Abstract
BACKGROUND
Gastric cancer (GC) remains an aggressive malignancy with a high rate of mortality, being the third leading cause of cancer-related death. More than one million newly diagnosed cases and 782685 deaths due to GC were reported in 2018. GC is characterized by limited effective treatment options and the lack of consistent biomarkers for the diagnosis and prognosis of these patients. The discovery of new biomarkers useful in the early diagnosis of GC is mandatory.
AIM
To evaluate the potential of COL10A1 as a circulating biomarker for the diagnosis and prognosis of gastric adenocarcinoma patients.
METHODS
Plasma and tissue obtained from 49 patients with gastric adenocarcinoma have been used in exploring the expression of COL10A1. Real-time PCR and western blot techniques were used to evaluate COL10A1 level in gastric tumor tissue compared to normal adjacent tissue. The circulating level of COL10A1 was also evaluated by ELISA in plasma of gastric adenocarcinoma patients. Survival analysis was made in order to evaluate the potential of COL10A1 as a biomarker for the diagnosis and prognosis of gastric adenocarcinoma patients.
RESULTS
Our results showed a significant increase in COL10A1 gene expression and protein levels in gastric tumor tissue compared to adjacent normal tissue (P < 0.05). COL10A1 seems to show an elevated expression from the beginning of carcinogenesis, in the early stages, and its increased level remains elevated during cancer progression. A significant increase of COL10A1 plasma level in gastric adenocarcinoma patients was also identified. Moreover, increased COL10A1 plasma level was associated with poor survival of the patients. Plasma COL10A1 performed a diagnostic value in GC with area under the receiver operating characteristic curve (AUC) of 0.9171 (P = 0.0002), sensitivity of 87.76%, and specificity of 100.0%. Furthermore, this study demonstrated the potential role of plasma COL10A1 in the early detection of GC, as in the early stage, we obtained an AUC of 0.8789 (P = 0.0030), sensitivity of 81.25%, and specificity of 100.0%.
CONCLUSION
Circulating expression level of COL10A1 is significantly increased in gastric adenocarcinoma patients being associated with poor survival and is a potential biomarker for early detection of GC.
Keywords: Gastric cancer, COL10A1, Circulating biomarkers, Early diagnosis, Poor prognosis, Tumor stage
Core tip: Gastric cancer remains an aggressive malignancy with a high rate of mortality, characterized by limited effective treatment options and the lack of consistent biomarkers for the diagnosis and prognosis of the patients. The aim of this study was to evaluate the potential of COL10A1 as a circulating biomarker for the diagnosis and prognosis of gastric adenocarcinoma patients. Our results suggest that COL10A1 could be considered a good biomarker for prognosis and also for early detection, as its elevated expression occurred early and remained elevated during cancer progression.
INTRODUCTION
Despite the major advances in the field of personalized medicine, gastric cancer (GC) remains a disease with a high rate of mortality, representing the third leading cause of cancer-related deaths, with approximately one million newly diagnosed cases reported every year. According to GLOBOCAN data, 782685 deaths due to GC were reported in 2018. GC is still characterized by late diagnosis, limited effective treatment options, and lack of reliable biomarkers for the patient outcome prediction and response to therapy. The main issue in the management of this disease is represented by the high molecular heterogeneity that results in the phenotypical aggressiveness of GC and limits the antitumor efficacy of the targeted therapy[1-3].
Gastric adenocarcinomas represent about 90% of GC cases and can be subdivided, based on Lauren’s criteria, in two major histologic subtypes: intestinal type (54%) and diffuse type (32%) adenocarcinoma, plus indeterminate type (15%) as an uncommon variant. Unfortunately, this classification system has a limited clinical utility, and the necessity of introducing molecular testing became obvious[4]. In 2014, Bass et al[5] made a comprehensive molecular evaluation of 295 primary gastric adenocarcinomas as part of The Cancer Genome Atlas project. Using several modern molecular assays such as copy number analysis, whole-exome sequencing, DNA methylation profiling, and messenger RNA-sequencing, they successfully identified many genomic alterations (insertions, deletions, CNV), DNA hypermethylation and amplifications[5]. That allowed the development of a new classification of GC into four molecular subtypes and also pointed out several biomarkers that can be used for the development of new screening strategies and targeted therapies[6,7]. These biomarkers hold the key to improve the early detection of GC and survival rates.
Currently, the diagnosis of gastrointestinal tumors relies on an invasive technique such as endoscopy and on several tumor markers used in the clinic for early tumor detection without high specificity such as carcinoembryonic antigen (CEA), the carbohydrate antigens (CA): CA19-9, pepsinogen, and also α-fetoprotein (AFP). Due to these inconveniences, the diagnostic rate of early-stages GC is very low. Circulating biomarkers that can be detected by serological tests are considered simpler due to non-invasive sample collection method and to high-throughput screening application.
We identified COL10A1 in a previous study focused on the molecular characterization of gastric tumorigenesis, among other most up-regulated genes, as the second overexpressed gene (fold change, 72.55) in gastric tumor tissue compared to normal one[8]. This gene microarray data have been deposited in the Gene Expression Omnibus database, accession no. GSE103236. COL10A1, a member of the collagen family, is a gene with limited expression in most normal tissues and elevated expression in several tumor types[9-11]. Interesting, COL10A1 gene expression is also found to be overexpressed in all the four GC subtypes described by Bass et al[5]. Therefore, we intended to propose COL10A1 (collagen type X alpha 1 chain) as a new circulating biomarker for the diagnosis and prognosis of this disease.
MATERIALS AND METHODS
Clinical plasma and tissue samples
Gastric adenocarcinoma tissue samples and adjacent normal tissues were collected from 49 patients (33 men and 16 women, mean age 61 years) during surgery at the Center of General Surgery and Liver Transplantation of Fundeni Clinical Institute, after written informed consents and approval of the Ethical Committee were obtained. None of the patients had received preoperative chemotherapy or radiotherapy. Pathologists confirmed all GC diagnoses and selected fresh tissue samples from tumor and adjacent tissue taken from the proximal resection margin. The GC samples were classified according to the American Joint Committee on Cancer tumor, node, and metastasis (TNM) staging system. The tissue samples were frozen in liquid nitrogen immediately after excision and stored at -80 °C. The peripheral venous blood was collected from GC cases, prior to surgery, and cancer-free controls.
Evaluation of the COL10A1 gene expression level in gastric tumor tissue
Total cellular RNA samples were obtained from GC and normal tissue samples using Tri-Reagent (Sigma-Aldrich) and purified with RNeasy mini columns (Qiagen) according to standard procedure. The quality of RNAs was assessed using a 2100 Bioanalyzer (Agilent Technologies). Reverse transcription was performed using 2 μg RNA and Access Quick RTPCR System (Promega) according to the manufacturer protocol, and 50 ng of cDNA from each sample was used in real-time PCR reaction. Real-time PCR was performed on an ABI 7300 real-time PCR System using pre-validated Taqman Gene Expression Assays kits for COL10A1 and 18S (endogenous control). The ΔΔCT method was used to compare the relative expression levels.
Evaluation of the COL10A1 protein expression level in gastric tumor tissue
Whole protein extracts were obtained using T-PER Tissue Protein Extraction Reagent (ThermoFisher) supplemented with Complete O, Mini, EDTA-free Protease Inhibitor Cocktail (Roche Applied Science), and the concentration of total proteins was determined using BCA Protein Assay Reagent (Pierce). A quantity of 60 µg of total proteins for each sample was electrophoretically separated by SDS-PAGE and transferred onto PVDF membranes that were subsequently blocked in Tris-buffer saline - 0.5% Tween 20 with 2% bovine serum albumin and then incubated with the primary antibodies against COL10A1 and β-actin at 4 °C overnight. The antibodies used were rabbit polyclonal anti-collagen X (Abcam, ab58632, 1:500 dilutions) and mouse monoclonal anti-b-actin clone Ac-74 (Sigma Aldrich, 1:1000 dilution). Proteins of interest were detected with the appropriate secondary antibodies (1:1000 dilutions) conjugated with HRP: anti-rabbit IgG (RD Systems), and anti-mouse IgG (RD Systems). Signals were developed using ECL HRP chemiluminescent substrate (Invitrogen) and captured using a MicroChemi 4.2 system (Bio-Imaging Systems).
Measurement of COL10A1 circulating level
Plasma samples from 49 GC cases and 10 cancer-free controls were obtained by centrifugation of the peripheral venous blood for 15 min at 900 × g and stored at -80 °C. The level of COL10A1 expression in plasma of the GC patients and cancer-free controls was measured using Human Collagen alpha-1 (X) chain (COL10A1) ELISA kit (Cusabio) according to the manufacturer instructions.
Statistical analysis
Data analyses were performed using GraphPad Prism 7.0. Statistical significance between the two groups was determined by Student’s t-test. Data are expressed as the mean ± SD. Univariate analyses of survival were performed using the Kaplan-Meier method. The data were censored from the analysis for the surviving patients at the date of the last follow-up. For the determination of the cut-off point in survival analysis, the Cutoff Finder online tool developed by Budczies et al[12] was applied. Receiver operating characteristic (ROC) curves were plotted and area under the ROC curve (AUC) with 95% confidence interval (CI) was calculated to analyze the accuracy of diagnostic value. The cut-off levels on the ROC curves were selected using the Youden’s index [(sensitivity + specificity) -1]. Significance was set at P < 0.05.
RESULTS
In order to identify new biomarkers for diagnosis and prognosis of GC, we analyzed the expression level of COL10A1 in gastric tumor tissues and plasma obtained from 49 patients with gastric adenocarcinoma. The main characteristics of the gastric adenocarcinoma patients included in the study are presented in Table 1.
Table 1.
Clinical and pathological details of patients included in the study, n (%)
| Characteristics | Patients (n = 49) |
| Age (yr): Median | 61 |
| Sex | |
| Men | 33 (67.35) |
| Women | 16 (32.65) |
| Tumor size | |
| ≤ 5 cm | 20 (40.8) |
| 5-7 cm | 17 (34.7) |
| > 7 cm | 12 (24.5) |
| Site of tumor | |
| Upper stomach | 3 (6.1) |
| Middle stomach | 31 (63.3) |
| Lower stomach | 15 (30.6) |
| Type of gastrectomy | |
| Total | 34 (69.4) |
| Subtotal | 15 (30.6) |
| Pathological tumor stage | |
| T1 | 2 (4.1) |
| T2 | 19 (38.7) |
| T3 | 21 (42.9) |
| T4 | 7 (14.3) |
| Pathological nodal stage | |
| N0 | 6 (12.2) |
| N1 | 24 (49) |
| N2 | 15 (30.6) |
| N3 | 4 (8.2) |
| AJCC stage | |
| IB | 1 (2) |
| II | 14 (28.6) |
| IIIA | 15 (30.6) |
| IIIB | 5 (10.2) |
| IV | 14 (28.6) |
| Histological type | |
| Differentiated | 5 (10.2) |
| Undifferentiated | 44 (89.8) |
COL10A1 shows an increased expression level in GC tissue
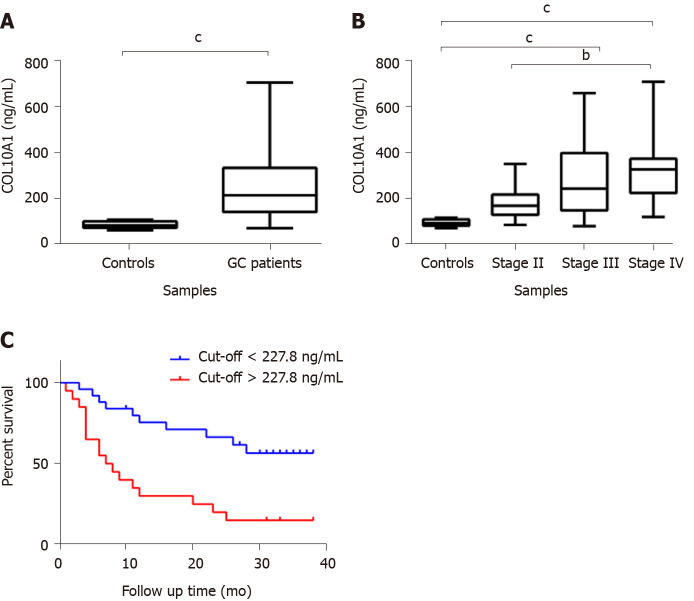
The results obtained from gene expression analysis showed a significantly increased level of COL10A1 in gastric tumor tissue compared to adjacent normal tissue (P < 0.05). Interesting COL10A1 seems to show an elevated expression from the beginning of carcinogenesis, in the early stages, and this increased level remains elevated during cancer progression (Figure 1A). The protein expression level of COL10A1 in the normal and tumor samples was evaluated by Western blot analysis. The obtained data revealed an increased expression of COL10A1 in gastric tumor tissue compared to normal adjacent tissue, as shown in Figure 1B.
Figure 1.
COL10A1 expression in gastric cancer tissue. A: Overexpression of COL10A1 gene in gastric tumor tissue compared to normal gastric tissue. Values are represented as mean ± SD; B: Overexpression of COL10A1 protein in gastric tumor tissue compared to normal gastric tissue. aP < 0.05. TNM: Tumor, node, and metastasis.
COL10A1 shows an increased circulating level in plasma of GC patients
In order to evaluate the potential of COL10A1 as a biomarker, the COL10A1 level in plasma of 49 gastric adenocarcinoma patients compared to cancer-free controls was analyzed using ELISA technique. Samples were divided according to their TNM stage in early (AJCC stage II) and advanced gastric adenocarcinoma (AJCC stage IV). The results showed a significant increase of COL10A1 plasma level in gastric adenocarcinoma patients compared with cancer-free controls (P = 0.0029) (Figure 2A). In addition, a correlation between COL10A1 plasma level and tumor progression was observed. As shown in Figure 2B, a significant increase of COL10A1 plasma level was found in GC stage III vs controls (P = 0.007), in GC stage IV vs controls (P = 0.0011), as well as in GC stage II vs stage IV (P = 0.0168). Furthermore, the Kaplan-Meier survival analysis showed that patients with COL10A1 plasma levels lower than 227.8 ng/mL had significantly better survival compared with patients than presents COL10A1 levels higher than 227.8 ng/mL (P = 0.0006) (Figure 2C).
Figure 2.
COL10A1 expression in gastric cancer plasma. A: Overexpression of circulating COL10A1 in gastric adenocarcinoma patients compared to cancer-free controls. Values are represented as mean ± SD; B: Increased COL10A1 plasma level is correlated with tumor progression. C: Kaplan-Meier survival plots for gastric adenocarcinoma patients. Tick marks represent the time of the last follow-up. High COL10A1 plasma levels (> 227.8 ng/mL) were significantly associated with shorter survival in gastric adenocarcinoma patients. bP < 0.02, cP < 0.007. GC: Gastric cancer.
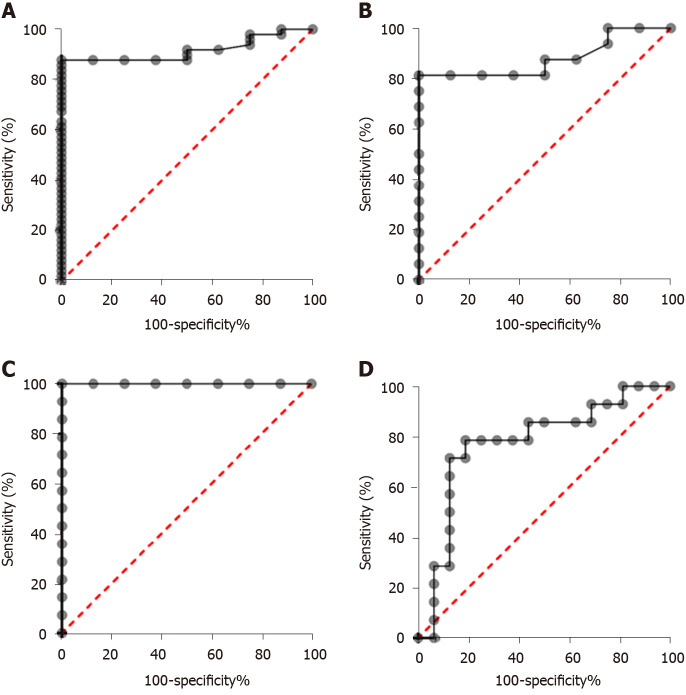
In order to assess the diagnostic significance of plasma COL10A1 in GC patients, we generated ROC curves. The cut-off levels on the ROC curves were selected using the Youden index [(sensitivity + specificity) - 1]. Comparisons of the COL10A1 plasma levels between GC patients and cancer-free controls showed an AUC of 0.9171 (95%CI: 0.8443 to 0.9899) and a P = 0.0002, with a sensitivity/specificity of 87.76% (95%CI: 75.76%-94.27%) / 100.0% (95%CI: 67.56%-100%), allowing to distinguish between patients with early gastric adenocarcinoma and cancer-free controls. In the early stage, we obtained an AUC of 0.8789 (95%CI: 0.7385 to 1.000) and a P = 0.0030, with a sensitivity/specificity of 81.25% (95%CI: 56.99%-93.41%) / 100.0% (95%CI: 67.56%-100%). The obtained results are summarized in Table 2 and in Figures 3A, 3B, 3C, and 3D.
Table 2.
Evaluation of the detection value of COL10A1 in the diagnosis of gastric cancer patients.
| AUC | P value | Cut-off values (ng/mL) | Sensitivity | Specificity | |
| GC vs CFC | 0.9171 (0.8443 to 0.9899) | 0.0002 | 113.3 | 87.76% (75.76%-94.27%) | 100% (67.56%-100.0%) |
| Early stage GC vs CFC | 0.8789 (0.7385 to 1.000) | 0.003 | 118.1 | 81.25% (56.99%-93.41%) | 100% (67.56%-100.0%) |
| Advanced stage GC vs CFC | 1.000 (1.000 to 1.000) | 0.0001 | 113.3 | 100% (78.47%-100.0%) | 100% (67.56%-100.0%) |
| Early stage vs Advanced stage | 0.7768 (0.5977 to 0.9558) | 0.0100 | 225.3 | 78.57% (52.41%-92.43%) | 81.25% (56.99%-93.41%) |
95%CI was given in brackets for each group. GC: Gastric cancer; CFC: Cancer-free controls; AUC: Area under the Receiver operating characteristic curve; Early stage: AJCC stage II; Advanced stage: AJCC stage IV.
Figure 3.
Receiver operating characteristic curve analysis in the diagnosis of gastric cancer. A: Patients vs control; B: Early-stage gastric cancer (GC) patients vs control; C: Advanced-stage GC patients vs control; D: Early-stage GC patients vs advanced-stage GC patients.
Therefore, circulating COL10A1 level appears to be an important diagnostic and prognostic biomarker in patients with gastric adenocarcinoma. If confirmed in further studies, it could be considered for treatment decisions in these patients.
DISCUSSION
GC represents one of the most common human cancers worldwide with a low 5-year survival rate. Gastric adenocarcinoma, the main type of GC is characterized by many genomic and proteomic alterations that sustain the aggressiveness of this disease and the early development of drug resistance[13,14]. Despite the development of innovative targeted therapies and the recent advances in the molecular GC characterization, the majority of GC patients are still diagnosed at advanced stages and their prognosis remains extremely poor. Currently, the most frequent tumor markers used in the clinic for early detection of GC comprise CEA, CA: CA19-9, CA72-4, CA125, CA24-2, CA50, and also pepsinogen and AFP. However, the specificity and sensitivity of these serum biomarkers are poor and so far, none of them is unique for GC diagnosis. Therefore, identification of biomarkers in the early stage of this malignancy could improve diagnosis, prognosis, prediction of recurrence, and treatment response[15-17].
Recent studies suggest that COL10A1 is a disease progression-associated gene. COL10A1 is a member of the collagen family involved in tissue architecture and acts as a barrier to the migration of epithelial cells under normal conditions. However, data sustain that increased stromal collagen microenvironment significantly increases tumor formation and results in a significantly more invasive phenotype in mammary tissue[18]. COL10A1 was identified in tumor vasculature of the breast tumors, presenting an increased gene expression[9,19]. Increased level of stromal COL10A1 was correlated with poor pathologic response in ER+/HER2+ breast tumors[20]. Another study suggested that circulating COL10A1 could be considered a useful biomarker in diagnostic assessment of suspicious breast nodules[21]. In esophageal squamous cell carcinoma, the expression of COL10A1 was reported upregulated along with other collagen-related genes[22]. In colorectal cancer, COL10A1 was found to be abnormally over-expressed and associated with the progression of cancer and the process of epithelial-mesenchymal transition. Moreover, high-level expression of COL10A1 was found to be an independent risk factor of prognosis and overall survival in these patients[10]. Further, Sole et al[23] reported that COL10A1 protein levels in serum of colon cancer patients can detect both adenoma lesions and tumors. In lung cancer increased plasma levels of COL10A1 were also detected, but no significant association was observed between plasma levels and the clinicopathological features or survival[11,24].
Considering previous findings, we analyzed the expression of COL10A1 in tissue and plasma of gastric adenocarcinoma patients as a possible biomarker for diagnosis and prognosis. COL10A1 tissue level was found to be increased in gastric adenocarcinoma patients and this increased level was associated with tumor stage. The major finding was a significantly increased circulating level of COL10A1 in gastric adenocarcinoma patients compared to cancer-free controls. Moreover, Kaplan–Meier curves of overall survival showed that GC patients with an elevated COL10A1 plasma level had a significantly negative prognostic with shorter survival.
The AUC on ROC curve of 0.9171 (P = 0.0002), with sensitivity of 87.76% and specificity of 100.00%, makes plasma COL10A1 level a promising diagnostic biomarker. Moreover, this study demonstrated the potential role of plasma COL10A1 in the early detection of GC since in this case the obtained AUC value was 0.8789 (P = 0.0030), with sensitivity of 81.25% and specificity of 100.00%. The result can be very important since there is a high difference (2.43-fold change) between early stage samples and cancer-free controls, positioning this biomarker as a promising candidate. The same early elevated level of COL10A1 was reported by Sole et al[23] in plasma of patients with adenomas and colon cancer when compared to controls.
The discovery of new biomarkers that can be detected in serum/plasma presents interest since they can be easier used in daily clinical practice. Currently, the diagnosis of solid tumors is based on the screening of several tumor markers such as CEA and CA19-9. However, the applicability of CEA and CA19-9 for detection, prognosis, and progression of GC is low. At a specificity of 89.5%-95%, CA19-9 sensitivity varies between 26.3%-54.8% (AUC = 0.58)[25,26]. Similar, CEA has a low sensitivity of 21% (AUC = 0.52)[25].
Further, we report an increase in serum and tissue level of COL10A1 in GC with tumoral stage, similar to previous reports on breast[21], lung[11], and colon cancer[23]. Interestingly, all of them are epithelial cancers where epithelial to mesenchymal transition (EMT) is involved in tumor progression and metastasis. There is evidence that COL10A1 acts as a potential inducer of EMT via SOX9. In a series of experiments of knocking down COL10A1 expression, Li et al[27] showed that COL10A1 was directly associated with cell migration, invasion, and metastasis in GC. Moreover, it had been reported that collagen type I is able to initiate a disruption of the E-cadherin cell-to-cell adhesion complex and to promote EMT and proliferation of pancreatic carcinoma cells[28]. Willumsen et al[29] demonstrated that during EMT, the extracellular matrix (ECM) is actively remodeled by matrix metalloproteinases, and ECM components (e.g., collagens) are released into circulation and can be potentially used as biomarkers for the early detection of cancers.
Another important finding of our study is related to the association between high-level expression of COL10A1 in plasma and negative prognostic in GC. This correlation is reported here for the first time. In colon cancer study the authors showed that high expression level of COL10A1 is associated with poor prognosis, but the quantification was done on tissue through immunohistochemistry and real-time quantitative polymerase chain reaction.
Through our findings, we indicate the serum level of COL10A1 as an early diagnostic biomarker with high sensitivity and specificity, a risk factor of prognosis and overall survival indicator in GC patients. The identification of circulating biomarkers is important since their detection is non-invasive and can be easily implemented in daily clinical practice without higher costs being based on such techniques as ELISA. On the other side, the use of a cheap and specific test for population screening could improve early diagnosis rate and contribute to the decrease of the GC burden.
ARTICLE HIGHLIGHTS
Research background
Despite the major advances in the field of personalized medicine, gastric cancer (GC) remains an aggressive malignancy with a high rate of mortality, being the third leading cause of cancer-related death. The main issue in the management of this disease is represented by the high molecular heterogeneity that results in the phenotypical aggressiveness of GC and limits the antitumor efficacy of the targeted therapy.
Research motivation
GC is still characterized by late diagnosis, limited effective treatment options, and lack of reliable biomarkers for the patient outcome prediction and response to therapy. The discovery of new circulating biomarkers useful in the early diagnosis of GC is mandatory.
Research objectives
The present study aimed to evaluate the potential of COL10A1 as a circulating biomarker for the diagnosis and prognosis of gastric adenocarcinoma patients.
Research methods
Plasma and tissue samples obtained from 49 patients with gastric adenocarcinoma have been used in exploring the expression of COL10A1. Real-time PCR and western blot techniques were used to evaluate COL10A1 level in gastric tumor tissue compared to normal adjacent tissue. The circulating level of COL10A1 was also evaluated by ELISA in plasma of gastric adenocarcinoma patients. Survival analysis was made in order to evaluate the potential of COL10A1 as a biomarker for the diagnosis and prognosis of gastric adenocarcinoma patients.
Research results
Our results showed a significant increase in COL10A1 gene expression and protein levels in gastric tumor tissue compared to adjacent normal tissue. COL10A1 seems to show an elevated expression from the beginning of carcinogenesis, in the early stages, and its increased level remains elevated during cancer progression. This significant increase of COL10A1 was observed also in plasma of gastric adenocarcinoma patients. Moreover, increased COL10A1 plasma level was associated with poor survival. Plasma COL10A1 performed a diagnostic value in GC with an area under the receiver operating characteristic curve (AUC) of 0.9171 (P = 0.0002), sensitivity of 87.76%, and specificity of 100.0%. Furthermore, this study demonstrated the potential role of plasma COL10A1 in the early detection of GC, as in the early stage we obtained an AUC of 0.8789 (P = 0.0030), sensitivity of 81.25%, and specificity of 100.0%. If confirmed in further studies, circulating COL10A1 level could be considered for treatment decisions in these patients.
Research conclusions
We reported for the first time an increased circulating expression level of COL10A1 in gastric adenocarcinoma patients that is associated with poor survival. The high sensitivity and specificity obtained suggest that COL10A1 could represent a potential biomarker for early detection of GC.
Research perspectives
The identification of circulating biomarkers is important since their detection is non-invasive and can be easily implemented in daily clinical practice without higher costs, being based on such techniques as ELISA. On the other hand, the use of a cheap and specific test for population screening could improve early diagnosis rate and contribute to the decrease of the GC burden.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Stefan S. Nicolau Institute of Virology.
Conflict-of-interest statement: This work was supported by a grant of the Romanian Authority for Scientific Research and Innovation, CNCS - UEFISCDI, No. PN-III-P4-ID-PCCF-2016-0158 (contract PCCF 17/2018), within PNCDI III. All other authors have nothing to disclose.
Manuscript source: Invited manuscript
Peer-review started: December 28, 2019
First decision: January 19, 2020
Article in press: May 28, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ju SQ, Tanabe S S-Editor: Liu M L-Editor: A E-Editor: Ma YJ
Contributor Information
Laura Necula, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania; Titu Maiorescu University, Faculty of Medicine, Bucharest 040441, Romania.
Lilia Matei, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania.
Denisa Dragu, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania.
Ioana Pitica, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania.
Ana Iulia Neagu, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania.
Coralia Bleotu, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania.
Simona Dima, Fundeni Clinical Institute, Bucharest 022328, Romania.
Irinel Popescu, Fundeni Clinical Institute, Bucharest 022328, Romania.
Carmen C Diaconu, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania.
Mihaela Chivu-Economescu, Department of Cellular and Molecular Pathology, Stefan S. Nicolau Institute of Virology, Bucharest 030304, Romania. mihaela.economescu@virology.ro.
Data sharing statement
No additional data are available.
References
- 1.Duarte HO, Gomes J, Machado JC, Reis CA. Gastric cancer: Basic aspects. Helicobacter. 2018;23 Suppl 1:e12523. doi: 10.1111/hel.12523. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Soni P, Garg M, Kamholz S, Chandra AB. Emerging Therapies in the Management of Advanced-Stage Gastric Cancer. Front Pharmacol. 2018;9:404. doi: 10.3389/fphar.2018.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fei HJ, Chen SC, Zhang JY, Li SY, Zhang LL, Chen YY, Chang CX, Xu CM. Identification of significant biomarkers and pathways associated with gastric carcinogenesis by whole genome-wide expression profiling analysis. Int J Oncol. 2018;52:955–966. doi: 10.3892/ijo.2018.4243. [DOI] [PubMed] [Google Scholar]
- 4.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget. 2017;8:57654–57669. doi: 10.18632/oncotarget.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chivu-Economescu M, Matei L, Necula LG, Dragu DL, Bleotu C, Diaconu CC. New therapeutic options opened by the molecular classification of gastric cancer. World J Gastroenterol. 2018;24:1942–1961. doi: 10.3748/wjg.v24.i18.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chivu Economescu M, Necula LG, Dragu D, Badea L, Dima SO, Tudor S, Nastase A, Popescu I, Diaconu CC. Identification of potential biomarkers for early and advanced gastric adenocarcinoma detection. Hepatogastroenterology. 2010;57:1453–1464. [PubMed] [Google Scholar]
- 9.Chapman KB, Prendes MJ, Sternberg H, Kidd JL, Funk WD, Wagner J, West MD. COL10A1 expression is elevated in diverse solid tumor types and is associated with tumor vasculature. Future Oncol. 2012;8:1031–1040. doi: 10.2217/fon.12.79. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Li T, Ye G, Zhao L, Zhang Z, Mo D, Wang Y, Zhang C, Deng H, Li G, Liu H. High expression of COL10A1 is associated with poor prognosis in colorectal cancer. Onco Targets Ther. 2018;11:1571–1581. doi: 10.2147/OTT.S160196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andriani F, Landoni E, Mensah M, Facchinetti F, Miceli R, Tagliabue E, Giussani M, Callari M, De Cecco L, Colombo MP, Roz L, Pastorino U, Sozzi G. Diagnostic role of circulating extracellular matrix-related proteins in non-small cell lung cancer. BMC Cancer. 2018;18:899. doi: 10.1186/s12885-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Barzi A, Rajdev L. Biomarker-driven targeted therapies for gastric/gastro-esophageal junction malignancies. Semin Oncol. 2018;45:133–150. doi: 10.1053/j.seminoncol.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Venerito M, Vasapolli R, Rokkas T, Malfertheiner P. Gastric cancer: epidemiology, prevention, and therapy. Helicobacter. 2018;23 Suppl 1:e12518. doi: 10.1111/hel.12518. [DOI] [PubMed] [Google Scholar]
- 15.Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029–2044. doi: 10.3748/wjg.v25.i17.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818–2832. doi: 10.3748/wjg.v24.i26.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battaglin F, Naseem M, Puccini A, Lenz HJ. Molecular biomarkers in gastro-esophageal cancer: recent developments, current trends and future directions. Cancer Cell Int. 2018;18:99. doi: 10.1186/s12935-018-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makoukji J, Makhoul NJ, Khalil M, El-Sitt S, Aldin ES, Jabbour M, Boulos F, Gadaleta E, Sangaralingam A, Chelala C, Boustany RM, Tfayli A. Gene expression profiling of breast cancer in Lebanese women. Sci Rep. 2016;6:36639. doi: 10.1038/srep36639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodsky AS, Xiong J, Yang D, Schorl C, Fenton MA, Graves TA, Sikov WM, Resnick MB, Wang Y. Identification of stromal ColXα1 and tumor-infiltrating lymphocytes as putative predictive markers of neoadjuvant therapy in estrogen receptor-positive/HER2-positive breast cancer. BMC Cancer. 2016;16:274. doi: 10.1186/s12885-016-2302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giussani M, Landoni E, Merlino G, Turdo F, Veneroni S, Paolini B, Cappelletti V, Miceli R, Orlandi R, Triulzi T, Tagliabue E. Extracellular matrix proteins as diagnostic markers of breast carcinoma. J Cell Physiol. 2018;233:6280–6290. doi: 10.1002/jcp.26513. [DOI] [PubMed] [Google Scholar]
- 22.Karagoz K, Lehman HL, Stairs DB, Sinha R, Arga KY. Proteomic and Metabolic Signatures of Esophageal Squamous Cell Carcinoma. Curr Cancer Drug Targets. 2016 Epub ahead of print. [PubMed] [Google Scholar]
- 23.Solé X, Crous-Bou M, Cordero D, Olivares D, Guinó E, Sanz-Pamplona R, Rodriguez-Moranta F, Sanjuan X, de Oca J, Salazar R, Moreno V. Discovery and validation of new potential biomarkers for early detection of colon cancer. PLoS One. 2014;9:e106748. doi: 10.1371/journal.pone.0106748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frezzetti D, De Luca A, Normanno N. Extracellular matrix proteins as circulating biomarkers for the diagnosis of non-small cell lung cancer patients. J Thorac Dis. 2019;11:S1252–S1256. doi: 10.21037/jtd.2019.02.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Fernández L, Tejero E, Tieso A, Rabadán L, Munoz M, Santos I. Receiver operating characteristic (ROC) curve analysis of the tumor markers CEA, CA 19-9 and CA 72-4 in gastric cancer. Int Surg. 1996;81:400–402. [PubMed] [Google Scholar]
- 26.Patai A, Héber S, Döbrönte Z, Kovács LG. [Diagnostic value of CA 19-9 and CEA in gastrointestinal pathology] Orv Hetil. 1992;133:1301–1304, 1307. [PubMed] [Google Scholar]
- 27.Li T, Huang H, Shi G, Zhao L, Li T, Zhang Z, Liu R, Hu Y, Liu H, Yu J, Li G. TGF-β1-SOX9 axis-inducible COL10A1 promotes invasion and metastasis in gastric cancer via epithelial-to-mesenchymal transition. Cell Death Dis. 2018;9:849. doi: 10.1038/s41419-018-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662–4671. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- 29.Willumsen N, Bager CL, Leeming DJ, Smith V, Christiansen C, Karsdal MA, Dornan D, Bay-Jensen AC. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med. 2014;3:1136–1145. doi: 10.1002/cam4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.