Abstract
Background
Transanal Minimally Invasive Surgery (TAMIS) has revolutionized local excision of mid and high rectal lesions; benign or malignant. It is a technique that is developed as a hybrid between Transanal Endoscopic Microsurgery (TEM) and laparoscopic surgery for resection of rectal lesions.
Methods
We retrospectively reviewed prospectively collected data on patients who underwent TAMIS for benign and early malignant rectal lesions between Jan 2015 and Sept 2019, at Hamad General Hospital, Doha, Qatar.
We assessed the following outcomes: feasibility, fragmentation of specimen, operative time, length of stay (LOS) post-operative complications, and margin negativity.
Results
Seventeen consecutive patients underwent TAMIS for benign and malignant rectal lesions. The average length of stay (LOS) is 1.5 days (1–6 days). Seven patients had different types of benign adenomas, five patients had proven adenocarcinoma, three patients had well-differentiated neuroendocrine tumors, one patient with hyperplastic polyp, and one patient had inflammatory polyp. No fragmentation occurred or detected by histopathologic examination, except in a patient who had inflammatory polyp, where the lesion removed in two fragments.
Conclusion
TAMIS procedure is feasible and safe even in a relatively low-volume colorectal unit. Using this tool, many patients can avoid unnecessary radical surgery. Therefore, we believe that TAMIS should form part of every specialized colorectal service repertoire. To our knowledge, this is the largest series in the gulf region.
Keywords: TAMIS, Rectal tumors
Background
Management of rectal lesions, benign or malignant, evolved with the improvement of surgical tools and techniques. Total mesorectal excision (TME) and proctectomy remain the gold standard curative procedure [1, 2]. Based on evidence from multiple large randomized controlled trials (RCTs), minimally invasive TME operations using laparoscopy and robotic systems have been implemented and used widely across the globe.
Rectal lesions are increasingly detected with increased screening and awareness. This is connected to improved tools of diagnosis and management of these lesions.
Local excision (LE) of benign and early malignant rectal lesions was traditionally described to remove lesions within 8 cm from the anal verge [3]. Higher lesions in the rectum rendered amenable for local excision using Transanal Endoscopic Microsurgery system described by Buess et al. more than three decades ago [4]. It became more attractive for many reasons; such as better functional outcomes, less morbidity, faster recovery and avoidance of radical resections [3].
Transanal Minimally Invasive Surgery (TAMIS) has revolutionized local excision of mid and high rectal lesions; benign or malignant. Since its introduction in 2009 by Atallah et al., this hybrid tool between single port laparoscopy and Transanal Endoscopic Microsurgery (TEM), has gained wider acceptance and popularity than TEM or the traditional Parks Transanal Excision (TAE), due to its superior surgical outcomes, shallow learning curve, and low cost, in addition to easier setup and more flexibility during the procedure [5].
Methods
We retrospectively reviewed prospectively collected data on patients who underwent TAMIS for benign and early malignant rectal lesions between Jan 2015 and Sept 2019, at Hamad General Hospital, Doha, Qatar. All cases were discussed in colorectal multidisciplinary team (MDT) and the procedures were done by a single colorectal surgeon or under his direct guidance.
For malignant lesions, preoperative staging accomplished by CT scan and Rectal MRI. All cases were discussed in the tumor board before the procedure.
We assessed the following outcomes: feasibility, fragmentation of specimen, operative time, length of stay (LOS) post-operative complications, and margin negativity.
All patients underwent TAMIS in Lioyd-Davies position under general anesthesia (GA), except one patient who refused GA and had spinal anesthesia. We used GelPOINT path transanal access platform, and maintained pneumorectum to pressure of 15 mmHg with AirSeal system insufflator. As a standard operative preparation; all patients were positioned in Lloyd Davis position with a Bean Bag underneath the patient, in case laparoscopic access to the peritoneal cavity was to be required. All patients were given rectal phosphate enema approximately 4 h before the procedure. All patients received standard DVT prophylaxis. Prophylactic antibiotics were also administered at induction of anesthesia.
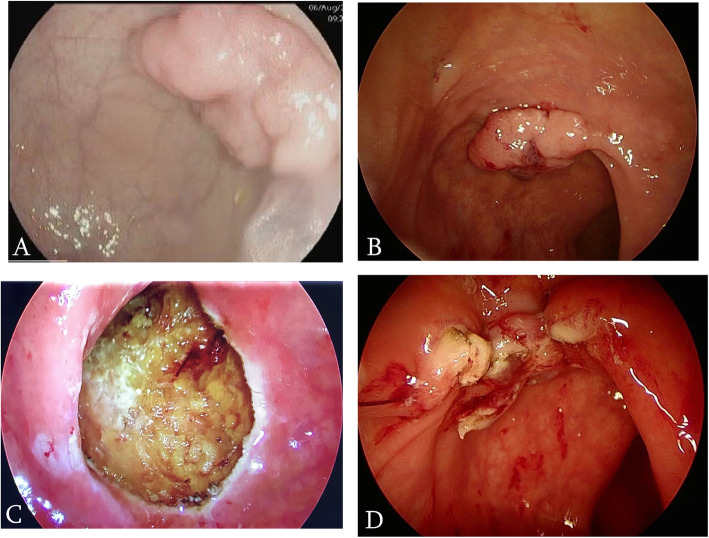
We used the GelPOINT path transanal access platform (Applied Medical, Rancho Santa Margarita, CA), AirSeal port (Conmed, NY, USA) to establish and sustain pneumorectum, and 30 degrees scope. We always approximate the defect after excision with V-Loc™ Vicryl (Medtronic, Minneapolis, MN, USA) The specimen then sent oriented to the histopathology department (Fig. 1).
Fig. 1.
a Colonoscopic appearance of rectal sessile polyp. b TAMIS view of the same polyp. c the polyp post TAMIS resection before closure. d resection site post closure
Results
Seventeen consecutive patients underwent TAMIS for benign and malignant rectal lesions, in the period between Jan 2015 and September 2019. The patients’ population consisted of 6 women (34%) and 11 men (64%), with an average age of 52 years (28–88). Nine patients had an ASA score of 2, six patients of ASA score 3, and three patients with an ASA score of 1 (Table 1).
Table 1.
Patients demographics
| Patients, n | 17 |
| • Men | 11 (64%) |
| • Women | 6 (34%) |
| Age (years) | 52 (28–88) |
| ASA Score | |
| • I | 3 |
| • II | 9 |
| • III | 5 |
The average length of stay (LOS) is 1.5 days (1–6 days). Eleven out of the eighteen patients discharged on day 1 post operatively. One patient stayed 6 days because of post-operative hypotension which was managed conservatively.
The average operative time was 74.17 min (20–180 min). The average distance of the lesion from the anal verge is 7.47 cm (3–18 cm). Eight patients (47%) had the lesion > 7 cm from the anal verge.
Seven patients had different types of benign adenomas, five patients had proven adenocarcinoma, three patients had well-differentiated neuroendocrine tumors, one patient with hyperplastic polyp, and one patient had inflammatory polyp.
No fragmentation occurred or detected by histopathologic examination, except in a patient who had inflammatory polyp, where the lesion removed in two fragments. The average size of the excised lesions is 2.62 cm (1.2–7 cm). All resection margins were free, the nearest margin was 4 mm in a tubular adenoma specimen. Six patients had a discrepancy between the preoperative and the post-operative histopathology; one of the patients down-staged from polyp adenocarcinoma to polyp high-grade dysplasia. Another patient was diagnosed as moderately differentiated adenocarcinoma pre-operatively; which is located 5 cm from the anal verge, down-staged to benign lesion on final histopathology following TAMIS excision. Both of these patients were spared radical resections (Table 2).
Table 2.
Tumor characteristics and histopathology for locally excised lesions
| Distance from anal verge, cm (range) | 7.41 (3–18) |
| Tumor size, cm (range) | 2.62 (1.2–7) |
| Benign | |
| • Hyperplastic | 1 |
| • Adenoma | 6 |
| • Inflammatory | 1 |
| Cancer | |
| • T0 | 6 |
| • T1 | 1 |
| • T2 | 4 |
| Neuroendocrine Tumor | 3 |
| Discrepancy (pre-op to post-op) | |
| • Adenoma to Hyperplastic polyp | 1 |
| • Hyperplastic polyp to adenoma | 1 |
| • Adenoma to Polyp cancer | 1 |
| • Polyp cancer to polyp high-grade dysplasia | 1 |
| • Low-grade dysplasia to adenoma | 1 |
| • Polyp high grade dysplasia to polyp cancer | 1 |
| • Fragmentation | 1 |
| • Positive margins | None |
| • Procedure-related complications (30 days) |
Bleeding not requiring transfusion (one patient) Intraoperative peritoneal perforation, repaired immediately (one patient) |
In one patient, the peritoneum is entered during the excision of a tubular adenoma 10 cm from the anal verge. Laparoscopic closure of the peritoneum was achieved as well as transanal closure. The patient was discharged 2 days post operatively, with no complications.
There was one procedure-related complication during excision of a serrated adenoma located 10 cm from the anal verge, were the peritoneum was breached. Immediate laparoscopic repair was done, and the patient discharged home on day 2 post-operatively with no complications.
Another patient had minimal post-operative fresh bleeding per rectum which required no transfusion.
There were no other procedure-related immediate or 30-day complications in any of the other patients.
Discussion
Transanal Minimally Invasive Surgery (TAMIS) is a technique that is developed as a hybrid between Transanal Endoscopic Microsurgery (TEM) and laparoscopic surgery for resection of rectal lesions. Adoption of the technique has spread widely due to availability of the laparoscopic tools and insufflators and the single-site port, as well as the shallow learning curve, and most importantly the comparable safety and oncologic outcomes.
NCCN guidelines have defined the lesions which are appropriate for local excision using any system: mobile rectal tumors, less than 3 cm in size, occupying less than one-third of the circumference of the bowel, not extending beyond the submucosa, with well to moderate differentiation, and low-risk histopathological features. Transanal local excision is not appropriate for rectal tumors with high-risk characteristics, including lymphovascular invasion, perineural invasion, and mucinous components [1].
We have demonstrated a congruent result of safety and oncologic outcomes using TAMIS compared to the existing literature and case series [6–8]. All our malignant lesion resections as well as NET resections were margin negative. The reported positive margin in a recent large series of 200 patients was 7% [9], and up to 2 out of 3 NET resections in some series [10] (Tables 3 and 4).
Table 3.
Characteristics of individual cases
| No | Age group / Sex | Location (cm) | Pre-operative HP | Indication | Tumor size (cm) | Post-operative HP | Need for further management |
|---|---|---|---|---|---|---|---|
| 1 | 35–45/ S2 | 7 | Tubulovillous adenoma with low grade dysplasia | Treatment | 2.8 × 2.5 | Tubulovillous adenoma | None |
| 2 | 45–55/ S2 | 18 | Serrated adenoma with low grade dysplasia | Treatment | 1.5 × 0.5 | hyperplastic polyp | None |
| 3 | 55–65/ S1 | 5 | Moderately differentiated adenocarcinoma | Treatment | 2.2 × 1.7 | pT0Nx adenocarcinoma | None |
| 4 | 55–65/ S2 | 10 | Serrated adenoma | Treatment | 2.5 × 2.2 | Serrated adenoma | None |
| 5 | 55–65/ S2 | 10 | Hyperplastic polyp | Treatment | 2.5 × 2.2 | Tubular adenoma | None |
| 6 | 25–35/ S1 | 4 | Well differentiated NET | Treatment | 2.7 × 2.2 cm (tumor 4 mm) | Well differentiated NET | None |
| 7 | 55–65/ S1 | 10 | Tubulovillous adenoma | Treatment | 2.3 (cancer 2 mm) | Polyp Moderately differentiated adenocarcinoma (Haggit’s 1) | None |
| 8 | 85–95/ S1 | 3 | Moderately differentiated adenocarcinoma (cT2 / early T3 N0 M0) | Treatment | 3.9 × 2.3 | Moderately differentiated adenocarcinoma (pT2) | None |
| 9 | 55–65/ S2 | 8 | Well differentiated NET | Treatment | 1.2 × 0.6 mm | Well differentiated NET | None |
| 10 | 35–45/ S1 | 4 | Moderately differentiated invasive adenocarcinoma, arising in a tubular adenoma with high-grade dysplasia | Treatment | 1.5 × 0.7 | Tubular adenoma with high-grade dysplasia | None |
| 11 | 65–75/ S2 | 3 | Villous adenoma with at least high-grade dysplasia and suspicions cancer | Treatment | 3 × 2 × 0.5 | Moderately differentiated adenocarcinoma cT1N0M0 | None |
| 12 | 35–45/ S1 | 5 | Well differentiated NET | Treatment | 2.3 × 1.7 × 0.7 (nodule 0.8 cm) | Well differentiated NET | None |
| 13 | 25–35/ S1 | 9 | Low grade dysplasia | Treatment | 7 × 5 × 4 cm | Villous adenoma | None |
| 14 | 45–55/ S1 | 5 | Tubulovillous adenoma with focal high-grade dysplasia | Treatment | 3 × 2.5 × 1.5 | Tubulovillous adenoma | None |
| 15 | 35–45/ S1 | 10 | Well differentiated adenocarcinoma on tubulovillous adenoma (incomplete colonoscopic removal) | Treatment | 4.5 × 3.6 × 2 | Residual well differentiated adenocarcinoma on tubulovillous adenoma (SM3) | None |
| 16 | 45–55/ S1 | 5 | None | Diagnostic and treatment | 2.7 × 1.8 × 0.8 cm and 2.8 × 2 × 0.4 cm | benign inflammatory cloacogenic polyp/ mucosal prolapse. | None |
| 17 | 55–65/ S1 | 10 | Tubulovillous adenoma with focal high-grade dysplasia | Treatment | 2.5 × 1.7 × 1.8 cm | Moderately differentiated invasive adenocarcinoma with mucinous component in a background of tubulovillous adenoma with focal high grade dysplasia. Kikuchi SM2 | None |
Table 4.
Characteristics of malignant lesions excised using TAMIS
| No | Age group/sex | Location (cm) | Pre-operative HP | Colonoscopy findings | MRI findings | Indication | Tumor size (cm) | Post-operative HP | Need for further management |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55–65 / S1 | 5 | Moderately differentiated adenocarcinoma (reaching inked margin) | Sessile polyp in the rectum (snared) | No gross lesion found | Treatment | 2.2 × 1.7 | pT0, no residual; tumor | None |
| 2 | 55–65 / S1 | 10 | Tubulovillous adenoma | Flat polyp | Lesion in mid-rectum confined to muscularis propria | Treatment | 2.3 (cancer 2 mm) | Polyp Moderately differentiated adenocarcinoma (Haggit’s 1) | None |
| 3 | 85–95 / S1 | 3 | Moderately differentiated adenocarcinoma (cT2 / early T3 N0 M0) | Ulceroproliferative lesion in the rectum | No MRI. EUS showed T2 / early T3 | Treatment, patient unfit for radical resection | 3.9 × 2.3 | Moderately differentiated adenocarcinoma (pT2) | None |
| 4 | 35–45 / S1 | 4 | Moderately differentiated invasive adenocarcinoma, arising in a tubular adenoma with high-grade dysplasia | 2 cm rectal lesion (infiltrating the mucosa) | Definite polypoidal lesion at the lower rectum T1/T2 with thickened CRM | Treatment | 1.5 × 0.7 | Tubular adenoma with high-grade dysplasia | None |
| 5 | 75–85 / S2 | 3 | Villous adenoma with at least high-grade dysplasia and suspicions cancer | Rectal mass with query malignant features | No MRI. EUS: T3 lesion | Treatment | 3 × 2 × 0.5 | Moderately differentiated adenocarcinoma cT1N0M0 | None |
| 6 | 35–45 / S1 | 10 | Well differentiated adenocarcinoma on tubulovillous adenoma (incomplete colonoscopic removal) | Large rectal polyp 10 cm from anal verge, with broad base. Removed incompletely in fragments | Lesion in the upper rectum could not be clearly appreciated because of metallic clips placed after polypectomy | Treatment | 4.5 × 3.6 × 2 | Residual well differentiated adenocarcinoma on tubulovillous adenoma (SM3) | None |
| 7 | 55–65 / S1 | 10 | Tubulovillous adenoma with focal high-grade dysplasia | Sessile polypoid lesion with central depression, about 1.5 cm in size, at 10 cm from anal verge | Right posterolateral polypoidal wall thickening measuring approximately 15 mm, with central hyperintensity; no significant diffusion restriction or hyperenhancement. No extension beyond the muscularis. | Treatment | 2.5 × 1.7 × 1.8 cm | Moderately differentiated invasive adenocarcinoma with mucinous component in a background of tubulovillous adenoma with focal high grade dysplasia. Kikuchi SM2 | None |
Our series showed that despite the fact that a relatively small number of patients dispersed over a period of nearly 5 years, the surgical outcomes, operative time, and quality of specimens did not compare unfavorably. One of our patients had a lesion 10 cm from anal verge with malignant features of T3 tumor by MRI, which was difficult to diagnose using colonoscopy due to superficial biopsy. TAMIS used in this patient to obtain adequate biopsies, which confirmed adenocarcinoma enabling the patient to receive neoadjuvant chemoradiotherapy.
Conclusions
TAMIS procedure is feasible and safe even in a relatively low-volume colorectal unit. Using this tool, many patients can avoid unnecessary radical surgery. Therefore, we believe that TAMIS should form part of every specialized colorectal service repertoire. To our knowledge, this is the largest series in the gulf region.
Acknowledgements
The publication of this article was funded by the Qatar National Library.
Abbreviations
- TAMIS
Transanal Minimally Invasive Surgery
- TEM
Transanal endoscopic microsurgery
- TAE
Transanal excision
- LE
Local excision
- MDT
Multidisciplinary team
- NET
Neuroendocrine tumor
- LOS
Length of stay
- ASA
American College of Anesthesiologists Physical Status Classification
- RCT
Randomized controlled trial
- CT scan
Computerized tomography scan
- MRI
Magnetic resonance imaging
Authors’ contributions
AGA Data acquisition and analysis, drafting the manuscript, AA Data analysis and critical revision of manuscript. MA Data acquisition and critical revision of manuscript. MK: Study concept and design, data analysis, writing and revision of the manuscript. All authors have read and approved the manuscript.
Funding
The processing fees for publication of this article was funded by the Qatar National Library.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. Abutaka, Email: aabutaka@hamad.qa
A. Ahmed, Email: aahmed40@hamad.qa
M. Abunada, Email: mabunada@hamad.qa
M. Kurer, Email: mkurer@hamad.qa
References
- 1.Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol. 2014;5(5):345–352. doi: 10.3978/j.issn.2078-6891.2014.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller DS, Haas EM. Transanal minimally invasive surgery: state of the art. J Gastrointest Surg. 2016;20(2):463–469. doi: 10.1007/s11605-015-3036-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee TG, Lee SJ. Transanal single-port microsurgery for rectal tumors: minimal invasive surgery under spinal anesthesia. Surg Endosc. 2014;28(1):271–280. doi: 10.1007/s00464-013-3184-0. [DOI] [PubMed] [Google Scholar]
- 4.Buess G, Hutterer F, Theiss J, Bobel M, Isselhard W, Pichlmaier H. A system for a transanal endoscopic rectum operation. Chirurg. 1984;55(10):677–680. [PubMed] [Google Scholar]
- 5.Caycedo-Marulanda A, Jiang HY, Kohtakangas EL. Transanal minimally invasive surgery for benign large rectal polyps and early malignant rectal cancers: experience and outcomes from the first Canadian centre to adopt the technique. Can J Surg. 2017;60(6):416–423. doi: 10.1503/cjs.002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert MR, Atallah SB, DeBeche-Adams TC, Izfar S, Larach SW. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56(3):301–307. doi: 10.1097/DCR.0b013e31827ca313. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18(9):775–788. doi: 10.1007/s10151-014-1148-6. [DOI] [PubMed] [Google Scholar]
- 8.McLemore EC, Weston LA, Coker AM, et al. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg. 2014;208(3):372–381. doi: 10.1016/j.amjsurg.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Lee L, Burke JP, Debeche-Adams T, et al. Transanal minimally invasive surgery for local excision of benign and malignant rectal neoplasia. Ann Surg. 2018;267(5):910–916. doi: 10.1097/SLA.0000000000002190. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Peng YF, Yao YF, Gu J. Trans-anal minimally invasive surgery for rectal neoplasia: experience from single tertiary institution in China. World J Gastrointest Oncol. 2018;10(6):i-144. doi: 10.4251/wjgo.v10.i6.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.