Abstract
The most difficult impediments to transcutaneous optical sensing are the absorbance and scatter of light caused by skin and the lack of fluorescent sensing probes which can be excited at wavelengths over 600 nm. Furthermore, current optical sensing techniques rely on absorbance or fluorescence intensity measurements, both of which are sensitive to drifts in lamp intensity, changes in probe concentration and inner filter effects. We demonstrate oxygen sensing through a layer of skin by using red light which readily penetrates skin as diffusely scattered light. The oxygen sensitive osmium-ligand complex used in this study can be excited at 635–680 nm. In addition, we measure fluorescence lifetimes, which are inherently unaffected by factors that limit absorbance and fluorescence intensity measurements. By using phase fluorimetry and long lived fluorophores, we are able to demonstrate the potential for subdermal oxygen sensing with simple and inexpensive instrumentation. This work describes a paradigm for future non-invasive measurements of other analytes.
Keywords: Fluorescence lifetime, Non-invasive sensing, Oxygen measurement, Patient monitoring, Phase fluorimetry, Transcutaneous sensing
INTRODUCTION
Non- or minimally invasive physiological monitoring is a highly desirable goal for many analytes. In principle, the sensing element can be incorporated under the skin or in the tissue. The practical problem of transducing the signal and detecting it through the skin has proved to be a formidable challenge. Consider, for example, physiological oxygen measurement. The survival of patients through their stay in hospital intensive care units may well be determined by the speed and accuracy of determinations of their vital functions. Paramount among these are determinations of tissue and arterial oxygen levels. Currently, oxygen determinations are carried out using direct blood gas measurements as well as pulse oximetry. Blood gas analyzers permit a limited measurement frequency because of the need to permanently remove blood samples and are subject to significant delays thus being of limited utility in situations where large dynamic variations in physiological oxygen occur (Walton et al., 1981). Pulse oximeters measure the pulsatile change in absorbance of haemoglobin and oxyhaemoglobin in arterial blood at two discrete wavelengths using the constant component (that due to absorbance of tissue and other blood components) at each wavelength to normalize the signals (for a review see Kelleher, 1989). The measurements are carried out non-invasively at 660 nm and 940 nm, in perfused tissue (typically a finger). These measurements are subject to interferences from significant amounts of other haemoglobin types present in blood (Reynolds et al., 1993) such as carboxyhaemoglobin (present due to smoking or smoke inhalation) and methaemoglobin (formed due to the use of various anaesthetics).
Despite these limitations, both methods are widely used in clinical practice as well as intensive care. More importantly however, both methods measure arterial blood oxygenation. While these measurements describe pulmonary function, they reveal little about cellular or tissue oxygenation. When pulse oximetry and blood gas measurements are not mistakenly used to assess tissue oxygenation, which, unfortunately is often the case (Shoemaker, 1991; Ahrens, 1993), a combination of simultaneous measurements such as arterial and venous haemoglobin saturation, cardiac output and lactate level are used in critically ill patients to determine tissue hypoxia. Even so, this is an indirect method and the information obtained tends to be non-specific (Rashkin et al., 1985; Kelleher, 1989; Ahrens, 1993). The method requires longer evaluation times and does not identify accurately oxygen availability at the cellular level in tissues and organs.
We describe an alternative method that circumvents the above drawbacks. It is a generic optical technique, capable of real time transcutaneous sensing of oxygen in tissue and is based upon the measurement of changes in the oxygen dependent fluorescence lifetime of a metal-ligand complex fluorophore. Previously, fluorescence lifetime-based sensing of oxygen using ruthenium-ligand complexes has been described (Bacon & Demas, 1987; Lippitsch et al., 1988; Bemdt & Lakowicz, 1992; Bambot et al. 1994). The method yields rapid and reliable measurements of both gaseous and dissolved oxygen; is suitable for measuring oxygen in dense microbial cultures used in bioprocesses and provides an optical alternative to the widely used Clark electrode (Bambot et al., 1994). However, the ruthenium-ligand complex used in these studies requires blue light excitation (near 450 nm) making it unsuitable for transcutaneous sensing, since human skin is only translucent to red light beyond 600 nm (Anderson &. Parrish. 1982). This fact is clearly illustrated in Fig. 1 which shows the ability of red light to penetrate a finger. Yellow light (580 nm) and shorter wavelengths are absorbed at the skin surface (left). While red light at 600 nm partially penetrates (middle), light at 620 nm can be seen throughout the finger as diffusely scattered light (right). We have therefore focused our efforts on utilizing red sensitive probe molecules because of the potential for transcutaneous sensing.
Figure 1.
Human skin obsorbs fibre optic illumination at 580 nm but shows greater transmittance at 600 and 620 nm, as evidenced by successively deeper light penetration.
Current detection techniques such as pulse oximetry, as described above, rely on the measurement of the intrinsic absorbance of haemoglobin in blood. While measurements of fluorescence intensity are clearly superior to absorbance measurements, due to higher sensitivity (Lakowicz. 1983). selectivity (by being able to choose both excitation and emission wavelengths) and compatibility with laser excitation sources (making it possible to use fibre optics), such measurements are plagued by many factors that alter the observed fluorescence intensity, including light losses in the fibre, changes in lamp intensity, probe washout, photobleaching and inner filter effects in the sample. In this report we have described the use of fluorescence lifetime measurements as a significant improvement upon current optical sensing methods. Time resolved sensing is advantageous because such measurements are independent of probe concentration and/or intensity and are mostly independent of photobleaching and inner filter effects of the sample. Such measurements have hitherto been conducted in the time domain requiring complex electronics and expensive instrumentation. Fortunately, recent developments in the frequency-domain technique of phase-modulation fluorimetry, coupled with the availability of novel synthetic fluorophores, have made drastic cost reduction possible, permitting the development of simple and inexpensive measuring devices (Szmacinski & Lakowicz, 1994). In phase-modulation fluorimetry, sinusoidally modulated excitation light excites a fluorescent chemical probe. The fluorescence emission is, as a result, forced to oscillate at the same frequency. Due to the analyte-dependent fluorescence lifetime of the probe, the emission is phase shifted with respect to the excitation. A calibration of phase angle versus analyte concentration is easily obtained.
Our ability to implement phase fluorimetry for transdermal oxygen sensing was facilitated by the availability of red-sensitive long lifetime fluorescent probes for oxygen. In this study, we have used the metal complex Os(2, 2’, 2”-terpyridine)22+ (Kober et al., 1985) because of its strong red light absorbance and far-red emission and also because its fluorescence intensity and lifetime are dependent upon oxygen partial pressures. Long lifetime luminescent metal-complex probes (Lin et al., 1976; Demas & DeGraff, 1991), particularly those excitable by red light and which emit in the far red, are known from studies of their photophysics (Kober et al., 1985; Demas & DeGraff, 1991). The availability of long lifetime probes has been particularly instrumental in cost reduction. Our probe is a luminescent transition metal complex of the form Me(L2 or 3)2+ where Me is the transition metal atom and L is an organic ligand. These complexes, particularly those using the metals Ru, Os, Re, Rh and Ir have been shown to have many desirable features (for a review see Demas & DeGraff, 1991). Most important among these are their long fluorescence lifetimes (hundreds of nanoseconds to a few microseconds) which translate directly into low equipment cost, particularly with respect to phase modulation fluorimetry. Most organic fluorophores, besides requiring excitation in the blue and green regions of the spectrum, have nanosecond lifetimes which require modulation frequencies of around 100 MHz. Besides being difficult to excite, (one typically has to use external electro-optic or accousto-optic modulators with high powered dye lasers to provide sufficient modulated excitation) the high equipment cost prohibits construction of simple inexpensive prototypes suited to routine applications. It has been shown previously that measuring fluorescence lifetimes of metal ligand complexes by phase modulation fluorimetry is implemented easily without requiring the more complex timing circuitry necessary for conventional decay time measurements (Lippitsch etal., 1988; Bemdt & Lakowicz, 1992; Bambot et al., 1994). Other desirable features include thermal, chemical and photochemical stability resulting in robust sterilizable sensors as well as intense visible absorbtions and high quantum yields, which make these probes highly sensitive and amenable to excitation using inexpensive, low power laser diodes and LEDs.
For transcutaneous sensing applications it is necessary to immobilize the probe molecule in a suitable biocompatible matrix. Silicone is widely used as a biocompatible material and the potential for its use as a subcutaneous patch incorporating the probe molecules is promising. By permitting direct real time monitoring of oxygenation in subcutaneous tissue, this technique will obviate reliance on other less accurate methods. Silicone rubber has been used previously because of its high oxygen permeability and because it hydrophobicity excludes polar compounds and hydrated ions (Lippitsch et al., 1988; Berndt & Lakowicz, 1992; Bambot et al., 1994). The fluorophore can be readily incorporated into a preformed silicone rubber matrix. The hydrophobic matrix as well as the insolubility of the fluorophore in aqueous solution also prevent probe leaching, which is an important consideration for in vivo applications.
MATERIALS AND METHODS
Chemicals
The osmium complex used in this work was a kind gift from Dr. Thomas J. Meyer of the Department of Chemistry, University of North Carolina at Chapel Hill. All other reagents were purchased from Aldrich or Sigma. The silicone membrane was prepared by spreading a thin layer of GE Silicone II (stock# GE 5000, GE Silicones, General Electric Company, Waterford, NY) on a microscope slide, using a blade. The thin layer (about 0.5 mm thick) was allowed to cure overnight and was then removed from the glass surface, resulting in a thin transparent silicone membrane. The osmium complex was dissolved to saturation in methanol. Chloroform was then added in the ratio 8 : 2 chloroform : methanol by volume, resulting in a homogenous solution. The silicone membrane was soaked in this solution for 5 min allowing the silicone matrix to expand and absorb the fluorophore. The membrane was then removed and air dried for 5 min after which it shrank back to its original size. The membrane was washed with methanol to remove surface fluorophore molecules, dried and laid out on a rectangular glass window.
Spectroscopy, optics and electronics
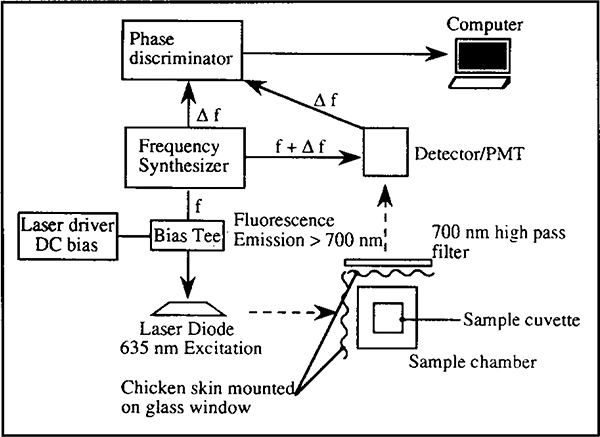
Figure 2 shows a schematic of the set-up used to obtain time resolved fluorescence measurements using phase fluorimetry on an ISS K2 phase fluorometer (Champaign, IL). Modifications, additions and components of the fluorometer, relevant to the measurement are illustrated. Modulated light at 635 nm was provided by a laser diode (Toshiba, model TOLD9520, Edison, NJ) terminated with a 47 ohm 2 W resistor. A 104.78 mA DC bias was supplied to the diode laser by an ILX Lightwave (Bozeman, MT) model LDX 3620 ultra low noise current source. Modulation current was applied to the external modulation port of the DC bias supply by a Marconi 2022A (Allendale, NJ) signal generator operated at −28 dBm. Temperature of the diode laser was thermoelectrically maintained at 20°C. Approximately 1 mW of 40% modulated light was produced, with a −3 dB bandwidth of approximately 0.07 to 1.7 MHz. Depth of modulation was limited by the current capability of the DC source and the value of the terminating resistor. The fluorometer uses cross correlation detection to simplify instrumentation (Lakowicz, 1983). By modulating the gain of the photomultiplier tube at the synthesizer frequency plus a small increment (f + Δf) the resulting photocurrent modulates at the lower frequency Δf, but contains the desired phase values.
Figure 2.
Schematic of experimental set-up used for obtaining phase angle measurements using an ISS K2 spectrofluorometer modified to accommodate excitation using a laser diode light source.
Modulated light was directed as shown into a cuvette containing either a solution of the fluorophore in methanol or a fluorophore embedded silicone membrane on a glass window, positioned at about 40 degrees to the incident light (membrane facing the incident light but facing away from the photodetector) in order to minimize scatter. Pieces of fresh chicken skin mounted on glass windows were positioned on both excitation and emission paths. The skin layers were doubled when required. A 700 nm long wave pass filter was placed in the emission path to eliminate scatter from the laser source. The reference phase was obtained by observing the scattering light from a solution of colloidal silica (LUDOX HS-30, DuPont Corp., Wilmington, DE) in a cuvette placed in the sample chamber. The reference measurement was made through neutral density filters and in the absence of the 700 nm filter.
Emission spectra were taken on the ISS K2 fluorometer using a monochromator. The excitation was provided by the laser diode powered by the DC source in the absence of modulation.
RESULTS AND DISCUSSION
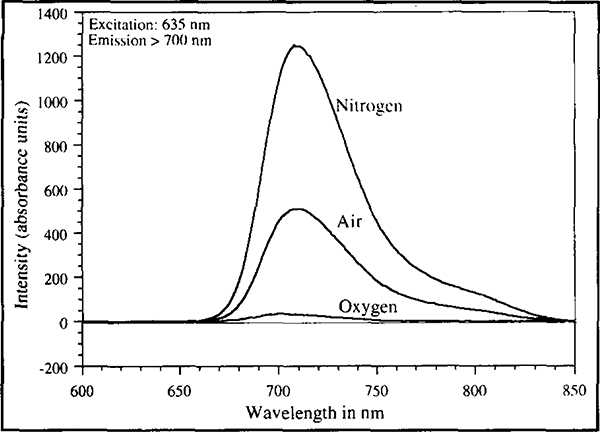
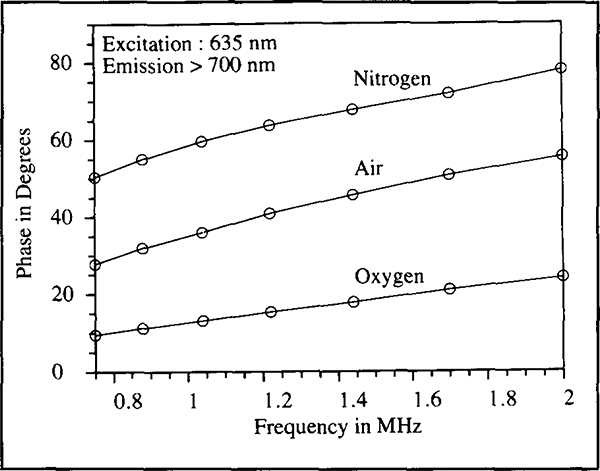
Figure 3 shows the oxygen partial pressure dependent changes in fluorescence emission spectra of Os(2,2’, 2”-terpyridine)22+. Asolution of the osmium complex in methanol was saturated first with oxygen, air and then nitrogen; spectra were taken in each instance. The spectra show broadband emissions which peak at 710 nm. The absorption spectrum of this complex shows strong absorbance between the wavelengths of 600 and 700 nm (Kober et al., 1985). The transparency of human skin to light at these wavelengths and the sensitivity of the probe’s fluorescence to oxygen, makes this complex ideally suited to applications in transcutaneous oxygen sensing. Changes in oxygen tension also showed changes in fluorescence lifetimes. Figure 4 shows the phase angles observed at various modulation frequencies for the probe solution in methanol saturated with oxygen, air and nitrogen. Large phase angle shifts across a broad range of frequencies was observed. Assuming the oxygen concentration in air to be around 21%, it is clear that the probe has a first order type response with oxygen partial pressure and is increasingly sensitive to oxygen at low oxygen tensions. Such behaviour is consistent with Stern°Volmer type kinetics, which describes the mechanism by which oxygen quenches the fluorescence of such complexes (Parker, 1968).
Figure 3.
Emission spectra of Os(2,2 ‘,2”-terpyridine)22+ in methanol solution saturated with oxygen, air and nitrogen. Excitation was provided by a 635 nm laser diode. The emission was observed through a 700 nm long wave pass filter.
Figure 4.
Frequency scans of the phase angles observed after saturation of the osmium complex solution in methanol with oxygen, air and nitrogen. The emission was observed through a 700 nm long wave pass filter.
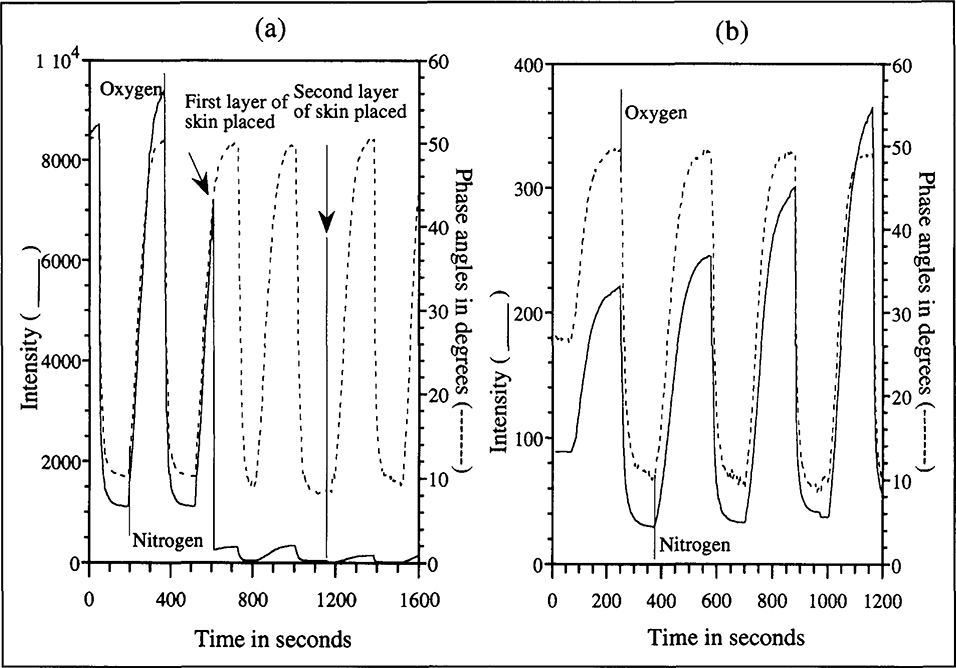
To demonstrate transcutaneous sensing, experiments were conducted using layers of chicken skin to block both excitation and emission light paths during spectra acquisition. Chicken skin was used because of its easy availability and its optical similarity to human skin (Anderson & Parrish, 1982). Figure 5(a) shows real time fluorescence intensity and phase angle measurements (at 750 kHz) as oxygen and nitrogen were altematingly bubbled through the fluorophore solution. The measurements were made first with no skin; one layer of skin and finally two layers of skin in both excitation and emission paths. While a 50 fold decrease in the intensity response occurred the phase angle measurements were unaffected by the presence of the skin. The experiment demonstrates the independence of phase angle measurements to changes in skin thickness and attenuation in excitation light intensity. Provided a minimum measurable emission is available (the quantity of light required for measurement at a tolerable signal to noise level, is governed entirely by the photodetector) the phase angles are independent of the attenuating effects that limit fluorescence intensity based measurements. Another factor that limits fluorescence intensity based measurements is changes in probe concentration due to washout or photobleaching. Figure 5(b) shows how phase angle measurements can be used to correct this dependence. During an experiment similar to that shown in Fig. 5(a), the purging gases caused a slow evaporation of the methanol, resulting in increasing fluorophore concentration. The measurements were made through one skin layer in both excitation and emission paths. While the increase in probe concentration resulted in increasing intensity extrema, phase angle profiles remained unchanged.
Figure 5.
(a). Intensity and phase angles (measured at 750 kHz) over time as oxygen and nitrogen pre-saturated with methanol are alternatingly bubbled through the fluorophore solution. The measurements were made first with no skin, one layer of skin and finally two layers of skin in both excitation and emission paths. While a 50-fold decrease in the intensity response occurred, the phase angle measurements were unaffected by the presence of the skin.
(b). The purging gases caused a slow evaporation of the methanol, resulting in increasing fluorophore concentration. The measurements were made through one skin layer in both excitation and emission paths. While probe concentration resulted in increasing intensity extrema, similar changes were not observed in the phase angles.
The results show the robustness of phase angle measurements as compared with conventional optical techniques. The measurements are completely reversible and rapid. Each phase angle measurement is completed in milliseconds and the response time of the measurement is limited only by the diffusion and mixing of oxygen in the fluorophore solution. In order to make a transcutaneous sensor, the osmium probe was immobilized in a silicone rubber matrix. A thin (about 1 mm) section of the probe immobilized silicone layer mounted on a glass window was placed in the sample cuvette. Phase angles were measured while altematingly passing oxygen and nitrogen gases through the cuvette. Figure 6 shows the phase angle and intensity profiles obtained. The osmium probe was found to partially retain its sensitivity to oxygen after immobilization in a silicone rubber, albeit with a lower dynamic range and reduced signal to noise (the oxygen to nitrogen change in phase angles was only 10° compared to about 40° in solution).
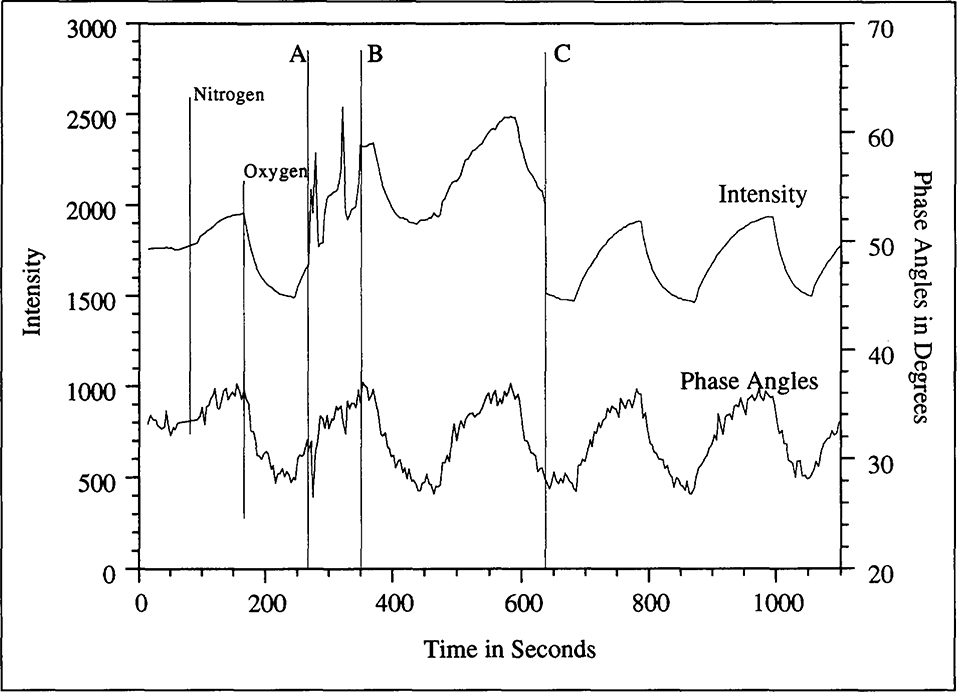
Figure 6.
Phase angle measurements for silicone embedded osmium complex demonstrating the independence of phase angle measurements to room light and other disturbances: During a regular experiment in which a silicone membrane incorporating the osmium dye was being subjected to alternating oxygen and nitrogen sparging, while recording the fluorescence lifetime (phase angles) and fluorescence intensity, a colleague tripped over the tubes supplying the gases (Instant A). After a few moments of attempting to fix the gas supply to the cuvette in the dark, the room lights were turned on (Instant B) and upon correcting the problem, the lights were turned back off again (Instant C).
Phase angle measurements are largely independent of factors that limit fluorescence intensity based measurements and this fact has been reiterated in Fig. 6. While recording the phase angles and fluorescence intensities in an ongoing experiment, a colleague tripped over the tubes supplying the gases (Instant A). After a few moments of attempting to fix the gas supply to the cuvette in the dark, the room lights were turned on (Instant B) and upon correcting the damage the lights were turned back off again (Instant C). The presence of extraneous light, besides generating some noise in the signal, raised the base intensity levels measured while phase angles were unaffected.
The Os probe suffered significant loss in sensitivity to oxygen after immobilization in a silicone rubber matrix. Nevertheless, reliable measurements of oxygen concentration could still be made. Such reductions in lifetime variation with oxygen tension accompanied with reduced signal to noise ratios are the result of the micro environment of the fluorophore in the silicone matrix. Some performance improvement may be achieved by choosing different silicones and by using fluorophores that have larger phase angle variations in solution. New red-sensitive osmium probes have recently become available with longer fluorescence lifetimes (Brewer et al., 1994).
CONCLUSIONS
This article describes the use of fluorescence lifetime measurement using phase fluorimetry in transcutaneous oxygen sensing. We have used the metal complex Os(2, 2’, 2”-terpyridine)22+ because of its strong red light absorbance, far-red emission, long fluorescence lifetimes and also because its fluorescence intensity and lifetime are dependent upon oxygen partial pressures. Lifetime-based sensing allows real time optical measurement of oxygen through skin, independent of factors that plague conventional absorbance and fluorescence intensity based measurements. While such measurements typically require expensive instrumentation and complex electronics, the availability of a long lifetime fluorophore, coupled with the technique of phase fluorimetry, can result in significant cost reductions. In addition to the use of a readily available inexpensive laser diode light source, the measurements were carried out using simple electronics. The recent availability of highly sensitive photodiodes as a replacement for expensive photomultiplier tubes enables even greater cost reduction. In principle, an inexpensive and compact hand held version of the instrumentation used in this study can be easily manufactured for routine use. Measurements of oxygen can be non-invasively made under physiological conditions and also in tissue reactors.
Further advances in probe synthesis will undoubtedly spur additional developments based on the principles described here. In addition to probes for oxygen, new lifetime probes which are sensitive to pH, pC02, Ca2+, Mg2+, Na+, K+, Cl− and glucose (Szmacinski & Lakowicz, 1994) have become available. Although none of them currently show long lifetimes or sensitivity to red light, the generic technology described can be directly ported over upon further advances in probe chemistry and development of effective immobilization methods. In addition, with specific monoclonal based assays using red-sensitive probes as the reporter molecule, one could in principle devise biosensors for a number of analytes. Biocompatible sensor patches containing the antibody-probe molecules could be implanted subcutaneously and various analyte concentrations could be monitored non-invasively through the skin with a compact, low-cost red diode laser based instrument.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Science Foundation (BCS-9157852, PYI to GR, and BES-9413262) and The National Institutes of Health (RR-08119 and RR-07510). Matching funds were generously provided by Artisan Industries, Inc. and Genentech Inc. We thank Dr. Thomas J. Meyer of the Department of Chemistry, University of North Carolina at Chapel Hill for the gift of the osmium complex.
Contributor Information
Shabbir B. Bambot, Department of Chemical and Biochemical Engineering, University of Maryland Baltimore County, Baltimore, MD 21228, USA
Govind Rao, Department of Chemical and Biochemical Engineering, University of Maryland Baltimore County, Baltimore, MD 21228, USA.; Medical Biotechnology Center of the Maryland Biotechnology Institute, University of Maryland at Baltimore, Baltimore, MD 21201, USA.
M. Romauld M., Department of Electrical Engineering, University of Maryland Baltimore County, Baltimore, MD 21228, USA..
Gary M. Carter, Department of Electrical Engineering, University of Maryland Baltimore County, Baltimore, MD 21228, USA.
Jeffrey Sipior, Center for Fluoresence Spectroscopy, Dept. of Biological Chemistry, University of Maryland at Baltimore, Baltimore, MD 21201 USA.
Ewald Terpetchnig, Center for Fluoresence Spectroscopy, Dept. of Biological Chemistry, University of Maryland at Baltimore, Baltimore, MD 21201 USA.
Joseph R. Lakowicz, Center for Fluoresence Spectroscopy, Dept. of Biological Chemistry, University of Maryland at Baltimore, Baltimore, MD 21201 USA. Medical Biotechnology Center of the Maryland Biotechnology Institute, University of Maryland at Baltimore, Baltimore, MD 21201, USA.
REFERENCES
- Ahrens T (1993). Changing perspectives in the assessment of oxygenation. Critical Care Nurse. 78–83. [PubMed] [Google Scholar]
- Anderson RR & Parrish JA (1982). Optical properties of human skin In: The Science of Photomedicine. Regan JD & Parrish JA (eds.), Plenum press, New York: pp. 149–194. [Google Scholar]
- Bacon JR & Demas JN (1987). Determination of oxygen concentrations by luminescence quenching of a polymer immobilized transition metal complex. Anal. Chem, 59, 2780–2785. [Google Scholar]
- Bambot SB, Holavanahali R Lakowicz JR, Carter GM & Rao G (1994). Phase fluorometric sterilizable optical oxygen sensor. Biotechnol. Bioeng, 43,1139–1145. [DOI] [PubMed] [Google Scholar]
- Berndt KW & Lakowicz JR (1992). Electroluminescent lamp-based phase fluorometer and oxygen sensor. Anal. Biochem, 201, 319–325. [DOI] [PubMed] [Google Scholar]
- Brewer RG, Jensen GE & Brewer KJ (1994). Long lived osmium (II) chromophores containing 2,3,5,6-tetrakis(2-pyridyl)pyrazine./norg. Chem, 33, 124–129. [Google Scholar]
- Clark Jr., L.C. (1956). Monitor and control of blood and tissue oxygen tension. Trans. Am. Soc. Artif. Int. Organs, 2, 41. [Google Scholar]
- Demas JN & DeGraff BA (1991). Design and applications of highly luminescent transition metal complexes. Anal. Chem, 63(17), 829–837. [Google Scholar]
- Dudell G, Cornish JD & Bartlett RH (1990). What constitutes adequate oxygenation? Pediatrics, 85, 39–41. [PubMed] [Google Scholar]
- Kelleher JF (1989). Pulse oximetry. J. Clin. Monit, 5, 37–62. [DOI] [PubMed] [Google Scholar]
- Kober EM, Marshall JL, Dressnick WJ, Sullivan BP, Caspar JV & Meyer T (1985). Synthetic control of excited states. Nonchromophoric ligand variations in polypyridyl complexes of osmium (II). J. Inorg. Chem, 24, 2755–2763. [Google Scholar]
- Lakowicz JR (1983). In: Principles of Fluorescence Spectroscopy. Plenum Press, New York. [Google Scholar]
- Lin C-T, Bôttcher W, Chou M, Creutz C & Sutin N (1976). Mechanism of quenching of the emission of substituted polypyridineruthenium (II) complexes by iron(II), chromium(III) and europium (III) ions. J. Am. Chem. Soc, 98, 6536–6544. [Google Scholar]
- Lippitsch ME, Pusterhofer J, Leiner MJP & Wolfbeis OS (1988). Fibre optic oxygen sensor with the fluorescence decay time as the information carrier. Anal. Chim. Acta, 205,1–6. [Google Scholar]
- Parker CA (1968). Photoluminescence of Solutions. Elsevier, Amsterdam. [Google Scholar]
- Rashkin M, Boxkin C & Baughman R (1985). Oxygen delivery in critically ill patients: relationship to blood lactate and survival. Chest, 87,580–584. [DOI] [PubMed] [Google Scholar]
- Reynolds KJ, Palayiwa E, Moyle JTB, Sykes MK & Hahn MA (1993). The effect of dyshaemoglobins on pulse oximetry. J. Clin. Monit, 9(2), 81–90. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC (1991). Tissue perfusion and oxygenation: a primary problem in acute circulatory failure and shock states. Crit. Care. Med, 19,595–596. [PubMed] [Google Scholar]
- Szmacinski H & Lakowicz JR (1994). Lifetime-based sensing, pp. 295–334. In: Probe Design and Chemical Sensing, Topics in Fluorescence Spectroscopy, Vol. 4 Lakowicz JR (ed.), Plenum Publishing Corp., New York. [Google Scholar]
- Walton JR, Shapiro BA, Wine C (1981). Pre-analytic error in arterial blood gas measurement. Abstract. Respir. Care, 21,1136. [Google Scholar]