Abstract
Introduction:
Although allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for hematologic neoplasms, one of its limiting toxicities continues to be graft versus host disease, both acute and chronic (aGVHD, cGVHD). Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor which has been found to be effective in GVHD prophylaxis, in combination with calcineurin inhibitors like tacrolimus. The impact of sirolimus on immune reconstitution has not been comprehensively investigated in-vivo thus far. We now present an ancillary analysis of the randomized study BMT-CTN 0402, examining the effect of sirolimus on immune subsets post-transplant. We further examine the association between different lymphocyte subsets and outcomes post-transplant in each arm.
Methods:
BMT-CTN 0402 was a randomized trial (n=304) which compared two GVHD prophylaxis regimens, tacrolimus/sirolimus (Tac/Sir) versus tacrolimus/methotrexate (Tac/MTX), in AML/ALL/MDS patients, undergoing myeloablative HLA-matched transplantation. There was no difference in 114-day GVHD free survival (primary endpoint) as well as acute or chronic GVHD, relapse or overall survival between arms. 264/304 patients had available samples for the current immune reconstitution analysis. Blood samples were collected at 1,3, 6, 12 and 24 months post-HCT. Multi-parameter flow cytometry was performed at the project lab (Esoterix Clinical Trials Services) in a blinded fashion, and results were compared between arms. Multivariable Cox regression models, treating each phenotypic parameter as a time dependent variable, were constructed to study impact of reconstitution on clinical outcomes.
Results:
There were no significant differences in patient and transplant characteristics between the Tac/Sir and Tac/MTX arms in this analysis. Absolute lymphocyte count (ALC), CD3+, CD4+ and conventional T cell counts were significantly decreased in the Tac/Sir arm upto 3 months postHCT while CD8+ cells recovered even more slowly (upto 6 months) in this arm. Interestingly there was no clear difference in the absolute number of regulatory T-cells (defined as CD4+ CD25+ cells) between arms at any point post-HCT. However the Treg:Tcon ratio was significantly greater in the Tac/Sir arm in the first 3 months after HCT. B-lymphocyte recovery was significantly compromised in the Tac/Sir arm from 1 to 6 months after HCT while NK cells reconstitution was not affected in the sirolimus arm. In the outcomes analysis, higher numbers of CD3+, CD4+. CD8+ and Tregs were associated with better overall survival. Neither Treg numbers nor Treg:Tcon ratio correlated with GVHD.
Conclusion:
Tac/Sir has a more profound T-cell suppressive effect than the combination of Tac/MTX in the early post-transplant period, and particularly compromises recovery of CD8+ T-cells which have been implicated in aGVHD. Sirolimus when used in-vivo with tacrolimus does not appear to result in increased absolute numbers of Tregs, but might have a beneficial effect on the Treg:Tcon balance in the first 3 months after transplantation. Despite this, it should be noted that no differences in aGVHD or cGVHD were observed between the two arms in the parent randomized trial. Calcineurin-inhibitor free, sirolimus containing GVHD prophylaxis strategies, incorporating other novel agents, should be investigated further to maximize the potential favorable effect of sirolimus on Treg:Tcon balance in the post-transplant immune repertoire. Sirolimus significantly compromises B-cell recovery in the first 6 months post-HCT with potential complex effects on cGVHD which merit further study.
Keywords: Sirolimus, Immune reconstitution, Myeloablative, Tregs, B-lymphocytes
Introduction:
Acute GVHD (aGVHD) occurs in 30–35% of HLA-matched hematopoietic stem cell transplantation (HSCT) while chronic GVHD (cGVHD) estimates have been in the 30–70% range1. The use of calcineurin inhibitor (CI) (tacrolimus/Tac, cyclosporine) based prophylaxis has resulted in improvement in aGVHD rates, although control of cGVHD with this regimen has been more challenging2. CI inhibitors are typically used in combination with methotrexate (MTX) with the potential downstream toxicities of nephrotoxicity, myelosuppression and mucositis. Hence, effective agents with better adverse effect profiles continue to be an area of active interest.
Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor with potent immunosuppressive properties, originally developed for use in solid organ transplantation. It binds to the same immunophilin as tacrolimus (FKBP12), however it acts at a later stage of T-cell cycle progression and blocks cytokine mediate signal transduction pathways3. It therefore prevents T-cell activation and proliferation in a synergistic manner with tacrolimus4. Consequently, sirolimus has been used in combination with tacrolimus for GVHD prophylaxis with promising results. Sirolimus was shown to be safe as a GVHD prophylaxis agent along with tacrolimus and low-dose methotrexate in the early 2000s5. It was then shown to be effective in HLA-matched related and unrelated donor transplants in combination with tacrolimus only6 as well as double umbilical cord blood transplantation7. Thrombotic microangiopathy and hepatic veno-occlusive disease were found to be associated with sirolimus use in this context, particularly when myeloablative regimens like Busulfan/Cyclophosphamide (BuCy) or total body irradiation (TBI) was used8.
A Phase II randomized controlled trial (RCT) comparing tacrolimus/sirolimus (Tac/Sir) to tacrolimus/methotrexate (Tac/MTX) as GVHD prophylaxis in 74 patients found that grade II-IV aGVHD and moderate/severe cGVHD were significantly better in the sirolimus arm. Overall survival (OS) and patient reported quality of life (QOL) was however, similar in both arms9. The largest RCT comparing the combination of Tac/Sir with Tac/MTX (standard of care) as GVHD prophylaxis (BMT-CTN 0402) (n=304) in MRD HSCT, used 114-day grade II-IV acute GVHD free survival as its primary end-point, in an intention to treat analysis10. Interestingly, there were no significant differences in the primary end-point or in grade II-IV acute GVHD, chronic GVHD, relapse-free survival (RFS) or overall survival (OS) in either arm. In a point-wise post-hoc analysis, severe (Gr III – IV) aGVHD was reduced in the sirolimus arm. Oropharyngeal mucositis was significantly less in the sirolimus arm. Hence Tac/Sir was thought to be an acceptable alternative to standard of care as a GVHD prophylaxis regimen but was not superior to Tac/MTX.
The effect of sirolimus on various T-cell subsets such as conventional T-cells (Tcon) and regulatory Tcells (Tregs) has been studied in mice. The addition of sirolimus led to reduced expansion of alloreactive Tcon and aGVHD lethality in mice11. Concomitantly, expansion of polyclonal Tregs was observed with conserved high FOXP3 expression. This differential effect on two major T-cell subsets was attributed to the reduced usage of the mTOR pathway in Tregs compared with Tcon. Limited analyses on the in-vivo effect of sirolimus on post-transplant immune reconstitution have been performed in smaller numbers of patients and have suggested that Treg reconstitution is better preserved with Tac/Sir compared to Tac/MTX9,12. However, a comprehensive analysis of the effect of sirolimus on immune reconstitution after HSCT has not been performed and is critical to understand how sirolimus affects T-cell and B-cell subsets as well as NK cells, in vivo.
BMT-CTN 0402 is the largest RCT comparing sirolimus-based GVHD prophylaxis (Tac/Sir) with MTX based prophylaxis (Tac/MTX) and provides the ideal platform to study the effect of sirolimus on posttransplant immune reconstitution. We present here the results of an analysis comparing immune reconstitution in the Tac/Sir and Tac/MTX arms. We further analyze the association between different immune subsets and post-transplant outcomes in each arm.
Methods:
Study Design:
An open-label, phase 3, multi-center RCT was performed by the Blood and Marrow Transplant Clinical Trials Network or BMT-CTN (BMT-CTN 0402) comparing the combination of Tac/Sir with Tac/MTX as GVHD prophylaxis regimens, following matched related donor (MRD) peripheral blood stem-cell (PBSC) HSCT (n=304). The primary end-point for this RCT was 114-day grade II-IV acute GVHD free survival, in an intention to treat analysis. Subjects under 60 years of age with acute leukemia in remission, myelodysplastic syndrome (MDS) or chronic myeloid leukemia (CML) in chronic or accelerated phase were eligible. All patients received TBI-based myeloablative conditioning (1200cGy) along with cyclophosphamide (Cy) or etoposide. Patients receiving BuCy conditioning were excluded from the analysis due to excessive toxicity when combined with sirolimus for GVHD prophylaxis and were not part of either the parent RCT or the current analysis. Tac was started on day −3 (0.02mg/kg/day intravenous, with a trough level maintained at 5–10 ng/ml) and sirolimus was started also on day −3 (loading dose of 12mg followed by 4mg daily to maintain a trough level of 3–12 ng/ml). MTX was administered iv on day +1 (15mg/m2), and on days +3, +6 and +11 (10mg/m2).
Of the 304 subjects, 264 had available samples for this analysis (Tac/Sir=132, Tac/MTX=132). Randomization was maintained for the flow cytometry analysis for this immune reconstitution analysis.
Flow cytometry:
Written informed consent was obtained for this immune reconstitution analysis prior to sample collection in accordance with the Declaration of Helsinki. Protocol approval was obtained from the Institutional Review Board (IRB) of contributing institutions. Blood samples were collected at months 1, 3, 6, 12 and 24 post-HCT for all subjects and included (a) 3ml EDTA peripheral blood sample (to calculate absolute cell population counts by flow cytometry) and (b) 10ml ACD peripheral blood sample (for immunophenotypic analyses by multi-parameter flow cytometry) which was shipped to the project lab (Esoterix Clinical Trials Services) for immediate analysis. Protocol-specified immunophenotypic analyses were performed with a panel of fluorophore-conjugated monoclonal antibodies (Mabs) specific for cell surface determinants. Flow cytometry was performed in a blinded fashion without knowledge of patient treatment.
The panel of Mabs used to define the various T-cell subsets were as follows: CD3+, CD3+CD4+, CD3+CD8+, regulatory T-cells (Tregs) CD3+CD4+CD25+, conventional T-cells (CD3+CD4+ minus CD3+CD4+CD25+) cells. B-cells were defined as CD19+ as well as a B-cell subset CD19+CD27+ cells. Natural Killer (NK) cells were defined as CD3-CD56+CD16+ as well as CD3-CD56+CD16- and NKT cells (NKT) as CD3+CD56+ cells. Sub-compartment analysis of T-cells included naive CD4+ T cells (CD4+CD45RA+CD62L+), effector CD4+ T-cells (CD4+CD45RA+CD62L−), naive CD8+ cells (CD8+CD45RA+CD62L+) and effector CD8+ cells (CD8+CD45RA+CD62L−). Proliferating naïve and effector cells in the CD4+ and CD8+ compartments were designated by Ki67+. The Treg:Tcon ratio was defined as Treg (CD4+CD25+)/Tcon (CD4+ minus CD4+CD25+) while the Treg:CD8 ratio was defined as Treg (CD4+CD25+)/CD8+.
Statistical Analysis:
Analysis included participants who were randomized and underwent transplant and had available samples only. Baseline characteristics were compared using the Fisher’s exact test, Chi-square test or Wilcoxon-rank-sum test, as appropriate. Wilcoxon-rank-sum test was also utilized to compare immune reconstitution data between two treatment arms at each time point. Multivariable Cox regression models treating each phenotypic parameter as a time dependent variable were constructed to study impact of reconstitution on clinical outcomes.
Clinical endpoints considered in this study included overall survival (OS), non-relapse mortality (NRM) relapse, acute GVHD (grade II-IV), acute GVHD (grade III-IV), chronic GVHD (cGVHD) and grade II-IV acute GVHD-free survival. These endpoints were defined previously10. For GVHD, NRM and relapse, cause-specific Cox regression analysis was performed treating each phenotypic parameter as a time dependent variable. Potential prognostic factors considered in the regression analysis included GVHD prophylaxis, age, recipient and donor sex, disease, disease risk, Karnofsky performance score, conditioning regimen, cytogenetic risk, and CMV serostatus of recipient and donor at HCT. All immune-phenotypic data were natural log transformed for regression analysis. Correlation analysis was performed using Spearman’s rankorder correlation. Since the primary focus of this ancillary immune reconstitution study was the effect of sirolimus on T subsets, a nominal p value of 0.01 was pre-set as an ad hoc adjustment for multiple comparisons of these subsets and their ratios (Tregs, Tcon, CD8, Tregs:Tcon, Tregs:CD8). All tests were two-sided.. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R version 3.2.2 (the CRAN project, www.cran.r-project.org).
This work is submitted on behalf of the Blood and Marrow Transplant Clinical Trials Network (BMT-CTN ).
Results:
Patient and transplant characteristics:
Patient and transplant characteristics for 264 subjects are summarized in Table 1. The median recipient age in the Tac/Sir group was 44 years (range 18, 59) while in the Tac/MTX group was 42 years (range 12, 55). Myeloid malignancies comprised the majority (65.9%) of diseases in the Tac/Sir group as well as the Tac/MTX group (55%). The proportion of acute lymphoblastic leukemia (ALL) patients, however, was disproportionately higher in the Tac/MTX group compared to the Tac/Sir group (45% versus 33.3%). Karnofsky performance status (KPS), donor-recipient gender match and conditioning regimen (Cy/TBI, VP-16/TBI) were comparable across both arms. The distribution of donor-recipient CMV status is detailed in Table 1.
TABLE 1.
BASELINE CHARACTERISTICS
| Tac/Sir (N=132) | Tac/MTX (N=132) | All (N=264) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| AGE, MEDIAN (RANGE) | 44 (18, 59) | 42 (12, 58) | 44 (12, 59) | |||
| RECIPIENT GENDER | ||||||
| MALE | 64 | 48.5 | 73 | 55 | 137 | 51.9 |
| FEMALE | 68 | 51.5 | 59 | 45 | 127 | 48.1 |
| GENDER OF DONOR | ||||||
| FEMALE | 56 | 42.4 | 48 | 36 | 104 | 39.4 |
| MALE | 76 | 57.6 | 84 | 64 | 160 | 60.6 |
| DONOR AGE, MEDIAN (RANGE) | 45 (14, 66) | 41 (13, 64) | 44 (13, 66) | |||
| PRIMARY DIAGNOSIS* | ||||||
| AML | 60 | 45.5 | 56 | 42 | 116 | 43.9 |
| CR1 | 49 | 49 | 98 | |||
| CR2 | 11 | 7 | 18 | |||
| ALL | 44 | 33.3 | 59 | 45 | 103 | 39.0 |
| CR1 | 37 | 48 | 85 | |||
| CR2 | 7 | 11 | 18 | |||
| CML | 9 | 6.8 | 11 | 8 | 20 | 7.6 |
| AP | 2 | 2 | 4 | |||
| CP | 7 | 9 | 16 | |||
| MDS | 18 | 13.6 | 6 | 5 | 24 | 9.1 |
| RA | 1 | 1 | ||||
| RARS | 2 | 2 | ||||
| RAEB1 | 3 | 3 | 6 | |||
| RAEB2 | 5 | 5 | ||||
| CMML | 3 | 2 | 5 | |||
| OTHER | 4 | 1 | 5 | |||
| ABL | 1 | 0.8 | 1 | 0.4 | ||
| CR1 | 1 | 1 | ||||
| DONOR-RECIPIENT GENDER MATCH | ||||||
| F/F | 29 | 22.0 | 21 | 16 | 50 | 18.9 |
| F/M | 27 | 20.5 | 27 | 20 | 54 | 20.5 |
| M/F | 39 | 29.6 | 38 | 29 | 77 | 29.2 |
| M/M | 37 | 28.0 | 46 | 35 | 83 | 31.4 |
| KARNOFSKY SCORE | ||||||
| >=90% | 89 | 67.4 | 98 | 74.3 | 189 | 70.8 |
| <90% | 43 | 32.6 | 34 | 25.7 | 77 | 29.2 |
| DONOR-RECIPIENT CMV STATUS** | ||||||
| +/+ | 54 | 40.9 | 43 | 33 | 97 | 36.7 |
| +/− | 10 | 7.6 | 24 | 18 | 34 | 12.9 |
| −/+ | 27 | 20.5 | 37 | 28 | 64 | 24.2 |
| −/− | 35 | 26.5 | 23 | 17 | 58 | 22.0 |
| UNK | 6 | 4.6 | 5 | 4 | 11 | 4.2 |
| CONDITIONING REGIMEN | ||||||
| CY-TBI | 107 | 81.1 | 105 | 79.6 | 212 | 80.3 |
| VP16-TBI | 25 | 18.9 | 27 | 20.5 | 52 | 19.7 |
: p=0.05,
:p=0.01.
p>0.05 for all other comparisions
Immune Reconstitution:
Total WBC count and absolute lymphocyte count (ALC ) after HSCT:
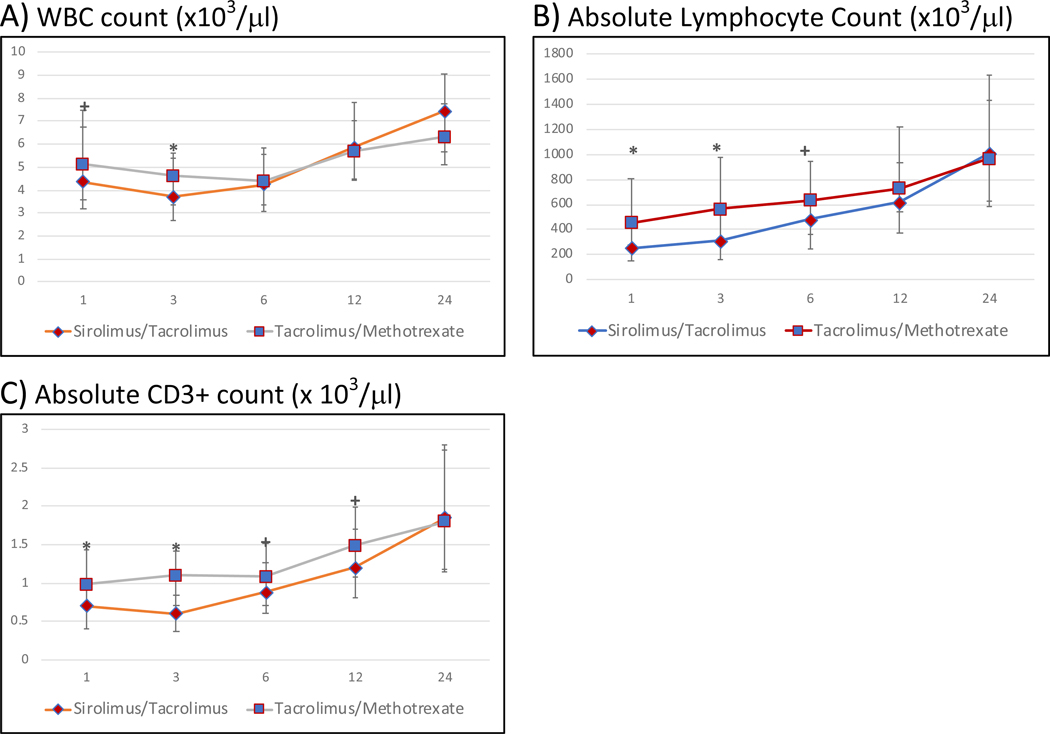
The recovery of total WBC count and absolute lymphocyte count (ALC) in each arm is summarized in Figure 1. Total WBC count recovery was similar in both arms at all time points post-HCT except at 3 months where it was significantly lower in the Tac/Sir arm (median= 3.71 × 103/ μl (Tac/Sir) versus 4.6 × 103/ μl (Tac/MTX), p=0.0053. The ALC was significantly lower in the Tac/Sir arm up to 3 months after HCT (p<0.0001). However, thereafter, ALC was not significantly different in the two arms at the 0.01 level. CD3+ recovery was significantly delayed in the Tac/Sir arm up to 3 months (median absolute CD3 count at 3 months: 315 × 103/ μl (Tac/Sir) versus 565 × 103/ μl (Tac/MTX) , p<0.0001), but absolute numbers were similar at later time points (Figure 2A).
Figure 1: Post-allogeneic transplant reconstitution of total WBC (A), absolute lymphocyte counts (ALC) (B) and absolute CD3+ cell count (/μl) (C) by treatment arm at months 1, 3, 6, 12 and 24.
The median cell counts for each population is represented at each time point. * denotes p-value<0.01 and + denotes 0.01<=pvalue<0.05. Median total WBC counts are significantly lower in the Tac/Sir arm at 3 months only. Median ALCcounts are significantly lower in the Tac/Sir arm upto 3 months post-transplant but not thereafter
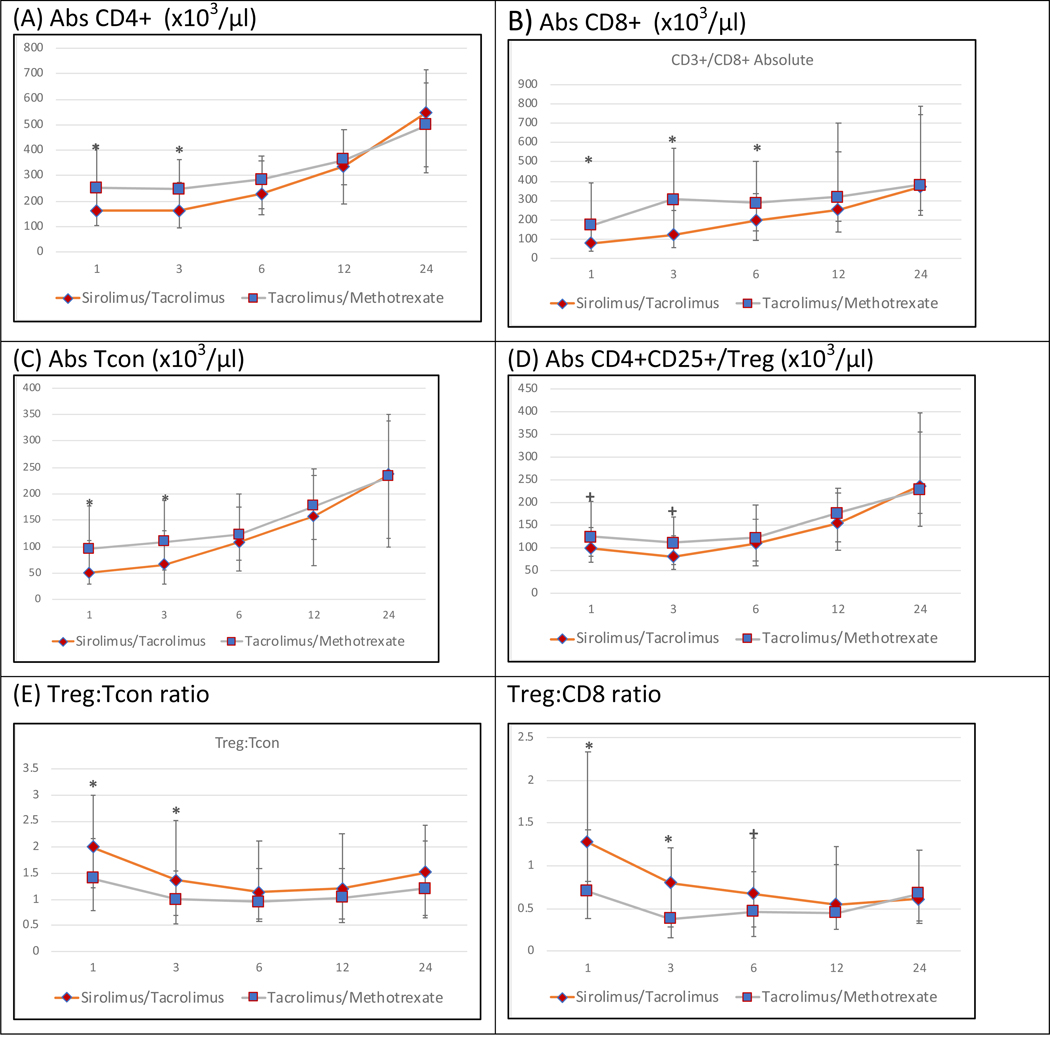
Figure 2 : Post-allogeneic transplant reconstitution of major T cell populations (CD4+, CD8+, CD4+25+/Tregs, CD4+25−/Tcon), Treg:Tcon ratio and Treg:CD8 ratio by treatment arm at months 1,3,6,12 and 24.
The median cell counts/μl for each population is represented at each time point.. * denotes p-value<0.01 and + denotes 0.01<=p-value<0.05. Absolute CD3+ and CD4+ counts are significantly lower in the Tac/Sir arm at 1 and 3 months post-transplant. Absolute CD8+ counts are significantly lower in the Tac/Sir arm from 1 to 6 months post-transplant but not thereafter. Treg counts are similar in both arms at all time-points. Treg:Tcon and Treg:CD8 ratios are significantly higher in the Tac/Sir arm upto 3 months post-transplant
Reconstitution of major T-cell populations:
The reconstitution of CD4+ T-cells, CD8+ T-cells, Tcon and Tregs (CD4+CD25+) are detailed in Figure 2. CD4+ cells followed the same trajectory as CD3+ T-cells and were significantly lower in the Tac/Sir arm in the first 3 months only (median absolute CD4 count at 3 months: 162 × 103/ μl (Tac/Sir) versus 246 × 103/ μl (Tac/MTX) , p<0.001 (Figure 2A). Absolute numbers of CD8+ T-cells were significantly lower in the Tac/Sir arm up to 6 months (i.e. at 1, 3 and 6 months). Median absolute CD8 count at 3 months: 121 (Tac/Sir) versus 304 (Tac/MTX) , p<0.0001 and median absolute CD8 count at 6 months: 195.5 (Tac/Sir) versus 287 (Tac/MTX) , p=0.009). Recovery of CD8+ cells was similar in each arm at 12 and 24 months (Figure 2B).
The absolute numbers of Tcon was derived by subtracting CD4+CD25+ cells from total CD4+ cells Conventional T cells also followed the same trajectory as CD3+ T-cells and were significantly lower in the Tac/Sir arm in the first 3 months only (median absolute Tcon count at 3 months: 66 (Tac/Sir) versus 109 (Tac/MTX), p<0.001 (Figure 2C). Tregs were defined as CD4+CD25+ cells in this analysis. Absolute numbers of CD4+ Tregs were not significantly different at the 0.01 level in the Tac/Sir and Tac/MTX arms at any point (1,3,6,12 or 24 months)(Fig 2D). In addition, absolute numbers of CD3+CD8+CD25+ cells were similar in both arms (data not shown).
Overall absolute numbers of CD3+ T cells were lower in the Tac/Sir arm in the early post-transplant period only (up to 3 months) and this was driven by both delayed Tcon, CD4+ and CD8+ cell recovery, with CD8+ cells recovering slowest. Tregs (CD3+CD4+CD25+) reconstitution was somewhat lower at 1 month and 3 months in the Tac/Sir arm compared to the Tac/MTX arm, however, the significance did not reach the 0.01 level (median level 98.5 vs 124.5, p=0.02 at 1 month for Tac/Sir and Tac/MTX respecitvely; 81 vs 111, p=0.029 at 3 months for Tac/Sir and Tac/MTX respecitvely p=0.02 and 0.029, respectively).
Treg:Tcon ratio and Treg:CD8 ratio :
The Treg:Tcon ratio was defined as CD4+CD25+/(CD4+ minus CD4+CD25+). These ratios were then compared in both arms at each time point. The Treg:Tcon ratio was significantly higher in the Tac/Sir arm compared with the Tac/MTX arm at 1 and 3 months, due largely to the delayed recovery of Tcon in the Tac/Sir arm, but similar at months 6, 12 and 24 (Fig 2E). The Treg:CD8 ratio followed a similar trajectory and was significantly higher in the Tac/Sir arm at 1 and 3 months, again reflecting the delayed recovery of CD8+ in the Tac/Sir arm. Thereafter the Treg:CD8 ratio was similar in both arms (Fig 2F).
Reconstitution of naïve and effector T-cells :
Absolute numbers of naive CD4+ T cells (CD4+CD45RA+CD62L+) were significantly lower at 1 month and 3 months in the Tac/Sir arm but were similar to the Tac/MTX arm thereafter (Figure 3A). Proliferating naive CD4+ T cells (CD4+CD45RA+CD62L+Ki67+) were significantly lower only at 1 month after transplantation (data not shown). Hence recovery of naive T-cells was more compromised than mature T-cells in the sirolimus arm. Recovery of effector CD4+ T-cells (CD4+CD45RA+CD62L−) was lower at 3 month and 6 months in the Tac/Sir arm (Figure 3B). Proliferating CD4+ effector cells (CD4+CD45RA+CD62L-Ki67+) recovered at the same rate in both arms (data not shown).
Figure 3: Post-allogeneic transplant reconstitution of T cell subsets (naiive CD4+ effector CD4+ T-cells, naiive CD8+ and effector CD8+ T-cells by treatment arm at months 1,3,6,12 and 24.
The median cell counts/μl for each population is represented at each time point. * denotes p-value<0.01 and + denotes 0.01<=pvalue< 0.05. Absolute naiive CD4+ cells are significantly lower in the Tac/Sir arm at 1 and 3 months post-transplant while absolute effector T-cells are significantly lower at 3 and 6 months. Absolute naiive CD8+ cells are significantly lower upto 3 months and then again at 12 months post-transplant while effector CD8+ cells are significantly lower in a sustained manner at 1,3 and 6 months post-transplant.
In the CD8 compartment, naive cells (CD8+CD45RA+CD62L+) were significantly lower in the Tac/Sir arm in the first 3 months and at one year after transplantation (Figure 3C) and proliferating naïve CD8+ cells (CD8+CD45RA+CD62L+Ki67+) followed the same trajectory (data not shown). CD8+ effector cells (CD8+CD45RA+CD62L−) were also significantly lower in the Tac/Sir arm in the first 6 months after transplantation (Figure 3D). Of these, the proliferating CD8 effector cells (CD8+CD45RA+CD62L-Ki67+) were significantly lower in the Tac/Sir arm at 1 month and 6 months after transplant (data not shown). Hence the effect of sirolimus on CD8 cell recovery was driven primarily by its effect on effector cells.
Recovery of B-lymphocytes, NK cells and NK/T cells post-HCT:
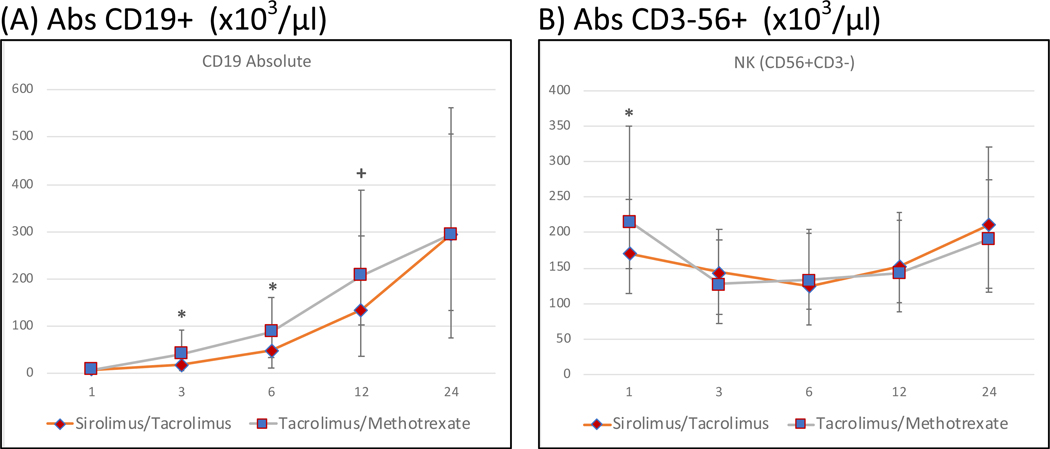
Immune reconstitution of CD19+ B-lymphocytes are demonstrated in Figure 4, panel A. Absolute numbers of CD19+ cells were similar very early after transplant (1 month), but significantly lower at 3 months and 6 months in the Tac/Sir arm. B-cell recovery however normalized thereafter and was similar to the control arm at 12 months and 24 months.
Figure 4: Post-allogeneic transplant reconstitution of B-cells and NK cells by treatment arm at months 1,3,6,12 and 24.
The median cell counts/μl for each population is represented at each time point. * denotes p-value<0.01 and + denotes 0.01<=p-value<0.05. Absolute CD19+ (B-lymphocyte) counts are significantly lower in the Tac/Sir arm at 3 and 6 months post-transplant. Absolute NK cells are significantly lower only at 1 month post-transplant in the Tac/Sir arm but not thereafter.
NK cell (CD3−CD16+CD56+ and CD3-CD16-CD56+) recovery in both arms is described in Figure 4, panel B. Absolute numbers of all NK cells (CD3−CD56+) cells were significantly lower in the Tac/Sir arm only at 1 month. Subsequently absolute numbers of NK cells were similar in both arms at all time-points.
Impact of immune reconstitution on clinical outcomes (using log-transformed absolute values for cell subtypes)
The impact of various immune subsets on clinical outcomes were analyzed by constructing multivariable Cox regression models using natural log-transformed absolute values of cell counts as time-dependent variables. Higher ALC, CD3+, CD3+CD4+, Tcon and Treg (CD4+CD25+) numbers were all associated with significantly improved overall survival and NRM at the 0.01 level (Table 2A) but not with relapse (Table 2A), acute GVHD of any grade or cGVHD (Table 2B). Higher CD3+CD8+ number was also associated with improved OS but had no effect on other outcomes including GVHD. The Treg:Tcon ratio and Treg:CD8 ratio did not correlate with any clinical outcome at the 0.01 level.
Table 2A:
Cox Multivariable Model for relapse, non-relapse mortality (NRM) and overall survival (OS) by immunophenotype (P< 0.01 is indicated in bold). DC=dendritic cell
| Cell type | Relapse | NRM | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% Cl | P-value | HR | 95% Cl | p-value | HR | 95% Cl | p-value | |
| CD3+ | 0.73 | 0.56, 0.97 | 0.03 | 0.56 | 0.40, 0.80 | 0.0015 | 0.55 | 0.44, 0.69 | <0.0001 |
| CD3+CD4+ | 0.76 | 0.57, 1.02 | 0.06 | 0.46 | 0.34, 0.64 | <0.0001 | 0.49 | 0.40, 0.62 | <0.0001 |
| CD3+CD8+ | 0.79 | 0.63, 0.99 | 0.04 | 0.72 | 0.53, 0.98 | 0.03 | 0.66 | 0.55, 0.80 | <0.0001 |
| CD3+CD4+CD25+ | 0.89 | 0.69, 1.13 | 0.33 | 0.68 | 0.55, 0.84 | 0.0004 | 0.67 | 0.57, 0.78 | <0.0001 |
| Tcon | 0.82 | 0.65 1.04 | 0.10 | 0.55 | 0.41 0.72 | <0.0001 | 0.57 | 0.47 0.69 | <0.0001 |
| Treg:Tcon | 1.13 | 0.69, 1.84 | 0.63 | 1.38 | 0.76, 2.51 | 0.29 | 1.54 | 1.03, 2.28 | 0.03 |
| Treg:CD8 | 1.29 | 0.72, 2.32 | 0.39 | 0.80 | 0.35, 1.82 | 0.60 | 1.12 | 0.66, 1.91 | 0.68 |
| CD19+ | 1.27 | 1.05, 1.53 | 0.0144 | 0.71 | 0.58, 0.86 | 0.0006 | 0.74 | 0.65, 0.84 | <0.0001 |
| CD3−CD56+ | 0.73 | 0.56, 0.96 | 0.02 | 0.65 | 0.46, 0.91 | 0.012 | 0.66 | 0.53, 0.81 | 0.0001 |
| Total WBC | 0.56 | 0.39, 0.82 | 0.0029 | 1.79 | 0.93, 3.47 | 0.08 | 0.81 | 0.56, 1.18 | 0.28 |
| ALC | 0.83 | 0.57, 1.20 | 0.33 | 0.64 | 0.38, 1.07 | 0.09 | 0.57 | 0.42, 0.76 | 0.0001 |
| cDC (Lin−/HLADR+/CD123−/CDllc+) | 0.991 | 0.875 1.123 | 0.89 | 0.995 | 0.838 1.182 | 0.96 | 1.012 | 0.903 1.134 | 0.833 |
| pDC (Lin−/HLADR+/CD123+/CDllc−) | 1.192 | 1.008 1.41 | 0.039 | 0.808 | 0.683 0.956 | 0.013 | 1.016 | 0.902 1.146 | 0.79 |
Table 2B:
Cox Multivariable Model for Acute and Chronic Graft-versus-host-disease by immunophenotype (P<0.01 is indicted in bold). DC=dendritic cell
| Cell type | Acute GVHD (grade II- IV) | Acute GVHD (grade III- IV) | Chronic GVHD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% Cl | P-value | HR | 95% Cl | P-value | HR | 95% Cl | p-value | |
| CD3+ | 0.96 | 0.70, 1.31 | 0.78 | 0.90 | 0.56, 1.43 | 0.65 | 1.22 | 0.98, 1.51 | 0.08 |
| CD3+CD4+ | 0.87 | 0.62, 1.21 | 0.40 | 0.80 | 0.50, 1.28 | 0.35 | 1.22 | 0.96, 1.54 | 0.11 |
| CD3+CD8+ | 0.99 | 0.77, 1.27 | 0.93 | 0.87 | 0.59, 1.28 | 0.48 | 1.16 | 0.98, 1.38 | 0.08 |
| CD3+CD4+CD25+ | 0.84 | 0.59, 1.17 | 0.30 | 0.75 | 0.49, 1.16 | 0.20 | 1.08 | 0.86, 1.35 | 0.51 |
| Tcon | 0.91 | 0.69 1.20 | 0.52 | 0.82 | 0.55 1.22 | 0.33 | 1.21 | 1.01 1.46 | 0.04 |
| Treg:Tcon | 0.99 | 0.56, 1.73 | 0.96 | 1.19 | 0.51, 2.78 | 0.69 | 0.71 | 0.49, 1.04 | 0.08 |
| Treg:CD8 | 0.71 | 0.37, 1.37 | 0.30 | 0.92 | 0.34, 2.53 | 0.87 | 0.73 | 0.46, 1.15 | 0.17 |
| CD19+ | 0.84 | 0.67, 1.06 | 0.14 | 0.77 | 0.56, 1.05 | 0.10 | 1.03 | 0.92, 1.15 | 0.66 |
| CD3−CD56+ | 0.99 | 0.71, 1.37 | 0.94 | 1.02 | 0.61, 1.69 | 0.95 | 0.90 | 0.74, 1.11 | 0.32 |
| Total WBC | 1.74 | 1.08, 2.81 | 0.02 | 0.90 | 0.45, 1.79 | 0.76 | 0.98 | 0.70, 1.36 | 0.89 |
| ALC | 0.93 | 0.62, 1.39 | 0.71 | 0.60 | 0.32, 1.12 | 0.11 | 1.15 | 0.88, 1.50 | 0.30 |
| cDC (Lin−/HLADR+/CD123−/CDllc+) | 0.992 | 0.881 0.117 | 0.89 | 0.995 | 0.821 1.206 | 0.96 | 1.009 | 0.92 1.107 | 0.84 |
| pDC (Lin−/HLADR+/CD123+/CDllc−) | 0.956 | 0.803 1.138 | 0.61 | 0.985 | 0.756 1.284 | 0.91 | 0.956 | 0.866 1.056 | 0.37 |
Among other cell subsets, increased number of B-lymphocytes (CD19+) was associated with improved OS and NRM but did not correlate with other outcomes; increased number of NK cells (CD3−CD56+) was also associated with overall survival. None of the cell subtypes were associated with relapse except WBC count, where increased counts were associated with reduced relapse rates. (Table 2A and 2B).
We repeated this analysis limiting immune subsets measured at early time points only (1 month and 3 months). The result remains largely similar except that the effect of CD19+ cells was not found to be significant (Supplementary Table 1), reflecting delayed (after 3 months) CD19+ reconstitution.
Discussion:
The recovery of various immune subsets following HCT is a gradual process and can take up to a year to approximate levels found in healthy individuals. Typically, the innate immune system (granulocytes, monocytes, NK cells) recovers in the first few weeks following transplant followed by T- and Blymphocyte recovery over a period of months. Usually CD4+ T-lymphocytes recover slower than CD8+ Tcells13. The recovery of various T-cell subsets has been studied more comprehensively in recent years, and there has been particular interest in Tregs. Tregs normally comprise 5–10% of circulating Tlymphocytes and are instrumental in controlling effector T-cell immune responses in sites of inflammation14. They have an important role in the immune system, where poor Treg recovery has been significantly associated with both acute and chronic GVHD14. In general, an imbalance between recovery of Tregs and Tcons has been associated with cGVHD13. In-vitro studies have suggested that sirolimus has a Treg sparing effect with subsequent beneficial effects on GVHD in murine models11,15. The effect of sirolimus in combination with a calcineurin-inhibitor (tacrolimus) in-vivo, however, may or may not reflect the effects seen in murine models.
We present here a comprehensive analysis of the randomized trial BMT-CTN 0402, comparing immune reconstitution in the Tac/Sir and Tac/MTX arms in an attempt to better delineate the in vivo effect of sirolimus. This was a unique opportunity to explore the effect of sirolimus on recovery of immune subsets without significant confounders and biases, since the arms were randomized, and flow cytometry was performed in a blinded fashion.
Patients who received Tac/Sir had compromised T-cell reconstitution in the early post-transplant period with significantly lower CD3+, CD4+ , Tcon and ALC in the first 3 months after transplantation compared with the Tac/MTX arm. Sirolimus specifically blocks T-cell proliferation via mTOR inhibition, by acting at a different point in the cell cycle than tacrolimus4; hence this synergistic effect on T-cell suppression is not unexpected. Interestingly the T-cell subset most profoundly affected in the Tac/Sir arm were CD8+ T-lymphocytes, which were significantly lower in this arm up to 6 months after transplantation.
We noted that there was no significant difference at the 0.01 level in Treg reconstitution when the Tac/Sir arm was compared with the Tac/MTX arm at any time-point. Although the Treg level was somewhat lower in the Tac/Sir arm early after HCT, the relative difference in the Treg level was much smaller than the significant differences seen in Tcon and CD3+CD8+.This is consistent with previous studies in murine models suggesting that sirolimus spares Tregs11,15,16. In humans, a calcineurin-inhibitor free transplant platform (Fludarabine/treosulfan/ATG-Fresenius conditioning with post-transplant cyclophosphamide and sirolimus for GVHD prophylaxis) in the haploidentical setting, showed that Treg numbers were significantly higher while patients were on sirolimus (day +30) compared to when they had been weaned off (day+180)17. This further suggests that the Treg sparing effect of sirolimus may have been more pronounced if tacrolimus had not been used concomitantly for GVHD prophylaxis, as it was in BMT-CTN 0402. To investigate this issue more comprehensively, we examined the Treg:Tcon ratio in the two arms and found that it was significantly higher in the Tac/Sir arm up to 3 months after transplantation, however this effect was lost at later time-points. We have shown in the past that a higher Treg:Tcon ratio at 90 days after transplantation is associated with lower rates of cGVHD13, and therefore the effect of sirolimus on this ratio may be indicative of its efficacy as a GVHD prophylaxis agent, at least in the context of cGVHD. The Treg:CD8 ratio was also significantly increased in the Tac/Sir arm up to 3 months after transplantation. Thus, although the absolute numbers of Tregs are not higher in the Tac/Sir arm in-vivo, sirolimus affects the balance between regulatory and effector cells in favor of regulatory T cells which has important implications for GVHD prevention. It should be noted that the effect of different rates of tapering of immunosuppression have not been accounted for in this analysis since this data was not available.
We performed further analysis of CD4+ and CD8+ T-cell recovery to determine whether sirolimus preferentially affected naïve or mature cell subsets. Within the CD4+ compartment, proliferation of naïve T-cells (Ki-67+) was lower in the first month after transplantation, and the number of naïve cells was significantly lower up to 3 months after transplantation in the Tac/Sir arm. CD4+ effector cells were significantly decreased in the sirolimus arm for only 1 month suggesting that naïve CD4 T cells were preferentially affected by sirolimus. In contrast, CD8+ effector cells were compromised up to 6 months indicating that sirolimus affected CD8 cell recovery more than CD4 T cells.
We found that the absolute numbers of CD19+ B-lymphocytes were similar in both arms at 1 month, but recovery was slower in the Tac/Sir arm at 3 and 6 months after transplantation. The effects of sirolimus on B-lymphocytes has been investigated in-vitro, but has never been analyzed in-vivo in the context of HCT. Using purified human B-lymphocytes from healthy volunteers, sirolimus profoundly inhibited B-cell proliferation at clinically relevant concentrations in-vitro. In contrast, tacrolimus had minimal effect on B-cells18,19. Hence the effect of B-lymphocytes seen in the Tac/Sir arm is likely a direct effect of sirolimus. The implications of this in the context of cGVHD are likely complex. The role of B-cells in the pathogenesis of cGVHD has been highlighted in recent years. A large diverse mature B-cell pool contains B-lymphocytes which can sequester B-cell activating factor (BAFF); subsequently autoreactive Blymphocytes which require BAFF to survive are not able to proliferate and mediate cGVHD20. It is possible that sirolimus depletes both alloreactive and autoreactive B-lymphocytes and therefore eventually does not have a significant effect on the incidence of cGVHD. Further studies with concomitant measurement of BAFF levels in patients who receive sirolimus may inform this issue further.
In the outcomes analysis performed on this subset, no cell subtype was associated with acute or chronic GVHD including Tregs and the Treg:Tcon ratio. However higher absolute numbers of all T-cell subtypes as well as B-lymphocytes were associated with better OS. This likely reflects more robust immune reconstitution. Relapse was not affected by any cell subtype at the 0.01 level although CD3+, CD8+, CD19+, and NK cells are borderline associated with relapse (0.01<p-values<0.05).
We acknowledge a limitation of our study in that we did not correlate infectious complications, specifically cytomegalovirus and other viral reactivations with immune reconstitution, and this should be studied further in the future. It should be noted that in the parent RCT, there was no difference between arms with regards to infectious complications/infectious dates10. We acknowledge that the definition used for Tregs (CD3+CD4+CD25+) could be refined further by current standards by the addition of Foxp3 or CD127 to the phenotypic definition and we will pursue this in follow-up studies. We further acknowledge that since this is a retrospective analysis of an existing dataset with multiple unplanned analyses, there is an increase in the possibility of a type 1 error.
In conclusion, we describe the effect of sirolimus used as GVHD prophylaxis on immune reconstitution post-transplant, in the context of a large randomized controlled trial. Sirolimus in combination with tacrolimus does have a more profound T-cell suppressive effect than the combination of tacrolimus/methotrexate in the early post-transplant period, and particularly compromises recovery of CD8+ T-cells with potential implications in the prevention of aGVHD. Sirolimus when used with tacrolimus does not appear to increase absolute numbers of Tregs (defined as CD4+CD25+ T-cells), but might have a beneficial effect on the Treg:Tcon balance in the first 3 months after transplantation. This also suggests that calcineurin-inhibitor free, sirolimus containing GVHD prophylaxis strategies, incorporating other novel agents (for e.g. OX40L blocking antibody KY1005 as shown by Kean et al21), should be investigated further to maximize the potential favorable effect of sirolimus on Treg:Tcon balance in the post-transplant immune repertoire. Tregs should be defined more rigorously (preferably as CD4+CD25+Foxp3+ cells) to more comprehensively validate these results. Finally, sirolimus significantly compromises B-cell recovery in the first 6 months after HCT with potential complex effects on cGVHD which merit further study.
Supplementary Material
Highlights.
Tacrolimus/Sirolimus (Tac/Sir) has a more profound T-cell suppressive effect than tacrolimus/methotrexate (Tac/MTX) post transplant
CD8+ T-cells are the slowest to recover among T-cell subsets with potential implications in the prevention of acute graft-versus-host disease (aGVHD)
Tregs are similar in the Tac/Sir and Tac/MTX arms, but the Treg:Tcon ratio is greater in the sirolimus arm in the first 3 months and could affect chronic GVHD
B-lymphocytes are significantly decreased in the Tac/Sir arm upto 6 months posttransplant
Acknowledgements:
Support for this study was provided by grant #U10HL069294 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute, along with contributions by Wyeth Pharmaceuticals Inc. BMT CTN 0402 biospecimens were obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the above mentioned parties
Additional support was provided by the Division of Allergy, Immunology, and Transplantation, National Institute of Allergy and Infectious Diseases for the ancillary study, ‘GVHD Prophylaxis – Immune Reconstitution
We would like to acknowledge the contributions of all the participating centers and principal investigators at each participating center and the patients who participated in BMT CTN 0402
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med 2017; 377: 2565–79. [DOI] [PubMed] [Google Scholar]
- 2.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062–8. [PubMed] [Google Scholar]
- 3.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003; 35: 7S–14S. [DOI] [PubMed] [Google Scholar]
- 4.Vu MD, Qi S, Xu D, et al. Tacrolimus (FK506) and sirolimus (rapamycin) in combination are not antagonistic but produce extended graft survival in cardiac transplantation in the rat. Transplantation 1997; 64: 1853–6. [DOI] [PubMed] [Google Scholar]
- 5.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 2003; 102: 1601–5. [DOI] [PubMed] [Google Scholar]
- 6.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 2004; 10: 328–36. [DOI] [PubMed] [Google Scholar]
- 7.Cutler C, Stevenson K, Kim HT, et al. Double Umbilical Cord Blood Transplantation with Reduced Intensity Conditioning and Sirolimus-Based GVHD Prophylaxis. Bone Marrow Transplant 2011; 46: 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood 2008; 112: 4425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pidala J, Kim J, Jim H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica 2012; 97: 1882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014; 124: 1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 2008; 111: 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Won J- H, Park SK, Kim SH, Yun J. The Effect of Sirolimus Based Regimen On Immune Reconstitution After Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant 2013; 19: S210. [Google Scholar]
- 13.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood 2016; 127: 646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koreth J, Ritz J. Tregs, HSCT, and acute GVHD: up close and personal. Blood 2013; 122: 1690–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiyama H, Maeda Y, Nishimori H, et al. Mammalian Target of Rapamycin Inhibitors Permit Regulatory T Cell Reconstitution and Inhibit Experimental Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant 2014; 20: 183–91. [DOI] [PubMed] [Google Scholar]
- 16.Coenen JJA, Koenen HJPM, van Rijssen E, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant 2007; 39: 537–45. [DOI] [PubMed] [Google Scholar]
- 17.Peccatori J, Forcina A, Clerici D, et al. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia 2015; 29: 396–405. [DOI] [PubMed] [Google Scholar]
- 18.Traitanon O, Mathew JM, La Monica G, Xu L, Mas V, Gallon L. Differential Effects of Tacrolimus versus Sirolimus on the Proliferation, Activation and Differentiation of Human B Cells. PloS One 2015; 10: e0129658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aagaard-Tillery KM, Jelinek DF. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol 1994; 156: 493–507. [DOI] [PubMed] [Google Scholar]
- 20.Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood 2015; 125: 1703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.