait speed in the current study raises two points for consideration: 1) PD may have a smaller than expected direct impact on these outcomes, and 2) interventions improving gait speed may also have a positive effect on these outcomes.
Several strengths and limitations should be noted. Strengths include a well screened for group of PD patients, and a set of outcomes encompassing several mobility domains (e.g. trunk ROM while walking, turning, step stability and regulation). An important limitation is that we only observed two walking speeds (comfortable and fast). This limits the ability to detect the curvilinear relationships that have been observed between gait velocity and some outcomes. A second limitation is that, due to our over-ground walking tasks, we were unable to fix gait speed, as can be done via treadmill walking. However, treadmill walking does not include turning and may act as a cue facilitating regularity of gait in PwPD [32].
Conclusions
Gait speed is an important outcome to assess mobility and function in both neurological and NTA. However, there is a complex relationship between gait speed and spatio-temporal gait outcomes, particularly in PwPD. A number of outcomes that are critical for PwPD (e.g. gait variability, turn kinematics, stability) do not have a uniform relationship with gait speed, and remain altered despite walking at a similar speed to healthy adults. These results underscore the importance of capturing a breadth of outcomes when quantifying gait in PwPD, as well as the need for development of quick, yet broad measures of gait ability for both clinical and research assessments.
ACKNOWLEDGEMENTS & FUNDING
We would like to thank the participants for generously donating their time to this study. Funding for this project included support from NIH 2R01 AG006457 (FH), VA Merit I01 RX001075 (FH), NIH Career Development Award R00 HD078492 (MM).
Footnotes
CONFLICT OF INTEREST
Dr. Horak has a significant financial interest in APDM, a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Oversight Council.
LITERATURE CITED
- [1].Combs SA, Diehl MD, Filip J, Long E (2014) Short-distance walking speed tests in people with Parkinson disease: reliability, responsiveness, and validity. Gait Posture 39, 784–788. [DOI] [PubMed] [Google Scholar]
- [2].Morris ME, Iansek R, Matyas TA, Summers JJ (1994) The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain 117 ( Pt 5), 1169–1181. [DOI] [PubMed] [Google Scholar]
- [3].Cole MH, Sweeney M, Conway ZJ, Blackmore T, Silburn PA (2017) Imposed Faster and Slower Walking Speeds Influence Gait Stability Differently in Parkinson Fallers. Arch Phys Med Rehabil 98, 639–648. [DOI] [PubMed] [Google Scholar]
- [4].Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM (2005) Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. J Neuroeng Rehabil 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bayle N, Patel AS, Crisan D, Guo LJ, Hutin E, Weisz DJ, Moore ST, Gracies JM (2016) Contribution of Step Length to Increase Walking and Turning Speed as a Marker of Parkinson’s Disease Progression. PLoS One 11, e0152469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morris ME, Iansek R, Matyas TA, Summers JJ (1996) Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 119 ( Pt 2), 551–568. [DOI] [PubMed] [Google Scholar]
- [7].Dipaola M, Pavan EE, Cattaneo A, Frazzitta G, Pezzoli G, Cavallari P, Frigo CA, Isaias IU (2016) Mechanical Energy Recovery during Walking in Patients with Parkinson Disease. PLoS One 11, e0156420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuhman D, Hammond KG, Hurt CP (2018) Altered joint kinetic strategies of healthy older adults and individuals with Parkinson’s disease to walk at faster speeds. J Biomech 79, 112–118. [DOI] [PubMed] [Google Scholar]
- [9].Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB (2001) Gait variability in community-dwelling older adults. J Am Geriatr Soc 49, 1646–1650. [DOI] [PubMed] [Google Scholar]
- [10].Menz HB, Lord SR, Fitzpatrick RC (2003) Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J Gerontol A Biol Sci Med Sci 58, M446–452. [DOI] [PubMed] [Google Scholar]
- [11].van Schooten KS, Pijnappels M, Rispens SM, Elders PJ, Lips P, van Dieen JH (2015) Ambulatory fall-risk assessment: amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci 70, 608–615. [DOI] [PubMed] [Google Scholar]
- [12].Herman T, Giladi N, Hausdorff JM (2011) Properties of the ‘timed up and go’ test: more than meets the eye. Gerontology 57, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stack E, Ashburn A (2008) Dysfunctional turning in Parkinson’s disease. Disabil Rehabil 30, 1222–1229. [DOI] [PubMed] [Google Scholar]
- [14].Mellone S, Mancini M, King LA, Horak FB, Chiari L (2016) The quality of turning in Parkinson’s disease: a compensatory strategy to prevent postural instability? J Neuroeng Rehabil 13, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55, 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- [17].Kroneberg D, Elshehabi M, Meyer AC, Otte K, Doss S, Paul F, Nussbaum S, Berg D, Kuhn AA, Maetzler W, Schmitz-Hubsch T (2018) Less Is More - Estimation of the Number of Strides Required to Assess Gait Variability in Spatially Confined Settings. Front Aging Neurosci 10, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fino PC, Mancini M, Curtze C, Nutt JG, Horak FB (2018) Gait Stability Has Phase-Dependent Dual-Task Costs in Parkinson’s Disease. Front Neurol 9, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bruijn SM, Meijer OG, Beek PJ, van Dieen JH (2013) Assessing the stability of human locomotion: a review of current measures. J R Soc Interface 10, 20120999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Schooten KS, Rispens SM, Elders PJ, van Dieen JH, Pijnappels M (2014) Toward ambulatory balance assessment: estimating variability and stability from short bouts of gait. Gait Posture 39, 695–699. [DOI] [PubMed] [Google Scholar]
- [21].Rosenstein MT, Collins JJ, De Luca CJ (1993) A practical method for calculating largest Lyapunov exponents from small datasets. Phys. D: Nonlinear Phenom 65, 117–134. [Google Scholar]
- [22].Cohen J (1988) Statistical power analysis for the behavioral sciences.
- [23].Rennie L, Lofgren N, Moe-Nilssen R, Opheim A, Dietrichs E, Franzen E (2018) The reliability of gait variability measures for individuals with Parkinson’s disease and healthy older adults - The effect of gait speed. Gait Posture 62, 505–509. [DOI] [PubMed] [Google Scholar]
- [24].Morris ME, Iansek R, Matyas TA, Summers JJ (1994) Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry 57, 1532–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kang HG, Dingwell JB (2008) Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J Biomech 41, 2899–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D (2001) Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. J Biomech Eng 123, 27–32. [DOI] [PubMed] [Google Scholar]
- [27].Jordan K, Challis JH, Newell KM (2007) Walking speed influences on gait cycle variability. Gait Posture 26, 128–134. [DOI] [PubMed] [Google Scholar]
- [28].Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR (2012) Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 18, 149–154. [DOI] [PubMed] [Google Scholar]
- [29].Mancini M, Smulders K, Harker G, Stuart S, Nutt JG (2018) Assessment of the ability of open- and closed-loop cueing to improve turning and freezing in people with Parkinson’s disease. Sci Rep 8, 12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM (2005) Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci 22, 1248–1256. [DOI] [PubMed] [Google Scholar]
- [31].Svehlik M, Zwick EB, Steinwender G, Linhart WE, Schwingenschuh P, Katschnig P, Ott E, Enzinger C (2009) Gait analysis in patients with Parkinson’s disease off dopaminergic therapy. Arch Phys Med Rehabil 90, 1880–1886. [DOI] [PubMed] [Google Scholar]
- [32].Warlop T, Detrembleur C, Stoquart G, Lejeune T, Jeanjean A (2018) Gait Complexity and Regularity Are Differently Modulated by Treadmill Walking in Parkinson’s Disease and Healthy Population. Front Physiol 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]


