Abstract
Evidence to date strongly suggests that poor inspiratory muscle performance is associated with dyspnea, poor exercise tolerance and poor functional status in patients with heart failure (HF). A growing body of literature has examined the effects of inspiratory muscle training (IMT) in HF patients with the majority of studies reporting favorable effects on several of the above limitations and a substantial number of related deficiencies due to inadequate inspiration and inspiratory muscle strength and endurance. The domains and manifestations of HF, which were significantly improved by IMT in one or more of the 18 out of 19 studies of IMT, included dyspnea, quality of life, balance, peripheral muscle strength and blood flow, peripheral muscle sympathetic nervous activity, heart rate, respiratory rate, peak VO2, 6-min walk test distance, ventilation, VE/VCO2 slope, oxygen uptake efficiency, circulatory power, recovery oxygen kinetics and several indices of cardiac performance. This paper will also review the available IMT literature with a focus on methods of IMT and clinical outcomes. Key differences between available IMT methods will be highlighted with a goal to improve IMT efforts and decrease the pathophysiological manifestations of heart disease and HF.
Keywords: breathing, heart failure, inspiratory muscle training, maximal inspiratory pressure
Inspiration is the initial task responsible for ventilation and respiration [1]. Ventilation is defined as the movement of air into and out of the lungs which facilitates pulmonary and peripheral gas exchange that subsequently enables the conversion of biochemical energy into ATP and the removal of biochemical waste products [1]. Inadequate inspiration can lead to limited ventilation, pulmonary and peripheral gas exchange, ATP availability and removal of biochemical waste [1–3]. Evidence to date strongly suggests that poor inspiratory muscle performance is associated with dyspnea, poor exercise tolerance and poor functional status in patients with heart failure (HF). Although a relatively limited literature has examined the effects of inspiratory muscle training (IMT) in heart disease and HF, the majority of studies have found favorable effects on several of the above limitations and a substantial number of related deficiencies owing to inadequate inspiration and inspiratory muscle strength and endurance (TABLE 1) [4–22]. Several different methods of IMT have been used to improve inspiratory muscle performance including Threshold® IMT (Philips Respironics, Andover, MA, USA), the test of incremental respiratory endurance (TIRE) and various breathing exercises with Threshold IMT being the most frequently used method. The purpose of this literature review is to describe the results of IMT in persons with heart disease and HF and to focus on the methods of IMT and outcomes. The literature review will end with a discussion of the TIRE, which will include a comparison with other forms of IMT and the novel outcome measures obtained with TIRE IMT. dyspnea (FIGURE 1) [23].
Table 1.
Studies of inspiratory muscle training in patients with heart disease and heart failure.
| Study (year) | n | Mode of IMT | Study measurements | Study outcomes | Ref. |
|---|---|---|---|---|---|
| Mancini et al. (1995) | Exp = 8 Con = 6 |
Threshold at 30% of MIP + a variety of BE for 12 weeks (90 min, 3×/week) | MIP, MEP, IME, 6-MWT, exercise duration, peak VO2, HR, BP, RR, VT, VE, dyspnea | Improved MIP, MEP, IME, 6-MWT, exercise duration, peak VO2, VE and dyspnea | [4] |
| Cahalin et al. (1997) | 8 | Threshold at 20% of MIP for 8 weeks (5–15 min, 3×/day, 7×/week) | MIP, MEP, dyspnea | Improved MIP, MEP and dyspnea | [5] |
| Johnson et al. (1998) | Exp = 8 Con = 8 |
Threshold at 30% of MIP for 8 weeks (15 min, 2×/day, 7×/week) | MIP, MEP, exercise duration, 100-m walk test and QoL | Improved MIP and MEP, but no significant change in other measures | [6] |
| Darnley et al. (1999)† | 9 | Hans-Rudolph valve attached to tubing with a progressive increase in the length of tubing each week of a 4-week study (four, 5-min BE/60-min session, 3×/week) | Exercise duration, PFTs, HR and diaphragmatic excursion and velocity | Improved exercise duration and diaphragmatic velocity | [7] |
| Weiner et al. (1999) | Exp = 10 Con = 10 |
Threshold at 60% of MIP for 12 weeks (30 min, 6×/week) | MIP, MEP, IME, PFTs, peak VO2, 12-MWT and dyspnea | Improved MIP, MEP, IME, PFTs, 12-MWT and dyspnea | [8] |
| Martinez et al. (2001)‡ | Exp = 11 Con = 9 |
Threshold at 30% of MIP for 6 weeks (15 min, 2×/day, 6×/week) | MIP, IME, peak VO2, 6-MWT and dyspnea | Improved MIP, IME, peak VO2, 6-MWT and dyspnea | [9] |
| Cahalin et al. (2001) | Exp = 6 Con = 6 Cross-over |
Threshold IMT at 40% of MIP and Threshold EMT at 5–15% of MEP for 12 weeks (30–40 min, 5×/week) | MIP, MEP, IME, PFTs, peak VO2, 6-MWT, cycling endurance, dyspnea and QoL | Improved MIP, MEP, IME, PFTs, peak VO2, 6-MWT, dyspnea and QoL | [10] |
| Laoutaris et al. (2004) | Exp = 20 Con = 15 |
TIRE at 60% of MIP/SMIP for 10 weeks (3×/week for an uncertain duration) | MIP, SMIP, peak VO2, VE, HR, 6-MWT, dyspnea and QoL | Improved MIP, SMIP, peak VO2, HR, 6-MWT, dyspnea and QoL | [11] |
| Hulzebos et al. (2006)§ | Exp = 139 Con = 137 |
Threshold at 30% of MIP for 14–90 days (mean = 30) prior to CABG surgery (20 min, 7×/week) | MIP, MEP, IME, and postoperative pulmonary complications | Improved MIP, IME and postoperative pulmonary complications | [12] |
| Dall’Ago et al. (2006) | Exp = 16 Con = 16 |
Threshold at 30% of MIP for 12 weeks (30 min, 7×/week) | MIP, MEP, IME, peak VO2, VE, VE/VCO2 slope, recovery O2, 6-MWT and dyspnea | Improved MIP, MEP, IME, peak VO2, VE, VE/VECO2 slope, recovery O2, 6-MWT and dyspnea |
[13] |
| Laoutaris et al. (2007) | Exp = 15 Con = 23 |
TIRE at 60% of MIP/SMIP for 10 weeks (3×/week for an uncertain duration) | MIP, SMIP, PFTs, peak VO2, VE, 6-MWT, dyspnea, TNF-α, TNF receptor I, IL-6, CRP, Fas and Fas ligand | Improved MIP, SMIP, PFTs, peak VO2, 6-MWT, dyspnea and TNF receptor I change | [14] |
| Laoutaris et al. (2008) | Exp = 14 Con = 9 |
TIRE at 60% of MIP/SMIP for 10 weeks (3×/week for an uncertain duration) | MIP, SMIP, peak VO2, dyspnea, VE, VE/VCO2 slope, HRV, NT-proBNP and endothelial vasodilation |
Improved MIP, SMIP, peak VO2 and dyspnea | [15] |
| Chiappa et al. (2008) | 18 | Threshold at 30% of MIP for 4 weeks (30 min, 7×/week) | MIP, MEP, diaphragm thickness and resting and exercise calf and forearm blood flow | Improved MIP, MEP, diaphragm thickness and resting and exercise calf and forearm blood flow | [16] |
| Padula et al. (2009)¶ | Exp = 15 Con = 17 |
Threshold at 30% of MIP for 12 weeks (10–20 min, 7×/week) | MIP, RR, dyspnea, self efficacy and QoL | Improved MIP, RR and dyspnea | [17] |
| Stein et al. (2009) | Exp = 16 Con = 16 |
Threshold at 30% of MIP for 12 weeks (30 min, 7×/week) | MIP, OUES | Improved MIP and OUES | [18] |
| Winkelmann et al. (2009) | Exp = 12 Con = 12 |
Threshold at 30% of MIP for 12 weeks (30 min, 7×/week) | MIP, MEP, IME, OUES, peak VO2, VE, VE/VCO2 slope, VE oscillation, recovery O2 kinetics, 6MWT and QoL | Improved MIP, MEP, IME, OUES, peak VO2, VE, VE/VCO2 slope, VE oscillation and recovery O2 kinetics | [19] |
| Bosnak-Guclu et al. (2011) | Exp = 16 Con = 14 |
Threshold at 40% of MIP for 6 weeks (30 min, 7×/week) | MIP, MEP, 6MWT, balance, quadriceps isometric strength, dyspnea, fatigue, PFTs, depression and QoL | Improved MIP, MEP, PFTs, 6MWT, balance, quadriceps isometric strength, dyspnea, depression and QoL | [20] |
| Mello et al. (2012) | Exp = 15 Con = 12 |
Threshold at 30% of MIP for 12 weeks (10 min, 3×/day, 7×/week); mostly home-based | MIP, peak VO2, VE/VCO2, VE/VCO2 slope, HRV, MSNA and QoL | Improved MIP, peak VO2, VE/VCO2, VE/VCO2 slope, HRV, MSNA and QoL | [21] |
| Laoutaris et al. (2012) | Exp = 13 Con = 14 |
TIRE at 60% of MIP/SMIP for 12 weeks (20 min, 3×/week) | MIP, SMIP, peak VO2, dyspnea, VE, VE/VCO2 slope, VT, CP, quadriceps isometric strength and endurance, LVEF, LVESD, LVEDD and QoL | Improved dyspnea, MIP, SMIP, peak VO2, VE/VCO2 slope, VT, CP, quadriceps isometric strength and endurance, LVEF, LVESD, LVEDD, QoL | [22] |
All but one of the nine subjects with ischemic heart disease had a LVEF <50%; the one other subject had a LVEF of 52% (mean LVEF: 46.6 ± 4%).
The control group performed IMT in the same manner as the experimental group except that the IMT workload was less (10% of MIP), which may have been responsible for the improvement in dyspnea, MIP, IME and peak VO2 in the control group. Only the experimental group had a significant improvement in the 6MWT distance ambulated.
Approximately, 40% of the subjects had a LVEF <50%; 63% of the IMT group and 76.5% of the usual care group experienced New York Heart Association class III symptoms and approximately 2% of the IMT group and usual care group experienced New York Heart Association class IV symptoms.
The control group consisted of patient education which included information on basic anatomy and physiology of the heart, diet, medication regimen, sleep, rest and activity patterns, and what and when to report to the doctor.
BE: Breathing exercises; BP: Blood pressure; CABG: Coronary artery bypass graft; Con: Control group; CP: Circulatory power (peak VO2 × peak systolic blood pressure); CRP: C-reactive protein; EMT: Expiratory muscle training; Exp: Experimental group; HR: Heart rate; HRV: Heart rate variability; IME: Inspiratory muscle endurance; IMT: Inspiratory muscle training; LVEDD: Left ventricular end-diastolic diameter; LVEF: Left ventricular ejection fraction; LVESD: Left ventricular end-systolic diameter; MEP: Maximal expiratory pressure; MIP: Maximal inspiratory pressure; MSNA: Muscle sympathetic nervous activity; MWT: Minute walk test; NT-proBNP: N-terminal fragment of basic natriuretic peptide; OUES: Oxygen uptake efficiency slope; PFTs: Pulmonary function tests; QoL: Quality of life; RR: Respiratory rate; SMIP: Sustained maximal inspiratory pressure; TIRE: Rest of incremental respiratory endurance; VE/VCO2: Ventilation to carbon dioxide ratio; VE: Ventilation; VO2: Oxygen consumption; VT: Ventilatory threshold.
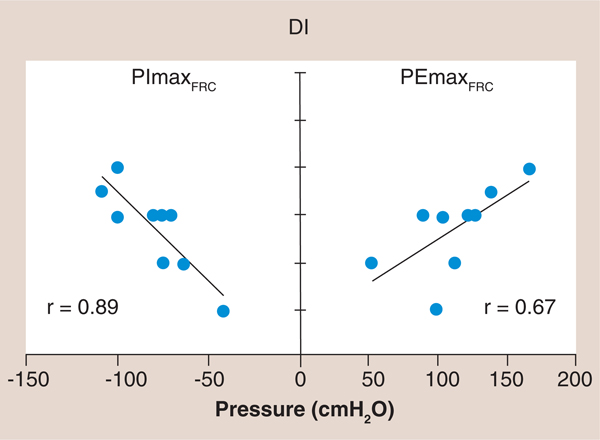
Figure 1. The relationship of maximal inspiratory and expiratory pressure to dyspnea using the Dyspnea Index in which a lower Dyspnea Index value is associated with greater dyspnea.
Note the higher correlation coefficient between PImax and DI (r = 0.89) compared with PEmax and DI (r = 0.67). Maximum inspiratory and expiratory mouth pressures along with the baseline DI were measured in nine stable, chronic heart failure patients who had no evidence of primary lung disease, which were compared with nine age- and sex-matched healthy control subjects. The chronic heart failure patients, when compared with their matched control subjects, had reduced inspiratory and expiratory muscle strength, and both inspiratory and expiratory muscle strength were significantly correlated with dyspnea during daily activity (r2 = 0.80, p = 0.001 and r2 = 0.45, p = 0.05, respectively). Inspiratory muscle strength accounted for all of the variance in dyspnea that was correlated with respiratory muscle strength when the relative contributions of inspiratory and expiratory muscle strength were examined.
DI: Dyspnea Index; FRC: Functional residual capacity; PEmax: Maximal expiratory pressure; PImax: Maximal inspiratory pressure. Reproduced with permission from [23].
Patients with HF have also been observed to have reduced inspiratory muscle endurance (IME) that is also related to their level of dyspnea [24]. Furthermore, inspiratory muscle strength has been found to be significantly correlated to peak oxygen consumption (VO2) and is an independent predictor of survival in patients with HF that is comparable to peak VO2 (FIGURE 2) [25,26].
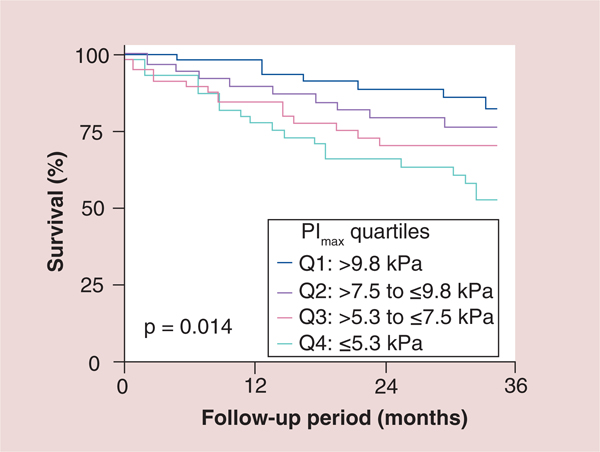
Figure 2. Kaplan–Meier survival curves of the total study population subdivided into quartiles according to maximal inspiratory pressure.
PImax was prospectively determined in 244consecutive patients (207 men) with CHF (ischemic: n = 75; idiopathic dilated cardiomyopathy: n = 169; age: 54 ± 11 years; left ventricular ejection fraction: 22 ± 10%). PImax was lower in the 244 patients with CHF than in 25 control subjects (7.6 ± 3.3 vs 10.5 ± 3.7 kPa; p = 0.001). The 57 patients (23%) who died during follow-up (23 ± 16 months; range: 1–48 months) had an even more reduced PImax (6.3 ± 3.2 vs 8.1 ± 3.2 kPa in survivors; p = 0.001). Kaplan–Meier survival curves differentiated between patients subdivided according to quartiles for PImax (p = 0.014). PImax was a strong risk predictor in both univariate (p = 0.001) and multivariate Cox proportional hazard analyses (p = 0.03).
CHF: Coronary heart failure; PImax: Maximal inspiratory pressure. Reproduced with permission from [26] © American Heart Association, Inc (2012).
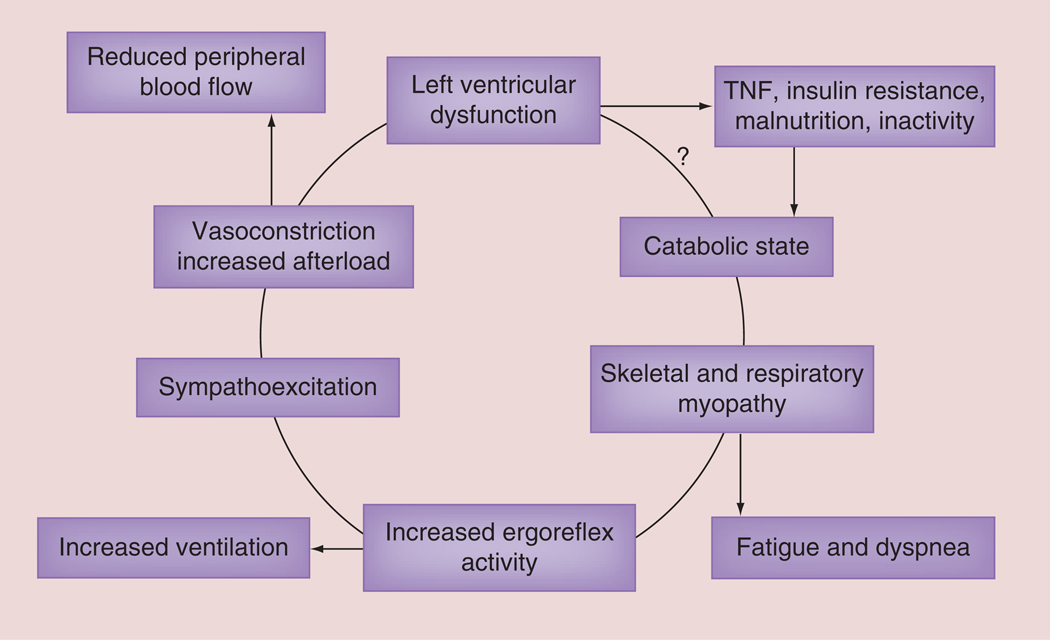
The muscle hypothesis of chronic HF places respiratory and peripheral muscle abnormalities as proximal components in the vicious cycle of this chronic cardiac disease that produces subsequent cardiorespiratory and vascular abnormalities which worsen HF (FIGURE 3) [27]. A secondary hypothesis generated from this conceptual model is that inadequate inspiration leads to limited ventilation, pulmonary and peripheral gas exchange, ATP availability and removal of biochemical waste with sub-sequent detrimental effects on the cardiovascular, pulmonary and neurohumoral systems all of which further impair heart function (FIGURE 3) [27,28].
Figure 3. The muscle hypothesis of chronic heart failure.
This paper presents some of the background evidence which leads to the hypothesis that a feedback loop links changes in skeletal muscle to abnormal reflex cardiopulmonary control, which may both limit exercise and be harmful in the progression of the syndrome.
?: It it is not completely known how left ventricular dysfunction causes a catabolic state. Reproduced with permission from [27].
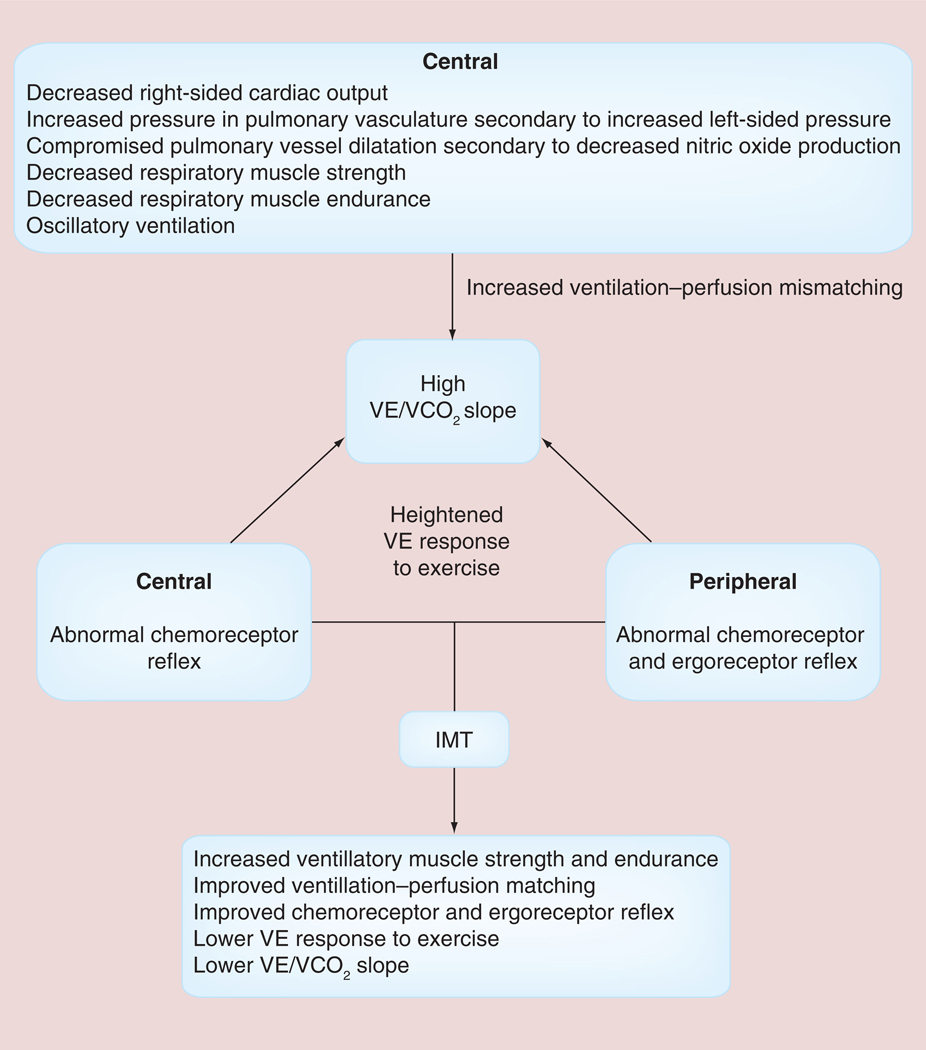
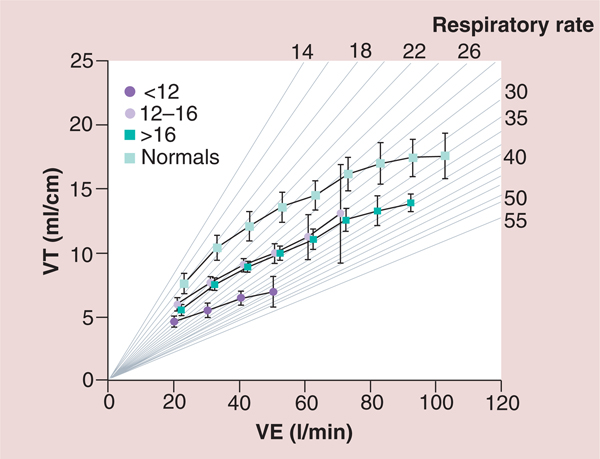
The pathophysiological consequences of HF and the potential role of IMT in patients with HF have been summarized in FIGURE 4 [29]. In addition to the impaired left ventricular performance described in the muscle hypothesis of chronic HF, FIGURE 4 highlights the role of impaired right ventricular performance in HF. Impaired right ventricular performance results in poor pulmonary perfusion, increased pulmonary vascular resistance and decreased nitric oxide production with the potential for the subsequent development of pulmonary hypertension [29]. Other central and peripheral factors are also responsible for the development of pulmonary hypertension and the abnormal ventilatory response found in patients with HF [29]. Impaired inspiratory muscle strength and endurance appears to be partly responsible for the altered ventilatory response to exercise including: decreases in tidal volume; end-tidal carbon dioxide; peak VO2; and tidal volume to ventilation ratio (VT/VE) and an increase in respiratory rate, minute ventilation, peak dead space ventilation to tidal volume ratio (VD/VT), ventilation to VO2 ratio (VE/VO2) and ventilation to carbon dioxide (VE/VCO2) slope [4–30]. Examples of the above abnormal ventilatory response to exercise in patients with HF are shown in FIGURES 5 –7 [30].
Figure 4. Pathophysiologic mechanisms of an elevated ratio of ventilation to carbon dioxide slope and methods by which inspiratory muscle training improves the elevated ratio of ventilation to carbon dioxide slope.
VE abnormalities found during exercise in patients with heart failure.
IMT: Inspiratory muscle training; VE: Ventilation; VE/VCO2: Ventilation to carbon dioxide ratio.
Adapted with permission from [29].
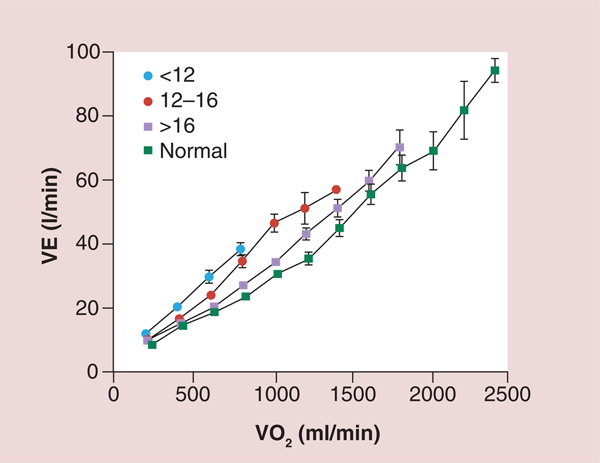
Figure 5. Ventilation as a function of oxygen consumption for normal subjects and three groups of heart failure patients based on peak oxygen consumption.
A total of 130 patients with chronic HF and 52 healthy subjects participated in the study. Spirometric and breath-by-breath gas exchange measurements were made during rest and increasing cycle exercise. Arterial blood was sampled for measurement of pH, PaCO2, PaO2 and lactate during exercise in 85 patients. Resting forced expiratory volume in 1 s and vital capacity were proportionately reduced at all levels of impairment. Patients with more severe HF had greater tachypnea and a smaller tidal volume at a given exercise expired volume per unit time. This was associated with an expiratory flow pattern characteristic of lung restriction. VE and carbon dioxide production as a function of VO2 were increased during exercise in HF patients. The increases were greater the lower the peak VO2/kg of body weight. The ratio of dead space ventilation (physiological dead space) to tidal volume and the difference between arterial and end-tidal PCO2 at peak VO2 also increased inversely with peak VO2/kg. By contrast,the difference between alveolar and arterial PO2 and PaCO2 were both normal, on average, at peak VO2 regardless of the level of impairment. The more severe the exercise limitation, the higher the lactate and the lower the HCO3− at a given VO2, although pH was tightly regulated.
HF: heart failure; PaCO2: Partial pressure of carbon dioxide in arterial blood; PaO2: Partial pressure of oxygen in the arterial blood; PCO2: Carbon dioxide partial pressure; VE: Ventilation; VO2: Oxygen consumption.
Reproduced with permission from [30] © American Heart Association, Inc. (2012).
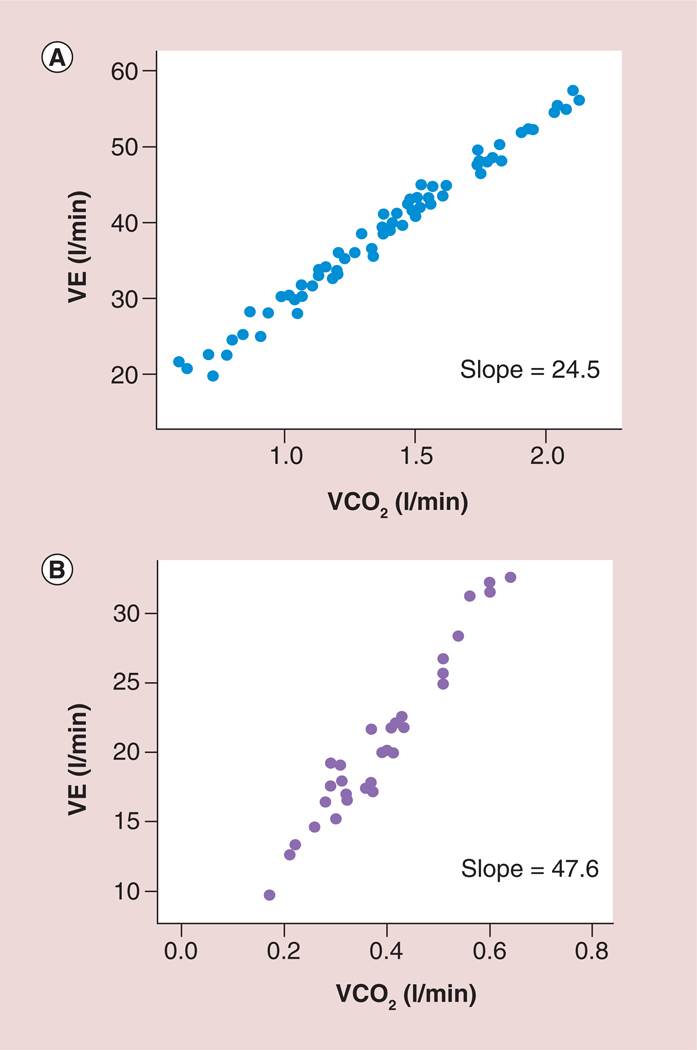
Figure 7. A normal and abnormal ventilation to carbon dioxide (VE/VCO2) slope.
(A) A normal ratio of ventilation to carbon dioxide slope in a patient with heart failure and (B) an abnormal ratio of ventilation to carbon dioxide slope in a patient with heart failure.
VE: Ventilation; VCO2: Carbon dioxide production.
Adapted with permission from [29].
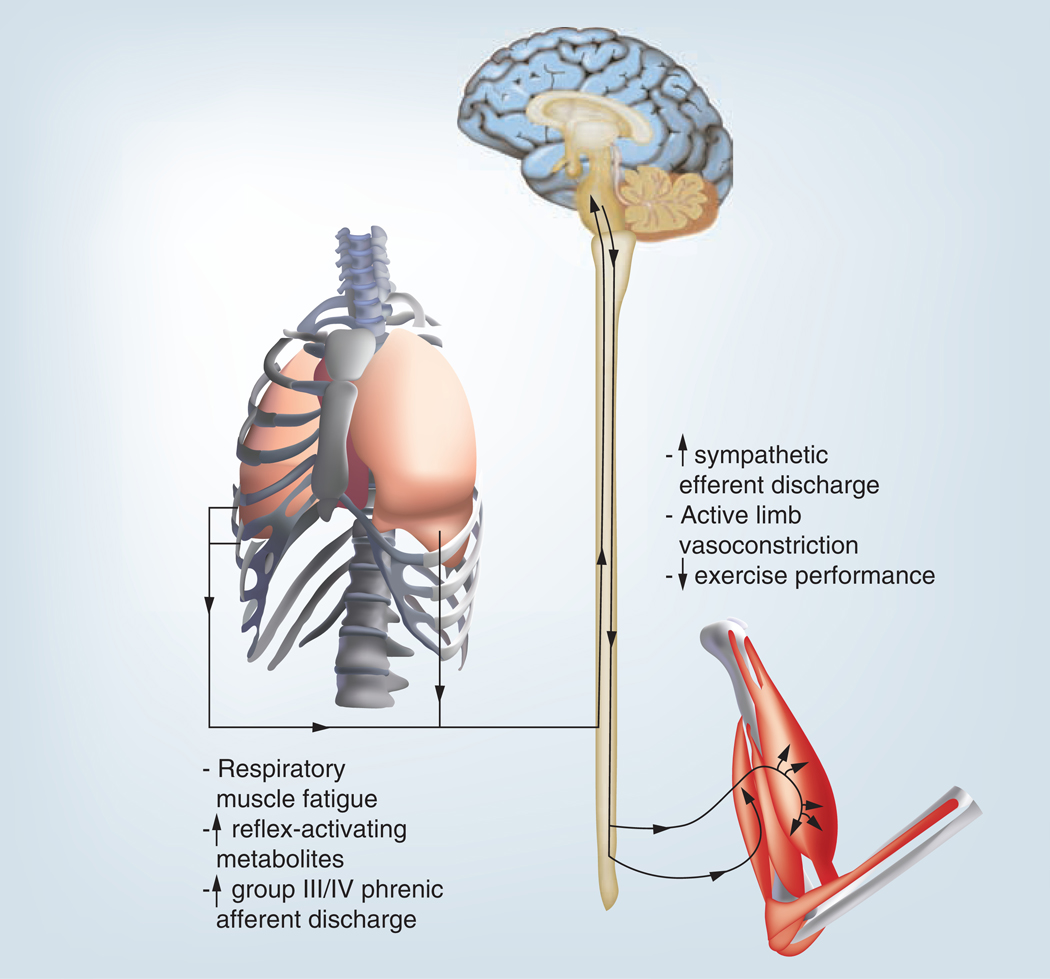
The abnormal ventilatory responses mentioned above and the heightened ergoreflex presented in FIGURES 3 –7 are associated with the respiratory muscle metaboreflex, which is significantly more sensitive in patients with HF [31]. The respiratory muscle metaboreflex is initiated when respiratory muscles fatigue which elicits the changes shown in FIGURE 8 including increased sympathetic nervous system activation, vasoconstriction in resting and exercising limbs and decreased exercise tolerance [31–33]. Thus, oxygenated blood is preferentially shunted to fatiguing respiratory muscles with less available oxygenated blood for exercising limb muscles, which further diminishes the functional capacity of the patient with HF [31–33]. IMT has the potential to improve the respiratory muscle metaboreflex as well as many of pathophysiologic responses described above [34]. Studies examining the effects of IMT in persons with HF have found significant improvements in many of the above areas and will be discussed below.
Figure 8. The major components and effects of the respiratory muscle metaboreflex.
Discusses the important findings by [62], who demonstrated that the respiratory muscle-limb reflex has the ability, at least under resting conditions, to reduce significantly limb blood flow and vascular conductance. Furthermore, the results of previous observations [63] provide compelling evidence for the existence of a metaboreflex, with its origin in the respiratory muscles, which can modulate limb perfusion via stimulation of sympathetic nervous system vasoconstrictor neurones.Reproduced with permission from [32].
Studies examining the effects of IMT in HF
Nineteen studies to date have examined the effects of IMT in persons with heart disease and HF (TABLE 1) [4–22]. Two of the studies included patients with heart disease who partially or fully met HF diagnosis criteria (e.g., left ventricular ejection fraction <50%, New York Heart Association [NYHA] class III–IV symptoms) [7,12]. Fifteen of the studies were randomized controlled trials [6,8–15,17–22] and the remaining four [4,5,7,16] were quasi-experimental studies. The sample size for the majority of studies was small except for one (n = 276) that administered IMT to patients with ischemic heart disease prior to coronary artery bypass graft surgery [12]. The mode of IMT in the majority of studies (n = 14) was the Threshold inspiratory muscle trainer at an intensity of approximately 30% of maximal inspiratory pressure (MIP) [4–6,8–10,12,13,16–21]. The duration and progression of IMT varied in each study that utilized the Threshold IMT device [4 – 6, 8–10,12 ,13,16 –21]. One study administered a variety of breathing exercises (isocapnic hyperpnea, inspiratory strength training and breathing calisthenics) in addition to Threshold IMT [4] and one study administered combined Threshold IMT and Threshold expiratory muscle training (EMT) [10]. Four studies performed by the same investigators administered IMT using the TIRE via the TRAINAIR® (Project Electronics Limited, Kent, UK) device at 60% of MIP/sustained MIP (SMIP) with an increasing work to rest ratio for 20–30 min, three times per week [11,14,15,22]. TRAINAIR IMT was performed to the point of exhaustion [11,14,15,22].
Effects of IMT on respiratory muscle performance & pulmonary function
All studies except one [7] measured maximal inspiratory muscle strength, which was consistently observed to increase significantly [4–6,8–22]. Ten of the studies measured maximal expiratory muscle strength, which increased significantly in nine of the studies [4–6,8,10,12,13,16,19,20], while the one remaining study did not report the change in maximal expiratory muscle strength [12]. IME was specifically measured in seven studies and was observed to significantly increase in each study [4,8–10,12,13,19]. A surrogate measure of IME (SMIP) was examined in four studies by the same group and was found to increase significantly in each study [11,14,15,22]. Pulmonary function tests were examined in five studies [7,8,10,14,20] with all but one study observing significantly greater pulmonary function [7].
Effects of IMT on clinical outcomes associated with HF
All but one of the 19 studies found IMT to improve other domains/manifestations of HF [6]. The one study not observing such an improvement had a small number of subjects in each group (n = 8) and administered IMT for only 15 min, 2×/day for 8 weeks at 30% of the MIP [6]. Four other studies with favorable results administered IMT at a similar duration and intensity, but had a greater number of subjects [9,12], administered IMT for a greater number of weeks [17] or administered IMT to patients with more severe HF [5]. All of the above factors appear to be important when administering IMT to improve other domains/manifestations of HF and will be discussed in subsequent sections.
The domains/manifestations of HF which were significantly improved by IMT in one or more of the 18 out of 19 studies of IMT included: dyspnea; quality of life (QoL); balance; peripheral muscle strength and blood flow; peripheral muscle sympathetic nervous activity; heart rate; respiratory rate; peak VO2; 6-min walk test (MWT) distance; ventilation; VE/VCO2 slope; oxygen uptake efficiency; circulatory power; recovery oxygen kinetics; and several indices of cardiac performance [4,5,7–22]. Dyspnea was significantly improved in all 12 studies in which it was measured [4,5,8–11,13–15,17,20,22]. QoL was measured in seven studies [6,10,11,17,19–22] and improved in all but two of the studies [17,19]. Resting and exercise heart rate were measured in four studies [7,11,14,15] and were observed to improve in one of the studies [11]. Heart rate variability was measured in two studies [15,21] and was significantly improved in one of the studies [21]. The respiratory rate was measured in two studies [4,17] and was observed to improve significantly in one study [17]. Peak VO2 was measured in 11 studies [4,8–11,13–15,19,21,22] and was observed to increase significantly in all but one of the studies [8]. The 6-MWT was administered in eight studies [4,9–11,13,14,19,20] and was observed to increase significantly in all but one of the studies [19]. The 12-MWT was administered in one study and was observed to increase significantly in this study [8]. Ventilation and ventilatory efficiency indices (e.g., VE/VCO2 and VE/VCO2 slope) were measured in eight studies [4,11,13–15,19,21,22] and were observed to improve significantly in five of the studies [4,13,19,21,22]. The three other studies, in which ventilation and ventilatory efficiency indices did not change, enrolled patients with mostly NYHA class II HF symptoms and were performed by the same group of researchers using a different form of IMT compared with all other studies [11,14,15]. The oxygen uptake efficiency slope and recovery oxygen kinetics were measured in two studies performed by the same investigators and were observed to improve significantly in both studies [18,19]. Two studies by the same investigators examined the effects of IMT on inflammation and the immune response as well as autonomic nervous system activity and found no significant change in TNF, C-reactive protein, heart rate variability, endothelial vasodilation or B-type natriuretic peptide after 10 weeks of IMT performed at 60% of MIP/SMIP for approximately 30 min, 3×/week [14,15].
A recent finding associated with the effects of IMT on the domains/manifestations of HF is a favorable peripheral muscle adaptation represented by significant improvements in quadriceps strength [20,22], resting and exercise calf and forearm blood flow [16] and peroneal nerve sympathetic nervous activity [21]. Such improvements may have been partially responsible for the significant improvement in balance reported by Bosnak-Guclu et al. [20]. Furthermore, it is probably that the increase in diaphragm thickness found after a relatively short period of IMT (4 weeks) in HF patients with inspiratory muscle weakness [16] combined with improved inspiratory muscle strength and endurance would contribute to improved balance, since the diaphragm and other muscles of respiration are responsible for maintaining both respiration and balance [16,20–22]. Thus, peripheral and central adaptations from IMT may improve the mechanisms responsible for respiration, ventilation and balance, and subsequently improve the synergy between each respective process.
Effects of IMT combined with other forms of exercise
The effect of IMT combined with other forms of exercise was examined in three studies [10,19,22]. Winklemann et al. demonstrated that 30 min of daily Threshold IMT performed at 30% of MIP combined with aerobic exercise (cycling for 20–45 min, 3×/week, at the first ventilatory threshold) resulted in additional significant improvements in MIP, peak VO2, circulatory power, efficiency of ventilation and oxygen uptake and recovery oxygen kinetics [19]. Similar results were observed by Laoutaris et al [22]. who found that 20 min of TIRE TRAINAIR IMT performed at 60% of MIP/SMIP combined with 15 min of resistance training (three exercise sets of 10–12 dynamic quadriceps repetitions at 50% of 1 RM as well as two exercise sets of 10–12 upper extremity repetitions using 1–2 kg dumbbells) and 20–30 min of aerobic exercise (cycling, 3×/week, at 70–80% of peak heart rate) resulted in additional significant improvements in the SMIP, quadriceps strength and endurance, exercise time, circulatory power, dyspnea and QoL [22].
Cahalin et al. combined IMT with EMT and compared combined IMT and EMT to cycle ergometry training (CET) and found results indicative of the specificity of training principle in that combined IMT and EMT improved IME and pulmonary function while CET improved cycling duration [10]. Similar improvements in respiratory muscle strength, 6-MWT distance, estimated peak VO2, symptoms and QoL were observed in the combined IMT and EMT group and CET group [10]. Administration of IMT combined with other modes of exercise appears promising for HF patients.
Effects of IMT on peripheral blood flow
The effects of IMT on peripheral blood flow are very important and appear to be directly related to inspiratory muscle weakness and the respiratory muscle metaboreflex [3,13,16,18,19,21,27–34]. Chiappa et al. were the first to document that HF patients with respiratory muscle weakness elicit a premature and more dramatic reduction in peripheral blood flow during fatiguing inspiratory muscle work compared with healthy control subjects [16], which are supported by the results of a previous study of a canine HF model [34] as well as the observation of respiratory muscle deoxygenation in HF patients during exhaustive exercise [35,36]. It appears that impaired delivery of oxygen to the diaphragm combined with increased inspiratory muscle work from HF produces an accumulation of inspiratory muscle metabolites that stimulates type IV nerve endings, resulting in an exaggerated sympathetic-mediated vasoconstriction and reduced peripheral blood flow [3,16,27–34]. Thus, IMT may have the potential to mediate the accumulation of inspiratory muscle metabolites with subsequent improvement in peripheral blood flow because of less sympathetic-mediated vasoconstriction [3,16,27–34].
Several important clinical factors regarding IMT and the potential for improved peripheral blood flow exist and include: the presence of inspiratory muscle weakness; strong relationship between hypertrophy of the diaphragm and MIP; and unloading the respiratory muscles by improving respiratory muscle strength and endurance or using other methods [3,13,16,18,19,21,27–34,37]. Chiappa et al. found a significant correlation (r = 0.88; p < 0.001) between the change in MIP and the change in diaphragm thickness after 4 weeks of IMT, identifying the morphologic and physiologic changes associated with a short period of IMT in HF patients with inspiratory muscle weakness [16]. Furthermore, Borghi-Silva et al. demonstrated that unloading ventilation (via proportional assisted ventilation) improved submaximal exercise capacity and skeletal muscle perfusion in patients with advanced HF (NYHA class III) [37]. Positive pressure ventilation decreased the work of the respiratory muscles and ‘unloaded them’ which improved peripheral skeletal muscle blood flow and submaximal exercise. Similarly, IMT appears to have an unloading effect on the respiratory muscles due to increased respiratory muscle size, strength and ability to work with less accumulation of respiratory muscle metabolite and less peripheral vasoconstriction, resulting in greater peripheral blood flow. However, this and other mechanistic effects of IMT on the pathophysiologic manifestations of HF require further investigation.
Important findings from the literature review
Several other important findings were observed from the above literature review including the method of IMT, IMT prescription, inclusion criteria for subjects performing IMT and the outcome measures from IMT in persons with HF [4–22]. Each of these areas will be briefly expanded on below.
Method of IMT
The method of IMT, which was most widely used and most effective in the studies of IMT in HF, was the Threshold IMT device [4–6,8–10,12,13,16–21]. Although the Threshold IMT device has a limited workload (−41 cm H2O), it did not appear to limit IMT or the effectiveness of IMT in persons with HF. The TIRE administered via the TRAINAIR IMT device has a number of psychologically and physiologically appealing characteristics, such as biofeedback, an increasing work to rest ratio and ability to easily alter IMT parameters [11,14,15,22]. However, the effects of TIRE IMT via the TRAINAIR produced outcomes that were slightly less favorable than those from IMT via the Threshold inspiratory muscle trainer (which may be due to healthier patients and will be discussed later) [11,14,15,22]. Thus, the consistently favorable outcomes from IMT using the Threshold IMT device as well as ease of administration in any setting (home or laboratory) suggests that IMT administered to persons with HF can be easily done with the Threshold IMT device. Furthermore, a relatively simple method of biofeedback can be applied to the Threshold IMT device (attaching one end of standard oxygen tubing to the oxygen port of the threshold and attaching the other end to a sphygmomanometer) that may facilitate greater IMT efforts and results. However, TRAINAIR IMT provides not only important biofeedback, but has the capacity for greater IMT workloads throughout the full range of inspiration using a decreasing rest period between breaths. Furthermore, detailed and accurate data are acquired through the computer software which is likely to enhance IMT efforts and may be useful for prognostic purposes.
IMT prescription
The above literature review also provided insight into the most effective IMT prescription which appears to be at 30% of the MIP for 30 min, 5–7 days per week for 4–12 weeks [4–22]. It is important to note that the greatest gain in maximal inspiratory muscle strength appears to be achieved after approximately 4 weeks of IMT with modest subsequent gains as IMT is continued [5,13,19]. Similarly, the greatest reduction in dyspnea in these studies was achieved in approximately 4 weeks, after which minimal to modest improvements occurred as IMT was continued [5,13,19]. The most likely reason for these findings includes motor learning and re-education of optimal breathing strategies as well as desensitization of dyspnea [5,13,19]. However, it should be noted that the patients who performed IMT in these three studies had advanced HF with one study performing IMT in patients awaiting cardiac transplantation and the two other studies enrolling patients with HF who had inspiratory muscle weakness (a MIP that was less than 70% of the predicted value) [5,13,19]. In view of these findings, patients with advanced HF may benefit substantially from a short program of IMT. However, such early improvements from IMT have not been found by Laoutaris et al. in studies using TIRE TRAINAIRE IMT [14,15,22].
Potential indications & contraindications for IMT in patients with HF
BOX 1 shows potential indications and contraindications for IMT in patients with HF. Although no specific relative or absolute indications or contraindications for IMT have been published, those listed in BOX 1 have been summarized from the available literature in patients with HF and related cardiorespiratory disorders [4–39]. Patients with HF who are dyspneic and have inspiratory muscle weakness and pulmonary hypertension may be ideal candidates for IMT. Two key contraindications to IMT appear to be markedly elevated left ventricular end-diastolic volume and left ventricular end-diastolic pressure and worsening signs/symptoms of HF after IMT has been initiated. These contraindications to IMT have been listed based on a previous investigation, which observed that several patients with advanced HF and markedly elevated left ventricular end-diastolic volume and left ventricular end-diastolic pressure, who were hospitalized awaiting cardiac transplantation, were unable to continue IMT owing to worsening HF signs and symptoms [5]. Other potential contraindications to IMT in patients with HF have been listed in BOX 1 as well as potential methods to facilitate IMT if potential contraindications are observed.
Box 1. Potential indications and contraindications for inspiratory muscle training in patients with heart failure.
| • Potential indications |
| – Dyspnea at rest or with exertion |
| – Inspiratory muscle weakness (<70% of the predicted value) |
| – Pulmonary hypertension |
| • Potential contraindications |
| – Markedly elevated left ventricular end-diastolic volume and pressure |
| – Worsening heart failure signs and symptoms after IMT is initiated† |
| – Desaturation with IMT‡ |
| – Paradoxical breathing pattern† |
| – Worsening inspiratory muscle performance†,§ |
| – Persistent diaphragmatic/abdominal/thoracic/inspiratory accessory muscle discomfort† |
Modify IMT prescription if observed.
Consider providing supplemental oxygen if oxygen saturation is observed to decrease from resting levels.
Worsening inspiratory muscle performance defined as an inability to repeatedly achieve MIP, sustained MIP, inspiratory endurance or inspiratory duration achieved during previous IMT sessions.
IMT: Inspiratory muscle training; MIP: Maximal inspiratory pressure.
As alluded to in several of the above sections and as shown in the literature, the severity of HF may dictate the observed outcomes in an intervention trial. For example, the IMT studies producing the most significant results were observed in patients with inspiratory muscle weakness defined as a MIP less than 70% of the predicted value [13,16,18,19,21]. Thus, HF patients with inspiratory muscle weakness are most likely to benefit from IMT. In addition, identifying a similar threshold level, but for IME, is likely to identify responders to IMT. Such a threshold level of IME does not appear to exist and requires further investigation.
It is important to note that although the results of the IMT studies examining autonomic nervous system activity, the immune system and inflammation as well as B-type natriuretic peptide may be disappointing, considering the high intensity of IMT (60% of MIP and SMIP) and increasing work to rest ratio of TIRE IMT, the results are actually somewhat encouraging [14,15]. It is possible that the higher workload and progressively shorter rest periods between breaths of the TIRE protocol may have worsened autonomic, inflammatory and immune system function, but this was not observed [14,15]. The most likely reason for the lack of change in these variables in the TIRE IMT TRAINAIR studies was due to enrollment of patients with less impairment [14,15]. This hypothesis is supported by a recent study of IMT in HF patients with inspiratory muscle weakness in whom autonomic nervous system activity was observed to significantly improve [21].
Key outcome measures to examine the effects of IMT
The outcome measures in the studies of IMT in persons with HF are numerous as shown in TABLE 1. From the above literature review, it appears that several important outcome measures should be included in future studies of IMT and in the care of HF patients including MIP, maximal expiratory pressure, IME, 6-MWT, peak VO2, QoL and dyspnea [4–22]. Additional outcome measures that appear to be important to include in the management of HF patients and in future studies of IMT in patients with HF include minute ventilation (VE), the VE/VCO2 slope, exercise oscillatory ventilation, the oxygen uptake efficiency slope, recovery oxygen kinetics, autonomic nervous system activity as well as inflammatory and immune system function and endothelial vasodilation. Exhaled nitric oxide may also be an important variable to measure in future studies of IMT in patients with HF, since it has been shown to be depressed in these patients and is significantly correlated to peak VO2 [38].
From the available literature, it appears that many of the above outcomes can be achieved with a short-term IMT program (4–8 weeks) [5–7,9,12,18,20], but that a longer-term IMT program is likely to elicit greater training adaptations to achieve a greater number of the above outcomes [4,8,10,11,13–15,17–19,21,22]. Investigation of the dose of IMT needed to maintain inspiratory muscle performance and clinical outcomes achieved from IMT is needed, since no such study has been performed in patients with HF. Furthermore, the effects of short-term (2×/week for 4–8 weeks), high intensity IMT (60–80% of MIP) in appropriate HF patients is needed, since such training would require fewer IMT sessions and enable IMT to be more easily included into a comprehensive rehabilitation program. The results of such studies are likely to provide insight into the maintenance of inspiratory muscle performance and clinical outcomes from IMT in patients with HF.
An additional concern when examining the effects of IMT on inspiratory muscle performance and clinical outcomes is the potential for concomitant medical therapy to improve inspiratory muscle strength as well as clinical outcomes. Because of this, the authors have listed, in BOX 2, various pharmacologic and medical therapies that have the potential to increase or decrease respiratory muscle performance. These are important considerations when examining the effects of IMT in patients with HF.
Box 2. Effects of pharmacologic and medical therapy on inspiratory muscle performance†.
| • Potential to decrease inspiratory muscle performance |
| – Respiratory depressants (e.g., benzodiazepines, barbiturates, sedatives) |
| – Nitrous oxide |
| – Corticosteriods |
| – Possibly statins |
| – Possibly LVAD |
| • Unlikely to effect inspiratory muscle performance |
| – β-blockers |
| • Potential to increase inspiratory muscle performance |
| – ACE inhibitors |
| – Theophylline |
| – Noninvasive positive pressure ventilation (e.g., CPAP/BiPAP)‡ |
| – Possibly β2-agonists |
| – Possibly digoxin |
Measures of inspiratory muscle performance include inspiratory muscle strength, inspiratory muscle endurance or inspiratory duration.
Short-term CPAP/BiPAP administration has the capacity to rest fatigued inspiratory muscles, but prolonged CPAP/BiPAP administration has the potential to atrophy inspiratory muscles.
ACE: Angiotensin-converting enzyme; BiPAP: Bi-level positive airway pressure; CPAP: Continuous positive airway pressure; LVAD: Left ventricular assist device.
TIRE versus Threshold: implications for IMT as well as clinical & research outcomes
Test of incremental respiratory endurance inspiratory muscle training
The TIRE IMT method employed in four of the studies discussed in the above literature review is worthy of further discussion because of the observed results and available outcome measures [11,14,15,22]. The TIRE method of training can be implemented via the TRAINAIR device (used in the above studies) [11,14,15,22] or via the RT2 device (DeVilbiss Healthcare, Wollaston, UK) [39–49]. Both the TRAINAIR as well as RT2 devices connect to a computer with specific software applications that provide the user biofeedback during IMT. The TRAINAIR software program provides the user with a game-like method of biofeedback during which the user attempts to maintain an on-computer-screen inspiratory effort with visual icons (i.e., clouds moving across the screen) and limited outcome measures can be obtained as shown in BOX 3 [11,14,15,22]. The RT2 device provides the user with a graphic representation of their inspiratory effort throughout all of inspiration and real-time biofeedback during which the user attempts to maintain an on-computer-screen inspiratory effort above a baseline template (FIGURE 9) and substantial research study outcome measures as shown in BOX 1 [39–49].
Box 3. Test of incremental respiratory endurance RT2 outcome measures.
| • MIP (cmH2O) |
| • SMIP (PTU) |
| • Accumulated SMIP (PTU) |
| • Peak inspiratory power (Watts) |
| • Inspiratory work (J/breath) |
| • SMIP power profile (Watts) |
| • SMIP power profile y-intercept, R value, R-squared and SEE |
| • SMIP IVC profile (liters) |
| • SMIP IVC profile y-intercept, R-value, R-squared and SEE |
| • Power at 25% of inspiratory work (Watts) |
| • Power at 50% of inspiratory work (Watts) |
| • Power at 75% of inspiratory work (Watts) |
| • Endurance ratio† |
| • IMTOC |
| • IVC (liters) |
| • RPE (6–20) |
Endurance ratio: power at 75% of inspiratory work/power at 25% of inspiratory work.
IMTOC: Inspiratory muscle time of contraction; IVC: Inspiratory vital capacity; MIP: Maximal inspiratory pressure; PTU: Pressure time units; RPE: Rating of perceived exertion; SEE: Standard error of the estimate; SMIP: Sustained maximal inspiratory pressure.
Figure 9. Test of incremental respiratory endurance RT2 inspiratory muscle training with computer screen images provided during training sessions.
MIP: Maximal inspiratory pressure; SMIP: Sustained MIP.
Regardless of IMT device (TRAINAIR or RT2), TIRE IMT is characterized by the serial presentation of submaximal isokinetic-like profiles based upon the MIP/SMIP [39–49]. These efforts are presented at an on-screen target of any desired percentage of MIP/SMIP using a progressive work to rest ratio, with rest periods decreasing from 60 s at level A to 45, 30, 15, 10 and 5 s at levels B through F, respectively. Each level has six resisted inspiratory efforts and the rest periods can be modified (increased or decreased) based on desired outcomes with particular patient populations. Subjects continue to inspire and match the on-screen target that is typically set at 60–80% of MIP/SMIP while wearing a noseclip (FIGURE 9). Thus, the examination session continues until task failure indicated by an inability to match the on-screen target, or until a maximum of 36 resisted inspirations have been performed [39–49]. It is important to note that each subsequent IMT session with the TIRE is also a testing session providing the same variety of outcome measures when the TIRE is incorporated with the RT2 device. Measurement of inspiratory performance via the TIRE has been shown to be valid and reliable as an examination and training device in a variety of patient populations [39–49].
It is also important to differentiate the type of IMT that is per- formed with the TIRE (both the TRAINAIR and RT2 devices) as well as the P-flex® (Respironics, Inc., PA USA) and DHD devices (Diemolding Healthcare Division, NY, USA) compared with the Threshold inspiratory muscle trainer. The form of IMT provided by the TIRE is referred to as inspiratory flow resistive loading (IFRL), which provides resistance to the inspiratory muscles by manipulating airflow [39–49]. During TIRE IMT subjects inhale through a 2-mm leak within a mouthpiece that limits airflow and measures pressure. Resistance with IFRL is dependent on the velocity/or flow of the inspiration. A faster airflow correlates with greater inspiratory muscle power which has been corrected for and adjusted with specific software within the TRAINAIR and RT2 devices that pro- vides a very accurate workload to the inspiratory muscles [39–49]. In fact, as a result of the above correction and adjustment, the workload imposed on the inspiratory muscles is constant and equivalent to the inspiratory force throughout all of inspiration. Thus, IMT via the TRAINAIR, RT2 and other IFRL devices provides a workload that is isokinetic-like yielding consistent work throughout all of inspiration [39–49]. The diagnostic and rehabilitation attributes of isokinetic work in the peripheral skeletal muscles have been observed to be superior to other forms of testing or training [45–47].
Threshold IMT
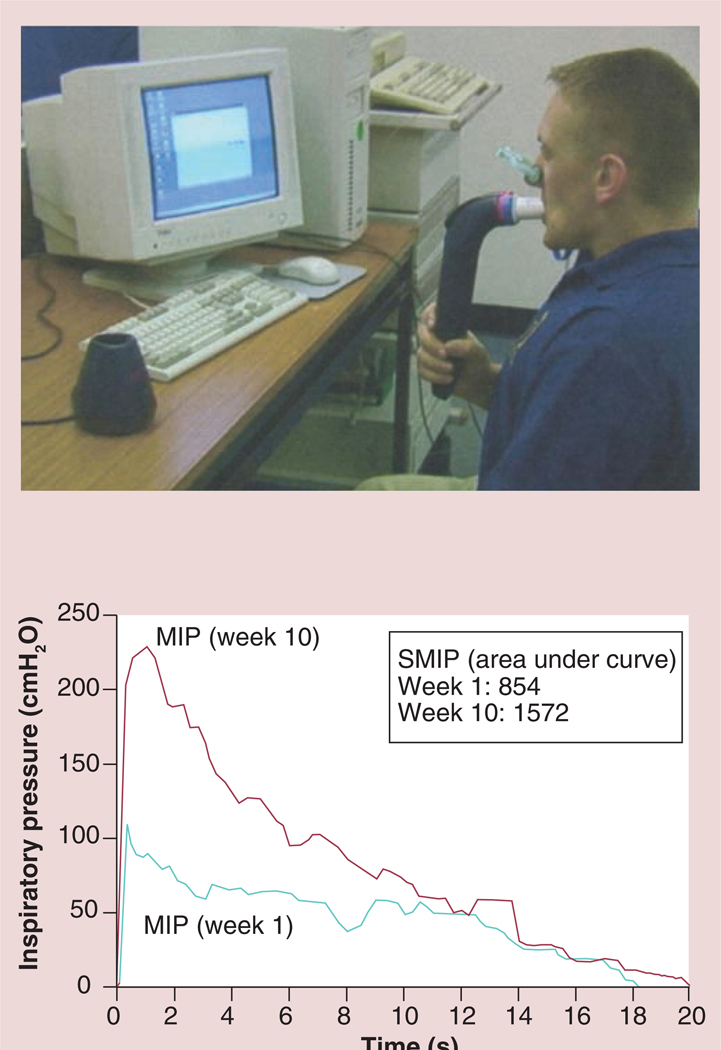
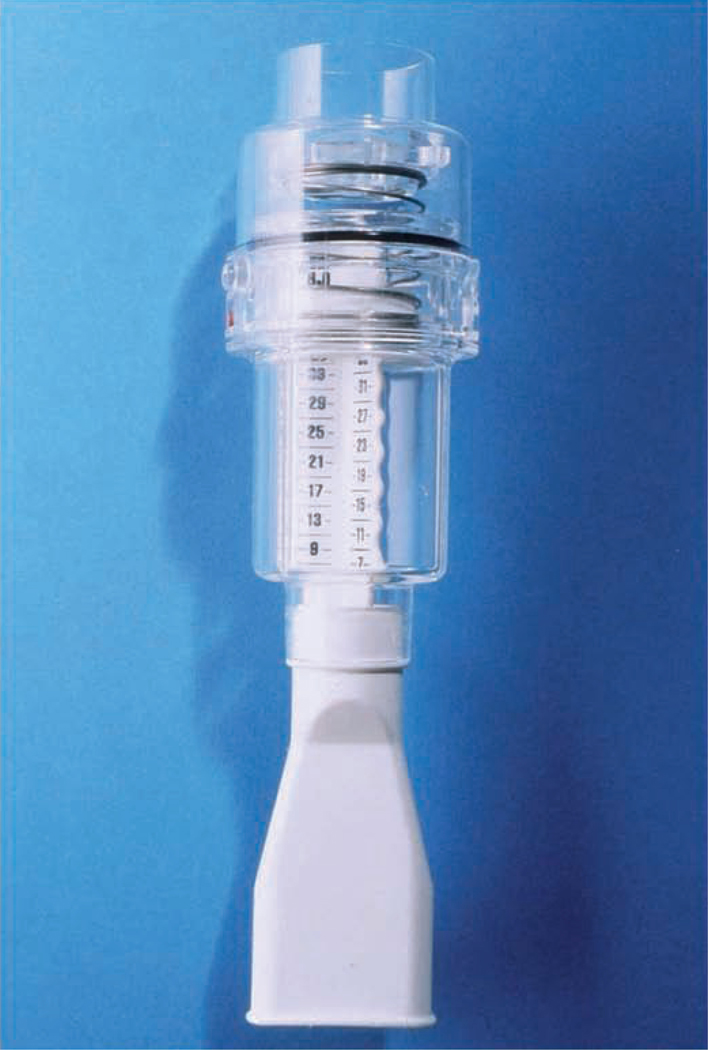
The form of IMT with the Threshold inspiratory muscle trainer is referred to as inspiratory pressure threshold loading (IPTL) which prohibits the subject from inhaling until a set negative pressure is achieved and overcome [4–6,8–10,12,13,16–21]. This is accomplished through the use of weighted plungers or spring-loaded valves. The Threshold inspiratory muscle trainer uses calibrated spring-loaded valves which appear to maintain their accuracy after repeated inspiratory efforts [4–6,8–10,12,13,16–21]. The Threshold inspiratory muscle trainer is a small hand-held device that consists of a mouthpiece and central body in which the spring is housed (FIGURE 10). The spring-loaded valve at the distal end of the Threshold device essentially acts as a door that does not open to allow air to flow through the mouthpiece until the user generates a pressure that is greater than that at which the tension of the spring is set (−9 to −41 cmH2O). The goal of IPTL IMT is that the threshold load can be set by adjusting the tension on the spring creating the opportunity to load the inspiratory muscles [4–6,8–10,12,13,16–21]. Inspiration cannot be achieved unless the muscles maintain a negative pressure greater than the threshold level. The disadvantage of IPTL is that variability in loading exists dependent on the speed of airflow which has not been corrected for with computer software or other methods. Nonetheless, IPTL via the Threshold inspiratory muscle trainer provides a workload on the inspiratory muscles that is sufficient to elicit favorable changes on the inspiratory muscles as shown in the above literature review [4–6,8–10,12,13,16–21]. One minor limitation with Threshold IMT is that fewer IMT outcomes are available compared with TIRE IMT.
Figure 10.
The Threshold inspiratory muscle trainer.
Comparing TIRE to Threshold
The primary advantage of TIRE IMT lies in its accurate workload reporting as well as amount and type (fixed) of workload provided. The TIRE method shows the exact resistance being applied and provides visual feedback to the user via a computer monitor and specialized software [39–49]. This enables more control and confidence with regards to workload prescription and adherence. With Threshold IMT, the exact amount of resistance being applied is not always known, but the Threshold device does enable the user to set the minimal threshold that must be achieved to inhale. Prior to reaching that threshold, however, the inspiratory muscles are generating an unknown force. After achieving the threshold and opening the valve, loading varies as a result of flow resistance and in many cases, drops off precipitously without a workload during the last and possibly most important portion of inspiration (FIGURE 11). Thus, with Threshold IMT, inhalation may stop abruptly when the inspiratory pressure drops below the set threshold as shown in FIGURE 11. By contrast, with TIRE IMT, the workload is applied evenly and consistently throughout the entire duration of a sustained inhalation. An example of the abrupt termination of IMT using the Threshold device is contrasted with the greater amount of inspiratory work achieved with the TIRE device on both the x and y-axis in FIGURE 11. As a result, TIRE IMT appears to generate a greater workload on the inspiratory muscles compared with Threshold IMT by requiring subjects to achieve a greater initial workload and to sustain a maximal effort throughout all of inspiration. Regardless of these differences, the mobility and ease of use of the Threshold inspiratory muscle trainer make it a much more practical and accessible tool than the TIRE method of IMT. However, it is likely that a combination of TIRE and Threshold IMT methods would elicit optimal inspiratory muscle performance with subsequent beneficial effects on other physiologic and functional measures. Perhaps, HF patients could undergo an initial 8-week supervised IMT program using the TIRE protocol after which they could continue IMT at home with the Threshold trainer. At least, TIRE testing should be employed at baseline and at strategic points during IMT (every 2–4 weeks) as well as during postintervention measurements. The reasons for these suggestions will be presented below, but in view of the apparent differences in IMT provided by the TIRE versus the Threshold device, a study examining the effects of IMT comparing both forms of IMT on clinical outcomes in patients with HF is needed, since no such study has been reported in the literature.
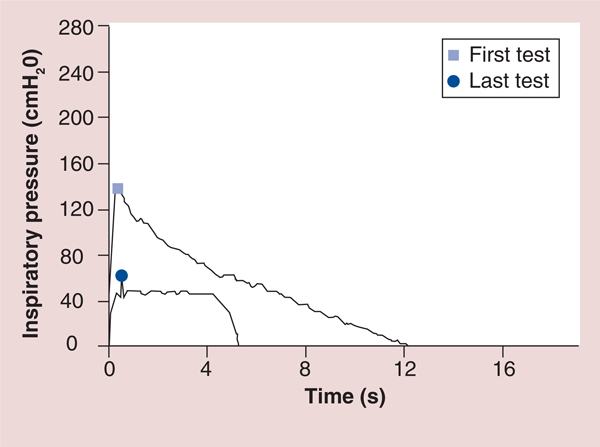
Figure 11. Graphic comparison of test of incremental respiratory endurance inspiratory muscle training to Threshold inspiratory muscle training in the same person.
The first test shows an example of TIRE IMT with a maximal inspiratory pressure of −139 cmH2O (shown on the y-axis) that continued for 12.1 s (shown on the x-axis). The last test shows an xample of Threshold IMT with the highest maximal inspiratory pressure possible using the Threshold device (−41 cmH2O which is shown on the y-axis with a slight and very short increase in pressure to −48 cmH2O shortly after inspiration) that continued for approximately 5 s before inspiration could no longer be maintained.
IMT: Inspiratory muscle training; TIRE: Test of incremental respiratory endurance.
Important outcomes from TIRE testing & training using the RT2 device
Several of the outcome measures listed in BOX 3 that are currently obtained only with the TIRE RT2 device will be briefly described below especially as they relate to patients with HF. The outcome measures with potential for the greatest diagnostic and rehabilitative value in persons with HF include the SMIP, accumulated SMIP, inspiratory muscle time of contraction (IMTOC), inspiratory vital capacity (IVC), peak inspiratory power, inspiratory work and the SMIP as well as IVC power profiles (yielding the y-intercept, R value, R-squared value and the standard error of the estimate) [39–49].
Since MIP has been found to be significantly correlated to peak VO2 in patients with HF [25,26], the authors hypothesize that the SMIP and accumulated SMIP will also be significantly correlated to peak VO2, at a level superior to MIP. As such, SMIP and accumulated SMIP may be superior predictors of survival and other outcomes in persons with HF compared with MIP. The SMIP profile before and after training is shown in FIGURE 9 and represents the work under the curve of ones inspiratory effort. In two separate and recent studies of healthy athletes, the SMIP was significantly correlated to exercise work performance (r = 0.58 and 0.61) and was more highly correlated to work than MIP [Wiwchar DM, Chatham K, Boothby DS, Clark SB, Cahalin LP. High-Intensity inspiratory muscle training improves skating performance and maximal oxygen consumption in division 1 college ice hockey players (2013), Submitted,50]. The accumulated SMIP (calculated via the RT2 software as the summation of work under the curve of each inspiration completed during the TIRE) was highly correlated to maximal VO2 in both of the recent studies in healthy athletes (r = 0.71 and 0.63; p < 0.05) and as apparent was more highly correlated to maximal VO2 than SMIP [Wiwchar DM, Chatham K, Boothby DS, Clark SB, Cahalin LP. High-Intensity inspiratory muscle training improves skating performance and maximal oxygen consumption in division 1 college ice hockey players (2013), Submitted,50].
The IMTOC and IVC do not appear to have been examined previously in patients with HF, but it is likely that these values are important rehabilitation outcomes that also have prognostic importance. The two recent studies of healthy athletes both found the IMTOC and IVC to be significantly correlated to maximal VO2 (r = 0.67 and 0.52 for IMTOC and r = 0.64 and 0.61 for IVC) [Wiwchar DM, Chatham K, Boothby DS, Clark SB, Cahalin LP. High-Intensity inspiratory muscle training improves skating performance and maximal oxygen consumption in division 1 college ice hockey players (2013), Submitted,50]. One could hypothesize that IMTOC and IVC are likely to be highly correlated to peak VO2 in patients with HF and that they will be significant predictors of survival in HF patients.
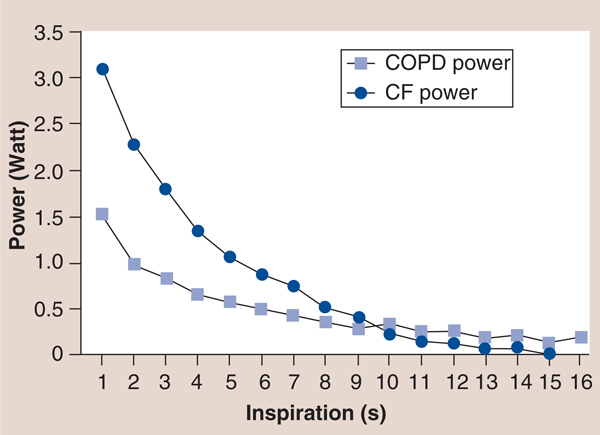
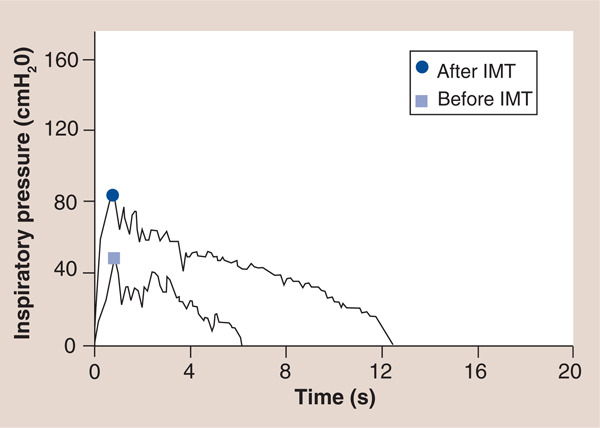
The inspiratory power indices available via the TIRE RT2 also appear to be important rehabilitation outcomes with prognostic value, since they incorporate flow and pressure as a result of previous work that established a calibration curve for the electronic manometer via the 2-mm leak of the mouthpiece, which provides the volume of air entering the manometer for a given pressure to be determined and enables the conversion of pressure to energy and power via: power (P) = p × Q where p = pressure expressed as N/m2 and Q = flow rate expressed in m3/second utilizing a calibration constant for the 2-mm leak of Q = 3.226 × 10–6 × <di>F0D6p</di> with inspiratory power expressed in Watts and inspiratory work expressed in Joules [39,43]. Furthermore, the measurement of inspiratory work per breath can be obtained from the power curve and can be expressed in Joules/breath of inspiratory work [39,43]. These characteristics of the power profile provide novel measures that may expand the clinical utility of SMIP. For example, in a study of patients with cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) matched by spirometry results (forced expiratory volume in 1 s and forced vital capacity), the inspiratory power profile of the patients with CF was significantly different than that of the patients with COPD [CHATHAM K, LINES TA, CAHALIN LP. UNPUBLISHED DATA]. Most striking was the slope of the inspiratory power curve which was significantly greater in patients with CF compared with patients with COPD (FIGURE 12) [CHATHAM K, LINES TA, CAHALIN LP. UNPUBLISHED DATA]. The authors believe that these findings are supported by changes in the inspiratory power curve during training and de-training of the inspiratory muscles [CAHALIN LP, BOYKIN M, WIWCHAR DM, CHATHAM K. POWER PROFILES DURING SUSTAINED MAXIMAL INSPIRATORY PRESSURE (SMIP) MEASUREMENTS ASSOCIATED WITH TRAINING AND DE-TRAINING OF THE INSPIRATORY MUSCLES (2013), SUBMITTED.], previous observations of respiratory muscle fiber type change in COPD patients [51] and peripheral skeletal muscle power curves obtained from exercise tests like the Wingate test [52]. Other characteristics of the inspiratory power profile and SMIP also appear to be important for persons with HF, since the results of both can be subjected to linear regression and can provide important information about the oscillatory characteristics of inspiration (i.e., R value, R-squared value and the standard error of the estimate) and the possible relationship of the ominous presence of exercise oscillatory ventilation in patients with HF [53]. An example of oscillatory inspiration in a patient with HF before and after a short period of IMT is shown in FIGURE 13. Improvement in all TIRE RT2 inspiratory parameters after only 2 weeks of IMT performed 3×/week occured and included a greater MIP (−84 vs −48 cm H2O), SMIP (533 vs 152 PU), accumulated SMIP (6423 vs 3620 PTU) and IMTOC (12.6 s vs 6.2 s). Furthermore, after 2 weeks of TIRE IMT performed 3×/week, the magnitude of initial inspiratory oscillation decreased slightly and decreased even more during the latter portion of inspiration at which time the inspiratory power profile appeared relatively smooth and much more normal in appearance. It is possible that the decrease in inspiratory oscillation may have occurred from the above improvements as well as nervous system adaptation from motor learning (owing to breathing retraining) and improved autonomic nervous system activity. Improvements in exercise oscillatory ventilation in HF patients have been observed with both exercise training and administration of sildenafil [53–55]. IMT has also been observed to improve exercise oscillatory ventilation in HF patients in one study, but further investigation of the relationship between inspiratory oscillation and exercise oscillatory ventilation observed during cardiopulmonary exercise testing is warranted. These measurements and those described above that are obtained from the TIRE RT2 device are in need of further investigation in persons with HF, but appear to have promise in testing, training and predicting outcomes in these patients.
Figure 12. Test of incremental respiratory endurance RT2 power profiles of patients with cystic fibrosis and chronic obstructive pulmonary disease.
CF: Cystic fibrosis; COPD: Chronic obstructive pulmonary disease.
Figure 13.
An example of oscillatory inspiration in a patient with heart failure before and after a short period of inspiratory muscle training.
Expert commentary
In summary, IMT has been observed to improve many clinical outcomes related to the pathophysiological manifestations of HF including dyspnea, QoL, balance, peripheral muscle and peripheral blood flow, peripheral muscle sympathetic nervous system activity, heart rate, respiratory rate, peak VO2, 6-MWT distance, ventilation, VE/VCO2 slope, oxygen uptake efficiency, circulatory power, recovery oxygen kinetics and several indices of cardiac performance [4,5,7–22]. Despite the improvements in many of the above outcomes associated with the pathophysiological manifestations of HF, IMT has not been included in guidelines for the examination and management of patients with HF. Strong evidence exists for the inclusion of inspiratory muscle performance measures to be added to examination guidelines for patients with HF [23–28]. Furthermore, strong evidence exists for the inclusion of IMT in management guidelines for patients with HF [4–22,56–59].
Five-year view
The five-year view of IMT in HF includes greater clinical application of IMT and IMT outcomes in the management of patients with HF. Specifically, we hypothesize that IMT will become a standard of care for patients with HF who have inspiratory muscle weakness (MIP <70% of the predicted value). Further investigation of a similar threshold level for IME in patients with HF is needed and will likely be determined within the next 5 years. This is extremely important since IME has been observed to be reduced despite preserved inspiratory muscle strength [60]. Furthermore, Tikunov et al. found that the diaphragm of patients with HF exhibits greater oxidative metabolism [61] for which reason combined measures of inspiratory muscle strength and endurance are important during the serial examination and management of patients with HF. In view of these findings, it is likely that IMT workloads should be alternated as with aerobic and resistance training to the peripheral skeletal muscles (or at least targeted to the major impairment found in each patient; strength, endurance or both). Thus, greater IMT workloads (60–80%) should facilitate greater strength gains while lesser workloads (≤60%) should facilitate endurance gains while also considering other components of the IMT prescription (e.g., number of repetitions and sets, duration and frequency). It is becoming apparent that IMT administered with aerobic and resistance training has a synergistic positive effect on the pathophysiologic manifestations of HF and will increasingly become a training modality combined with other exercise for patients with HF [10,19,22]. We also believe that IMT outcomes such as MIP, SMIP, accumulated SMIP, slope of the SMIP, inspiratory duration and presence of oscillatory inspiration will be used as prognostic markers for survival and management of patients with HF. The MIP has previously been observed to be highly correlated to peak VO2 and comparable to peak VO2 in predicting survival in patients with HF [25,26]. Recent findings in healthy persons suggest that SMIP and accumulated SMIP may have even greater clinical utility than MIP [Wiwchar DM, Chatham K, Boothby DS, Clark SB, Cahalin LP. High-Intensity inspiratory muscle training improves skating performance and maximal oxygen consumption in division 1 college ice hockey players (2013), Submitted,50]. The provision of IMT to patients with other cardiovascular diseases besides HF appears warranted in view of the beneficial effects of IMT on many of the pathophysiologic manifestations of HF, especially patients with compromised peripheral blood flow. Although research in such patients is needed, the largest IMT study performed to date provided approximately 4 weeks of IMT before high-risk patients underwent coronary artery bypass graft surgery and found significantly fewer postoperative complications in patients performing IMT [12]. These results appear to have occurred as a result of training targeted to the inspiratory muscles that improved not only inspiration, but many of the pathophysiologic manifestations of heart disease. The provision of IMT to such patients will likely increase within the next 5 years. However, the duration of IMT (as well as other components of the IMT prescription) needed to elicit specific adaptations to improve particular clinical outcomes is in need of further investigation, since the duration necessary to achieve specific clinical outcomes may vary. Nonetheless, a range of 4–8 weeks of IMT seems acceptable [5–7,12,13,16,19,20,56].
Figure 6. Tidal volume as a function of ventilation for normal subjects and three groups of patients with heart failure based on peak oxygen consumption.
VE: Ventilation; VT: Ventillatory threshold.
Reproduced with permission from [30] © American Heart Association, Inc. (2012).
Key issues.
Substantial evidence exists for inspiratory muscle training (IMT) to become a standard of care for patients with heart failure (HF), especially patients observed to have inspiratory muscle weakness.
Not all forms of IMT are the same with inspiratory flow resistive loading facilitating strength, power, and endurance training and inspiratory pressure threshold loading facilitating strength and endurance training.
Combining inspiratory flow resistive loading and inspiratory pressure threshold loading may be most effective in improving the pathophysiological manifestations of HF.
IMT performed with biofeedback is superior to IMT without biofeedback and facilitates patient adherence to perform IMT.
The number of testing and training outcome measures from computerized IMT (test of incremental respiratory endurance and TRAINAIR®) is greater than other forms of IMT, which can be used to better develop and target IMT efforts and provide prognostic information.
The role of expiratory muscle training (EMT) in HF is unclear, but may have beneficial effects in patients with HF. Combined IMT and EMT appear beneficial for patients with HF, but further research of EMT alone or combined with IMT is needed.
IMT combined with other modes of exercise appears warranted and further research examining the effects of IMT combined with other modes of exercise may provide insight into the best combinations of exercise for HF patients (and patients with other cardiovascular diseases).
Further research examining the role of IMT in other forms of heart disease and cardiovascular diseases is warranted in view of the beneficial effects of IMT in HF and high-risk patients undergoing coronary artery bypass graft surgery.
Future research examining the prognostic importance of key inspiratory (and possibly expiratory) muscle measures is also warranted in the HF population.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Lawrence P Cahalin, Department of Physical Therapy, Leonard M. Miller School of Medicine, University of Miami, 5915 Ponce de Leon Blvd. 5th Floor, Miami, Coral Gables, FL 33146-2435, USA.
Ross Arena, Division of Physical Therapy, Department of Orthopaedics and Rehabilitation, University of New Mexico School of Medicine Albuquerque, NM, USA; Division of Cardiology, Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque, NM, USA.
Marco Guazzi, Cardiology, I.R.C.C.S. Policlinico San Donato, University of Milano, San Donato Milanese, Italy.
Jonathan Myers, Division of Cardiology, VA Palo Alto Healthcare System, Stanford University, Palo Alto, CA, USA.
Gerson Cipriano, Department of Physical Therapy, University of Brasilia, Brasilia, Brazil.
Gaspar Chiappa, Exercise Pathophysiology Research Laboratory, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Carl J Lavie, Department of Cardiovascular Diseases, John Ochsner Heart and Vascular Institute, Ochsner Clinical School, The University of Queensland School of Medicine, New Orleans, LA, USA; Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA.
Daniel E Forman, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
References
Papers of special note have been highlighted as:• of interest
- 1.West JB. Respiratory Physiology: The Essentials (8th Edition). Lippincott Williams & Wilkins, Baltimore, MD, USA: (2008). [Google Scholar]
- 2.West JB. Pulmonary Pathophysiology: The Essentials (7th Edition). Lippincott Williams & Wilkins, Baltimore, MD, USA: (2008). [Google Scholar]
- 3.Ribeiro JP, Chiappa GR, Neder JA, Frankenstein L. Respiratory muscle function and exercise intolerance in heart failure. Curr. Heart Fail. Rep. 6(2), 95–101 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Henson D, La Manca J, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation 91(2), 320–329 (1995).• Study demonstrating a comprehensive approach to inspiratory muscle training (IMT) in patients with heart failure (HF).
- 5.Cahalin LP, Semigran MJ, Dec GW. Inspiratory muscle training in patients with chronic heart failure awaiting cardiac transplantation: results of a pilot clinical trial. Phys. Ther. 77(8), 830–838 (1997).• Study demonstrating a simple approach to IMT in patients with advanced HF.
- 6.Johnson PH, Cowley AJ, Kinnear WJ. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. Eur. Heart J. 19(8), 1249–1253 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Darnley GM, Gray AC, McClure SJ et al. Effects of resistive breathing on exercise capacity and diaphragm function in patients with ischaemic heart disease. Eur. J. Heart Fail. 1(3), 297–300 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin. Cardiol. 22(11), 727–732 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez A, Lisboa C, Jalil J et al. Selective training of respiratory muscles in patients with chronic heart failure. Rev. Med. Chil. 129(2), 133–139 (2001). [PubMed] [Google Scholar]
- 10.Cahalin L, Wagenaar R, Dec GW, Semigran MJ. Endurance training in heart failure – a pilot study of the effects of cycle versus ventilatory muscle training. Circulation 104(17), II–453 (2001). [Google Scholar]
- 11.Laoutaris I, Dritsas A, Brown MD, Manginas A, Alivizatos PA, Cokkinos DV. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 11(6), 489–496 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Hulzebos EHJ, Helders PJM, Favie NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery. JAMA 296, 1851–1857 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Dall’Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J. Am. Coll. Cardiol. 47(4), 757–763 (2006).• The first study showing the substantial beneficial effects of IMT on the pathophysiologic manifestations of HF.
- 14.Laoutaris ID, Dritsas A, Brown MD et al. Immune response to inspiratory muscle training in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 14(5), 679–685 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Laoutaris ID, Dritsas A, Brown MD et al. Effects of inspiratory muscle training on autonomic activity, endothelial vasodilator function, and N-terminal pro-brain natriuretic peptide levels in chronic heart failure. J. Cardiopulm. Rehabil. Prev. 28(2), 99–106 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Chiappa GR, Roseguini BT, Vieira PJ et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J. Am. Coll. Cardiol. 51(17), 1663–1671 (2008).• The first study showing the beneficial effects of IMT on peripheral blood flow and the respiratory metaboreflex.
- 17.Padula CA, Yeaw E, Mistry S. A home-based nurse-coached inspiratory muscle training intervention in heart failure. Appl. Nurs. Res. 22(1), 18–25 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Stein R, Chiappa GR, Güths H, Dall’Ago P, Ribeiro JP. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. J. Cardiopulm. Rehabil. Prev. 29(6), 392–395 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Winkelmann ER, Chiappa GR, Lima CO, Viecili PR, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am. Heart J. 158(5), 768.e1–768.e7 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Bosnak-Guclu M, Arikan H, Savci S et al. Effects of inspiratory muscle training in patients with heart failure. Respir. Med. 105(11), 1671–1681 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Mello PR, Guerra GM, Borile S et al. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J. Cardiopulm. Rehabil. Prev. 32(5), 255–261 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Laoutaris ID, Adamopoulos S, Manginas A et al. Benefits of combined aerobic/resistance/inspiratory training in patients with chronic heart failure. A complete exercise model? A prospective randomised study. Int. J. Cardiol. doi: 10.1016/j.ijcard.2012.05.019 (2012) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 23.McParland C, Krishnan B, Wang Y, Gallagher CG. Inspiratory muscle weakness and dyspnea in congestive heart failure. Am. Rev. Respir. Dis. 146, 467 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Walsh JT, Andrews R, Johnson P, Phillips L, Cowley AJ, Kinnear WJ. Inspiratory muscle endurance in patients with chronic heart failure. Heart 76(4), 332–336 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer FJ, Zugck C, Haass M et al. Inefficient ventilation and reduced respiratory muscle capacity in congestive heart failure. Basic Res. Cardiol. 95(4), 333–342 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Meyer FJ, Borst MM, Zugck C et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 103(17), 2153–2158 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br. Heart J. 72(Suppl. 2), S36–S39 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coats AJ. The ‘muscle hypothesis’ of chronic heart failure. J. Mol. Cell. Cardiol. 28(11), 2255–2262 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Arena R, Guazzi M, Myers J. Ventilatory abnormalities during exercise in heart failure: a mini review. Curr. Respir. Med. Rev. 3, 179–187 (2007). [Google Scholar]
- 30.Wasserman K, Zhang YY, Gitt A et al. Lung function and exercise gas exchange in chronic heart failure. Circulation 96(7), 2221–2227 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Scott AC, Francis DP, Davies LC, Ponikowski P, Coats AJ, Piepoli MF. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J. Physiol. (Lond.) 529(Pt 3), 863–870 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seals DR. Robin Hood for the lungs? A respiratory metaboreflex that ‘steals’ blood flow from locomotor muscles. J. Physiol. (Lond.) 537(Pt 1), 2 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harms CA, Wetter TJ, McClaran SR et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 85(2), 609–618 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Witt JD, Guenette JA, Rupert JL, McKenzie DC, Sheel AW. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J. Physiol. (Lond.) 584(Pt 3), 1019–1028 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 292(1), H580–H592 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Mancini DM, Ferraro N, Nazzaro D, Chance B, Wilson JR. Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near-infrared spectroscopy. J. Am. Coll. Cardiol. 18(2), 492–498 (1991). [DOI] [PubMed] [Google Scholar]
- 37.Borghi-Silva A, Carrascosa C, Oliveira CC et al. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 294(6), H2465–H2472 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Bussotti M, Andreini D, Agostoni P. Exercise-induced changes in exhaled nitric oxide in heart failure. Eur. J. Heart Fail. 6(5), 551–554 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Chatham K, Gelder CM, Lines TA, Cahalin LP. Suspected statin-induced respiratory muscle myopathy during long-term inspiratory muscle training in a patient with diaphragmatic paralysis. Phys. Ther. 89(3), 257–266 (2009).• Study describing the methods and outcomes of test of incremental respiratory endurance IMT.
- 40.Enright SJ, Unnithan VB. Effect of inspiratory muscle training intensities on pulmonary function and work capacity in people who are healthy: a randomized controlled trial. Phys. Ther. 91(6), 894–905 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Enright SJ, Unnithan VB, Heward C, Withnall L, Davies DH. Effect of high- intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys. Ther. 86(3), 345–354 (2006). [PubMed] [Google Scholar]
- 42.Enright S, Chatham K, Ionescu AA, Unnithan VB, Shale DJ. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest 126(2), 405–411 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Chatham K, Ionescu AA, Nixon LS, Shale DJ. A short-term comparison of two methods of sputum expectoration in cystic fibrosis. Eur. Respir. J. 23(3), 435–439 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Ionescu AA, Chatham K, Davies CA, Nixon LS, Enright S, Shale DJ. Inspiratory muscle function and body composition in cystic fibrosis. Am. J. Respir. Crit. Care Med. 158(4), 1271–1276 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Chatham K, Berrow S, Beeson C, Griffiths L, Brough D, Musa I. Inspiratory pressures in adult cystic fibrosis. Physiotherapy 80(11), 748–752 (1994). [Google Scholar]
- 46.Chatham K, Baldwin J, Griffiths H, Summers L, Enright S. Inspiratory muscle training improves shuttle run performance in healthy subjects. Physiotherapy 85(12), 676–683 (1999). [Google Scholar]
- 47.Chatham K Individualized fixed load inspiratory muscle training responses in a patient with severe restrictive lung disease and an elite sportsman. Physiotherapy 86(1), 28–30 (2000). [Google Scholar]
- 48.Mickleborough TD, Nichols T, Chatham K, Lindley MR, Ionescu AA, Shale DJ. The effect of high and low-intensity inspiratory muscle training on the physiological response to exercise in recreational runners. Scand. J. Med. Sci. Sports 20(3), 458–468 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Bruton A A pilot study to investigate any relationship between sustained maximal inspiratory pressure and extubation outcome. Heart Lung 31(2), 141–149 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Cahalin LP, Li R, Almeida AS, Keisling B, Corrado G. Inspiratory muscle performance is significantly related to 2K and maximal incremental ergometry rowing in division 1 women’s crew. Northeastern University Research Expo.April, 2011, Abstract 1989. [Google Scholar]
- 51.Ramirez-Sarmiento A, Orozco-Levi M, Guell R et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am. J. Respir. Crit. Care Med. 166(11), 1491–1497 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Inbar O, Bar-Or O, Skinner JS. The Wingate Anaerobic Test. Human Kinetics, Champaign, IL, USA: (1996). [Google Scholar]
- 53.Murphy RM, Shah RV, Malhotra R et al. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation 124(13), 1442–1451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zurek M, Corrà U, Piepoli MF, Binder RK, Saner H, Schmid JP. Exercise training reverses exertional oscillatory ventilation in heart failure patients. Eur. Respir. J. 40(5), 1238–1244 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Guazzi M, Vicenzi M, Arena R. Phosphodiesterase 5 inhibition with sildenafil reverses exercise oscillatory breathing in chronic heart failure: long-term cardiopulmonary exercise testing placebo-controlled study. Eur. J. Heart Fail. 14(1), 82–90 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Plentz RD, Sbruzzi G, Ribeiro RA, Ferreira JB, Dal Lago P. Inspiratory muscle training in patients with heart failure: meta-analysis of randomized trials. Arq. Bras. Cardiol. 99(2), 762–771 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Smart NA, Giallauria F, Dieberg G. Efficacy of inspiratory muscle training in chronic heart failure patients: a systematic review and meta-analysis. Int. J. Cardiol. (2012) doi: 10.1016/j.ijcard.2012.04.029 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 58.Laoutaris ID, Dritsas A, Brown MD et al. Inspiratory muscle training in a patient with left ventricular assist device. Hellenic J. Cardiol. 47(4), 238–241 (2006). [PubMed] [Google Scholar]
- 59.Laoutaris ID, Dritsas A, Adamopoulos S et al. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur. J. Cardiovasc. Prev. Rehabil. 18(1), 33–40 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Mancini DM, Henson D, LaManca J, Levine S. Evidence of reduced respiratory muscle endurance in patients with heart failure. J. Am. Coll. Cardiol. 24(4), 972–981 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation 95(4), 910–916 (1997). [DOI] [PubMed] [Google Scholar]
- 62.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J. Physiol. 537, 277–289 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St Croix C, Morgan B, Wetter T Dempsey J. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J. Physiol. 529, 493–504 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]