Abstract
Background
A growing body of evidence shows that hypothalamic inflammation is an important factor in the initiation of obesity. In particular, reactive gliosis accompanied by inflammatory responses in the hypothalamus are pivotal cellular events that elicit metabolic abnormalities. In this study, we examined whether MyD88 signaling in hypothalamic astrocytes controls reactive gliosis and inflammatory responses, thereby contributing to the pathogenesis of obesity.
Methods
To analyze the role of astrocyte MyD88 in obesity pathogenesis, we used astrocyte-specific Myd88 knockout (KO) mice fed a high-fat diet (HFD) for 16 weeks or injected with saturated free fatty acids. Astrocyte-specific gene expression in the hypothalamus was determined using real-time PCR with mRNA purified by the Ribo-Tag system. Immunohistochemistry was used to detect the expression of glial fibrillary acidic protein, ionized calcium-binding adaptor molecule 1, phosphorylated signal transducer and activator of transcription 3, and α-melanocyte-stimulating hormone in the hypothalamus. Animals’ energy expenditure was measured using an indirect calorimetry system.
Results
The astrocyte-specific Myd88 KO mice displayed ameliorated hypothalamic reactive gliosis and inflammation induced by injections of saturated free fatty acids and a long-term HFD. Accordingly, the KO mice were resistant to long-term HFD-induced obesity and showed an improvement in HFD-induced leptin resistance.
Conclusions
These results suggest that MyD88 in hypothalamic astrocytes is a critical molecular unit for obesity pathogenesis that acts by mediating HFD signals for reactive gliosis and inflammation.
Keywords: Myeloid differentiation primary response 88, Hypothalamus, Reactive gliosis, Obesity, Leptin resistance, High-fat diet, Proopiomelanocortin
Background
The increasing rate of obesity in the global population has become a major public health problem. Therefore, many investigations have been performed to identify the underlying mechanisms and pathological components of obesity [1, 2]. In particular, it has been proposed that perturbation of the hypothalamic neuronal circuit that controls whole-body energy metabolism is a primary cause of obesity development [3–5]. During the past decade, a great deal of attention has been paid to investigating the hypothalamic neuronal circuit linked to whole-body energy metabolism under the control of afferent inputs derived from metabolically involved peripheral organs [6–8].
Astrocytes are the most abundant cells in the central nervous system and dynamically participate in maintaining normal neuronal functions by playing multiple supportive roles. Thus, a growing body of evidence has emerged linking metabolic processes in hypothalamic astrocytes with the physiological or pathological control of body energy balance [9–11]. According to the recent literature, neuroinflammation and reactive gliosis can be observed in the hypothalami of mice exposed to a high-fat diet (HFD) before the occurrence of significant body weight gain, and this is sustained with continuous HFD feeding, suggesting that hypothalamic gliosis accompanied by inflammation is a crucial cellular event in obesity pathogenesis [12–15]. Thus, unmasking the underlying mechanism by which an HFD induces hypothalamic inflammation and gliosis is required to better understand the initiation and deterioration of metabolic disorders caused by over-nutrition.
Myeloid differentiation primary response 88 (MyD88) is a crucial adaptor molecule of Toll-like receptor (TLR) signaling that initiates innate immunity by mediating a variety of humoral factors and infectious pathogens [16–18]. In particular, the TLR and MyD88 axis in the hypothalamus is a key player in HFD-induced hypothalamic inflammatory responses. Mice fed with an HFD expressed high levels of TLR4 and MyD88 in the hypothalamus, which was coupled with intracellular inflammatory signaling cascades such as the Jun kinase and nuclear factor kappa B (NF-κB) pathways [19, 20]. A recent study reported that interaction between circulating saturated free fatty acids (sFFAs) and TLR4 was involved in the hypothalamic control of energy homeostasis and that mice bearing neuron-specific deletion of the Myd88 gene were protected against HFD-induced obesity through the alleviation of hypothalamic inflammation and leptin resistance [21]. However, it is unclear whether TLR and MyD88 signaling in astrocytes is triggered by over-nutrition and thus directly linked to the development of obesity in association with hypothalamic gliosis and inflammation. In this study, we investigated whether MyD88 signaling in astrocytes is involved in hypothalamic inflammation and reactive gliosis and whether altering the activity of MyD88 signaling in astrocytes, using mutant mice bearing an astrocyte-specific deletion of Myd88 gene expression, would affect the obesity phenotype and leptin resistance induced by HFD consumption.
Methods
Animals
Animals were fed a standard diet (STD, Feedlab, Gyeonggi-Do, Korea) or HFD (D12492, Research Diets, New Brunswick, NJ, USA) ad libitum and given free access to tap water. All animals were maintained in temperature- and humidity-controlled rooms with a 12 h/12 h light-dark cycle, with the lights on from 7:00 a.m. to 7:00 p.m. Myd88 floxed (Myd88fl/fl) mice (stock no. 008888), glial fibrillary acidic protein (Gfap)-CreERT2 mice (Stock No. 012849), and Ai14 reporter mice (stock no. 007914) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Myd88fl/fl mice were crossbred with Gfap-CreERT2 mice to generate Myd88 conditional knockout (KO) mice missing Myd88 specifically in cells expressing GFAP (Myd88ΔGFAP). Ai14 reporter mice were crossbred with Gfap-CreERT2 mice to label GFAP-positive astrocytes with tomato signals. Because the Gfap-CreERT2 mice expressed Cre recombinase under the control of the tamoxifen-inducible GFAP promoter, 6-week-old Myd88ΔGFAP mice and their littermate control (Myd88fl/fl) mice received daily injections for 5 days of tamoxifen (100 mg/kg, T5648, Sigma-Aldrich, St. Louis, MO, USA) dissolved in corn oil (C8267, Sigma-Aldrich). All animals and procedures used were in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee at the University of Ulsan (permission numbers: BJL-15-010, BJL-18-010, and BJL-19-010).
Ribo-Tag system
To analyze mRNA species that are specifically translated in hypothalamic astrocytes, we used the Ribo-Tag translational profiling system [22, 23]. In this study, we used Rpl22HA mice (Stock No. 011029, Jackson Laboratory), which have a loxP-flanked wild-type exon 4 followed by an identical exon 4 tagged with hemagglutinin (HA), as the Ribo-Tag animal. Crossbreeding Ribo-Tag mice with mice expressing Cre recombinase resulted in the deletion and replacement of the floxed wild-type exon 4 with the HA-tagged exon 4 in cells expressing Cre. The astrocyte-specific Myd88 KO (Myd88ΔGFAP) mice were crossbred with Rpl22HA mice to generate Myd88ΔGFAP;Rpl22HA mice that had both the HA-tagged ribosomal protein Rpl22 and the deletion of Myd88 in astrocytes. The Rpl22HA mice were also crossed with control Myd88+/+-Gfap-CreERT2 (Gfap-Cre) mice, which resulted in control mice bearing an astrocyte-specific Ribo-Tag system (Gfap-Cre;Rpl22HA mice).
RNA isolation with the Ribo-Tag system was conducted as previously described [22, 23]. Briefly, dissected hypothalamus samples were collected from animals and homogenized. RNA was extracted from 10% of the cleared lysate and used as input. The remaining lysate was incubated with mouse anti-HA antibody for 4 h at 4 °C followed by the addition of protein G agarose beads (LGP-1018B, Lugen, Gyeonggi-Do, Korea) and overnight incubation at 4 °C. The beads were washed three times in high-salt solution. The bound ribosomes and RNA were separated from the beads with 30 s of vortexing, and RNA was further purified using a QIAGEN RNeasy Micro Kit (74034, Qiagen, Hilden, Germany). After RNA isolation, we obtained 10–20 ng of RNA sample/hypothalamus. The RNA samples were then subjected to real-time PCR analysis.
Measurement of food intake and leptin administration
Five days before we began the food intake measurements, we moved the mice into individual cages and allowed them to acclimatize to their new environment. Food intake was measured for a week at 23–24 weeks of age during HFD feeding and calculated as an average daily food intake (Fig. 2a).
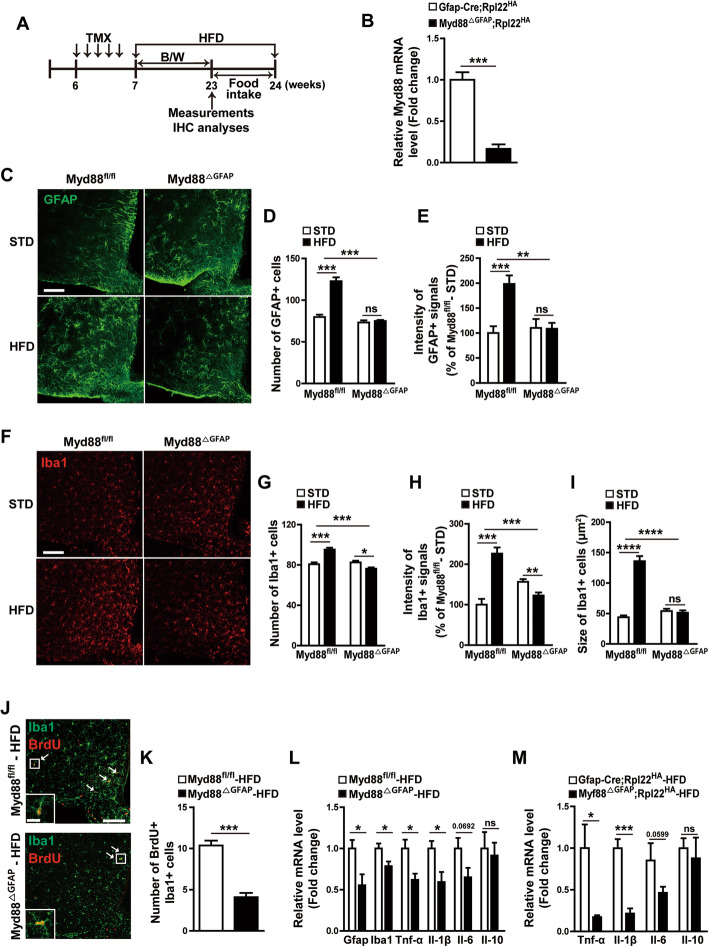
Fig. 2.
HFD-induced hypothalamic reactive gliosis is reduced by astrocyte-specific Myd88 KO. a Diagram depicts the experimental design. TMX, tamoxifen. B/W, body weight. IHC, immunohistochemistry. b The expression of Myd88 mRNA was determined using a real-time PCR analysis with RNA samples immunoprecipitated with HA antibody from hypothalamic extracts of Myd88△GFAP;Rpl22HA mice that were generated by cross-breeding astrocyte-specific Myd88 KO mice (Myd88△GFAP) with transgenic mice (Rpl22HA) expressing HA-tagged Rpl22. Gfap-Cre;Rpl22HA mice were used as the control (n = 4–5/group). c–i Control (Myd88fl/fl) mice and Myd88△GFAP mice were fed a STD or HFD for 16 weeks, and their astrocytes and microglia in the hypothalamic ARC were analyzed with immunohistochemistry using GFAP and Iba1 antibodies. Representative images (c, f) and calculated data (d, e, g, h, i) show the effect of astrocyte-specific Myd88 KO (Myd88△GFAP) on the HFD-induced increase in the number and intensity of GFAP-positive cells (c–e) and Iba1-positive cells (f–h), and the size of Iba1-positive cells (i) in the ARC compared with the control Myd88fl/fl mice (n=3–6 sections of 3–6 mice/group). j, k Representative images (j) and calculated data (k) indicate HFD-induced BrdU and Iba1 double-positive cells in the ARC of Myd88△GFAP mice and control Myd88fl/fl mice after HFD feeding for 8 weeks. White arrows indicate cells double positive for Iba1 and BrdU (n = 6 sections of 3 mice/group). l Real-time PCR analysis of RNA samples shows the expression of Gfap, Iba1, Tnf-α, Il-1β, Il-6, and Il-10 in the hypothalamus of Myd88△GFAP mice and control Myd88fl/fl mice after HFD feeding for 16 weeks (n = 3/group). m Hypothalamic expression of Tnf-α, Il-1β, Il-6, and Il-10 was determined using a real-time PCR analysis of RNA samples (immunoprecipitated with HA antibody) from hypothalamic extracts of Myd88△GFAP;Rpl22HA mice and control Gfap-Cre;Rpl22HA mice after HFD feeding for 16 weeks (n = 3–4/group). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. ns, not significant. Scale bar = 100 μm (20 μm for higher magnification view in insets)
Body weight was measured every week during HFD feeding. To determine how leptin affected feeding behavior, mice were intraperitoneally (ip) injected with vehicle (saline) or recombinant mouse leptin (2 mg/kg; R&D Systems, Minneapolis, MN, USA) after overnight fasting. The food intake of the individually caged animals was monitored for 24 h after the injection, and then their body weights were measured.
Cannulation and administration of palmitic acid
For intracerebroventricular (icv) cannula implantation, mice were anesthetized by ip injection of tribromoethanol (250 mg/kg, Sigma-Aldrich) and placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). The cannula (26 gage) was implanted into the right lateral ventricle (1.0 mm lateral, 0.3 mm posterior, and 2.4 mm ventral to the bregma) according to the Stereotaxic Mouse Brain Atlas (Paxinos G and Franklin KBJ, 2001, Academic Press, San Diego, CA, USA) and secured to the skull with dental cement. After 7 days of recovery, the mice were injected with vehicle [5% bovine serum albumin (BSA)] and palmitic acid (50 pmol/2 μl, Sigma-Aldrich) dissolved in BSA solution. Mice were sacrificed 1 h after the injection of palmitic acid.
Administration of 5-bromodeoxyuridine
For the analysis of Iba1-positive cell proliferation upon HFD, mice received daily ip injections with 5-bromodeoxyuridine (BrdU, 100 mg/kg, Sigma-Aldrich) dissolved in saline for 5 days after HFD feeding for 8 weeks. On the 5th day of injection, mice were sacrificed 1 h after BrdU injection, and their brain sections were analyzed with immunohistochemistry.
Immunohistochemistry
Animals were deeply anesthetized with tribromoethanol and transcardially perfused with phosphate buffer (PB, 0.1 M, pH 7.4), followed by a fresh fixative of 4% paraformaldehyde in PB. Brains were post-fixed overnight at 4 °C, sliced to a thickness of 50 μm using a vibratome (VT1000P; Leica Microsystems, Wetzlar, Germany), and then washed several times in PB. Coronal brain sections containing the hypothalamic arcuate nucleus (ARC) were preincubated with 0.2% Triton X-100 (T8787, Sigma-Aldrich) in PB for 30 min to permeabilize the tissues and cells. After further washing with PB, the sections were incubated overnight at room temperature (RT) with mouse anti-GFAP antibody (1:3000; G3893, Sigma-Aldrich), rabbit anti-Iba1 antibody (1:3000; 019-19741, Wako, Osaka, Japan), rabbit anti-pSTAT3 antibody (1:1000; 9145, Cell Signaling Technology, Beverly, MA, USA), and mouse anti-HA antibody (1:1000; MMS-101R, BioLegend, San Diego, CA, USA) or at 4 °C with sheep anti-α-melanocyte stimulating hormone (MSH) antibody (1:10,000; AB5087, Millipore, Billerica, MA, USA). For BrdU staining, sections were incubated with 0.01 mol/L citrate buffer for 10 min at 80 °C and washed in PB at RT. Sections were then incubated with 2 N HCl for 30 min and incubated with 0.2% Triton X-100 in PB for 30 min at RT. Afterward, sections were incubated with rat anti-BrdU antibody (1:200; ab74545, Abcam, Cambridge, MA, USA) overnight at RT. On the next day, sections were washed in PB. For immunofluorescence staining, sections were incubated with the following secondary antibodies for 2 h at room temperature: goat anti-rabbit Alexa Fluor 488 (1:500; A11008, Invitrogen, Carlsbad, CA, USA), goat anti-rabbit Alexa Fluor 594 (1:500; A11012, Invitrogen), chicken anti-rabbit Alexa Fluor 647 (1:500; A21443, Invitrogen), goat anti-mouse Alexa Fluor 488 (1:500; A11001, Invitrogen), goat anti-mouse Alexa Fluor 594 (1:500; A11005, Invitrogen), donkey anti-rat Alexa Fluor 594 (1:500; A21209, Invitrogen), and donkey anti-sheep Alexa Fluor 594 (1:500; A11016, Invitrogen). Stained brain sections were imaged using an FV-1200 confocal laser-scanning microscope (Olympus America, Inc., Center Valley, PA, USA).
IHC image analyses
The number of immuno-positive cells in the hypothalamic ARC was counted by an unbiased observer. The intensity of immuno-positive cells was measured using the ImageJ V 1.50 software (National Institutes of Health, Bethesda, MD). Region of interest (ROI) within an image was manually selected with the Mouse Brain Atlas for ARC or PVN (ARC: between − 1.46 and − 1.82 mm from bregma, PVN: between − 0.82 and − 1.06 mm from bregma). The images were converted to 8-bit images and threshold was applied. The images were binarized to separate the immuno-positive cells from the background. The fiber intensity and particle number of immuno-positive α-melanocyte-stimulating hormone (α-MSH) signals in the PVN were measured using the ImageJ software. The size of Iba1-positive cells in the ARC was measured using a thresholding parameter on the ImageJ software as a previous report [24].
Blood glucose measurement
Blood glucose was measured with a glucometer (One Touch Ultra, LifeScan, Milpitas, CA, USA). For glucose tolerance tests (GTTs), mice were given an ip injection of d-glucose (1 g/kg) after overnight fasting. For insulin tolerance tests (ITTs), mice were fasted for 4 h before ip injection of human insulin (0.75 IU/kg). Blood glucose levels were determined from the tail vein at 0, 15, 30, 60, and 120 min after injection.
Measurement of O2 consumption, CO2 production, and energy expenditure
Metabolic parameters, O2 consumption (VO2), CO2 production (VCO2), and energy expenditure, of MyD88fl/fl and MyD88ΔGFAP mice were analyzed using an indirect calorimetry system (Promethion, Sable Systems, Las Vegas, NV, USA). VO2 and VCO2 were measured at 10 min intervals for each mouse. Mice were acclimated in the chambers for 48 h prior to data collection. The average values during the light and dark periods were calculated. Data acquisition and instrument control were coordinated by the MetaScreen software (version 2.3.12), and the obtained raw data were processed using ExpeData (version 1.9.14, Sable Systems).
Real-time PCR
RNA was isolated from hypothalami using Trizol reagent (Sigma-Aldrich) or immunoprecipitation with HA antibody, as explained above, and reverse transcribed with MMLV reverse transcriptase (Beams Biotechnology, Gyeonggi-do, Korea). Gene expression was measured by real-time PCR using Evagreen qPCR Mastermix (TApplied Biological Materials Inc., Richmond, BC, Canada). The primers used were as follows: Myd88 sense primer, 5′-GCT ACT GCC CCA ACG ATA TC-3′; Myd88 antisense primer, 5′-ACA CAA CTT AAG CCG ATA GTC TG-3′; Il-1β sense primer, 5′-AGG GCT GCT TCC AAA CCT TTG AC-3′; Il-1β antisense primer, 5′-ATA CTG CCT GCC TGA AGC TCT TGT-3′; Il-6 sense primer, 5′-GAG ACT TCA CAG AGG ATA CCA C-3′; Il-6 antisense primer, 5′-TCT CAT TTC CAC GAT TTC CCA G-3′; Il-10 sense primer, 5′-TGG GTT GCC AAG CCT TAT CG-3′; Il-10 antisense primer, 5′-AAT CAC TCC TCA CCT GCT CCA CTG-3′; Tnf-α sense primer, 5′-TGG GAC AGT GAC CTG GAC TGT-3′; Tnf-α antisense primer, 5′-TTC GGA AAG CCC ATT TGA GT-3′; Gfap sense primer, 5′-CAG ACT TTC TCC AAC CTC CAG-3′; Gfap antisense primer, 5′-AAT CTG GTG AGC CTG TAT TGG-3′; Iba1 sense primer, 5′-TCT GCC GTC CAA ACT TGA AG-3′; Iba1 antisense primer, 5′-TCT AGG TGG GTC TTG GGA AC-3′; NeuN sense primer, 5′-ATG GTG CTG AGA TTT ATG GAG G-3′; NeuN antisense primer, 5′-CGA TGG TGT GAT GGT AAG GAT C-3′; β-actin sense primer, 5′-GAT CTG GCA CCA CAC CTT CT-3′; β-actin antisense primer, 5′-GGG GTG TTG AAG GTC TCA AA-3′; L19 sense primer, 5′-GGT GAC CTG GAT GAG AAG GA-3′; L19 antisense primer, 5′-TTC AGC TTG TGG ATG TGC TC-3′. Real-time PCR was performed using the StepOnePlusTM Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) for ~ 40 cycles. Relative mRNA expression was normalized with the β-actin or L19 mRNA level and calculated using the 2−ΔΔCT method [25].
Statistical analyses
Statistical analyses were performed in the GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). All data are expressed as the mean ± SEM. The statistical significance between two groups was analyzed by unpaired Student’s t test. Two-way analysis of variance (ANOVA) analyses followed by Bonferroni post hoc testing was performed to detect the significance of differences between two genotypes.
Results
Myd88 gene expression in astrocytes was increased by long-term HFD feeding
To validate that long-term exposure to HFD caused reactive gliosis in the hypothalamus, mice were fed an HFD for 16 weeks, and IHC using antibodies against GFAP, a molecular marker for astrocytes, and Iba1, a marker for microglia, was performed with brain sections containing hypothalamic ARC (Fig. 1a, d). Consistent with previous reports [11, 12, 26], HFD feeding increased the number and intensity of GFAP-positive cells (Fig. 1b, c) and Iba1-positive cells (Fig. 1e, f).
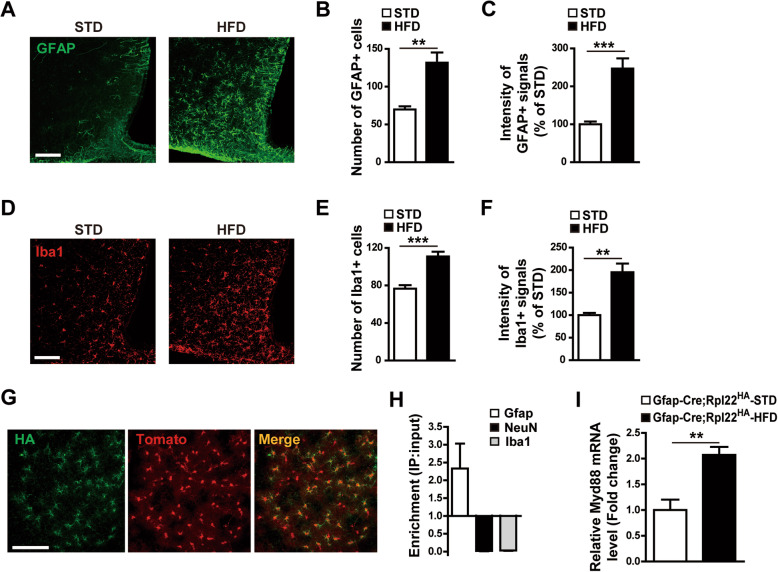
Fig. 1.
Increased Myd88 expression during hypothalamic reactive gliosis is induced by eating a high-fat diet (HFD). a–f Immunohistochemical analyses were performed to determine changes in astrocytes and microglia in the hypothalamic arcuate nucleus (ARC) caused by HFD feeding. Representative images (a, d) and calculated data (b, c, e, f) indicate that GFAP-positive astrocytes (a–c) and Iba1-positive microglia (d–f) were increased by an HFD compared with a standard diet (STD) for 16 weeks (n=6–12 sections of 3–6 mice/group). g–i Ribo-Tag analyses of Myd88 mRNA expression in the hypothalamic astrocytes of transgenic mice (Gfap-Cre;Rpl22HA) expressing hemagglutinin (HA)-tagged ribosomal protein Rpl22 in GFAP-positive cells. g Representative images showing co-expression of HA and astrocyte-specific tomato signals in the hypothalamic ARC of Gfap-Cre;Rpl22HA mice. h Real-time PCR data showing enrichment of Gfap mRNA (but not NeuN and Iba1 mRNA) in the RNA samples immunoprecipitated with HA antibody compared with the input RNA samples from hypothalamic extracts. i Ribo-Tag analyses showing that Myd88 mRNA expression in the hypothalamic astrocytes of Gfap-Cre;Rpl22HA mice were increased by HFD feeding, compared with STD feeding, for 16 weeks (n=3–4/group). Data are presented as mean ± SEM. **p<0.01 and ***p<0.001. Scale bar = 100 μm
To explore the function of MyD88 in hypothalamic astrocytes, we first used a Ribo-Tag system of transgenic (Gfap-Cre;Rpl22HA) mice that expressed HA-tagged ribosomal protein Rpl22 in astrocytes to identify the Myd88 mRNA species translated specifically in hypothalamic astrocytes. The IHC analysis identified specific HA signals in the GFAP-positive astrocytes (Fig. 1 g). Real-time PCR using the Ribo-Tag system further revealed that HA-mediated immunoprecipitation occurred in the cells expressing GFAP, but not in those producing NeuN or Iba1 (Fig. 1h). The amount of Myd88 mRNA translated in hypothalamic astrocytes was increased by HFD feeding (Fig. 1i), suggesting that astrocyte MyD88 could play a role in the response to over-nutrition.
Astrocyte-specific Myd88 knockout alleviated HFD-induced hypothalamic gliosis
It has been well established that coupling between TLRs and MyD88 initiates innate immunity in several types of peripheral cells and is also involved in the hypothalamic inflammatory responses linked to metabolic disorders [17, 18, 27]. To determine the effect of astrocyte MyD88 on HFD-induced astrogliosis and inflammation in the hypothalamus, we generated tamoxifen-inducible Myd88 gene KO specifically in GFAP-positive cells (Myd88ΔGFAP). More than 83% of Myd88 mRNA expression was eliminated in the hypothalamic astrocytes of Myd88ΔGFAP;Rpl22HA mice, compared with that in the hypothalamic astrocytes of control Gfap-Cre;Rpl22HA mice, as shown by a real-time PCR analysis of Ribo-Tag-purified mRNA (Fig. 2b).
Next, we measured the effect of this conditional Myd88 KO on HFD-induced astrogliosis in the hypothalamic ARC by counting GFAP-immuno-positive signals (Fig. 2c). The HFD-induced increase in the number and intensity of GFAP-positive cells was completely offset by Myd88 KO in astrocytes (Fig. 2d, e), suggesting the importance of MyD88 signaling in HFD-induced astrogliosis. The astrocyte-specific Myd88 KO also inhibited the HFD-induced increase in the number, intensity, and size of Iba1-positive cells in the hypothalamic ARC (Fig. 2f–i). Furthermore, the Myd88 KO resulted in a decreased number of HFD-induced Iba1-positive cells that were incorporated with BrdU (Fig. 2j, k). These results suggested that astrocyte MyD88 signaling also participates in HFD-induced microglial proliferation and activation. Consistent with the decrease in HFD-induced astrogliosis in the mutant animals, astrocyte-specific Myd88 KO decreased the HFD-induced mRNA expression of Gfap, Iba1, and proinflammatory cytokines in the hypothalamus (Fig. 2l). Real-time PCR analyses of hypothalamic mRNA purified with the Ribo-Tag system showed that astrocyte-specific Myd88 KO decreased HFD-induced expression of proinflammatory cytokines Tnf-α and Il-1β, but did not significantly affect the HFD-induced anti-inflammatory cytokines in the hypothalamic astrocytes (Fig. 2m).
Astrocyte-specific Myd88 KO affected hypothalamic gliosis triggered by palmitic acid
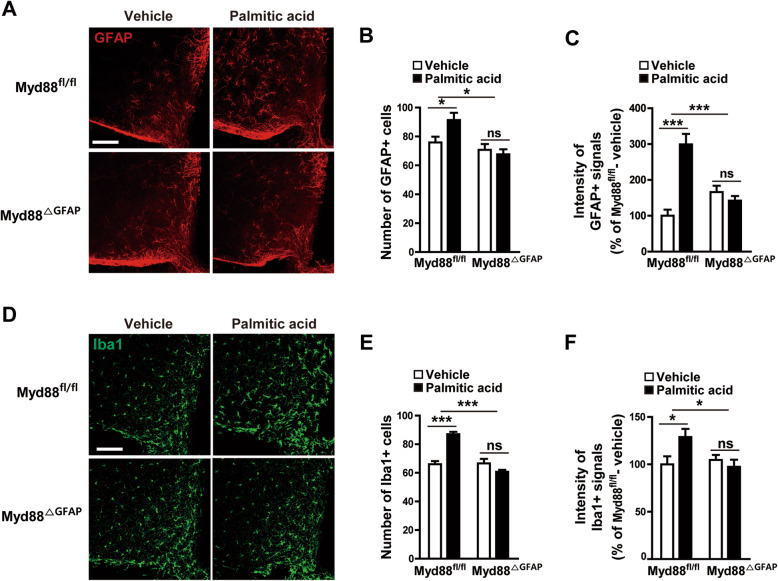
Because elevated levels of circulating sFFAs can cause hypothalamic reactive gliosis that is accompanied by inflammatory responses during over-nutrition [26, 28, 29], we next determined the effect of astrocyte-specific Myd88 KO on the hypothalamic astrogliosis induced by the administration of palmitic acid, an sFFA. Palmitic acid induced an increase in the number and intensity of GFAP-positive astrocytes in the hypothalamic ARC, which was attenuated by Myd88 KO in the astrocytes (Fig. 3a–c). Interestingly, astrocyte-specific Myd88 KO also caused a similar effect on palmitic acid–induced increase in the number and intensity of Iba1-positive microglia (Fig. 3d–f). Taken together, the current findings suggest that MyD88 signaling is a crucial molecular mediator of the hypothalamic gliosis induced by an elevation in circulating sFFAs.
Fig. 3.
Palmitic acid–induced hypothalamic gliosis is attenuated by ablation of Myd88 expression in astrocytes. To identify the effect of astrocyte MyD88 on saturated free fatty acid–induced hypothalamic reactive gliosis, astrocyte-specific Myd88 KO mice (Myd88△GFAP) and control Myd88fl/fl mice were icv administered palmitic acid (50 pmol/2 μl), and their astrocytes and microglia were immunohistochemically analyzed with GFAP and Iba1 antibodies. a, d Representative images show palmitic acid–induced changes in hypothalamic GFAP-positive cells (a) and Iba1-positive cells (d) in Myd88fl/fl mice and Myd88△GFAP mice. b, c, e, f Number and intensity of GFAP-positive cells (b, c) and Iba1-positive cells (e, f) observed in the hypothalamic ARC of Myd88fl/fl mice and Myd88△GFAP mice after icv injection of palmitic acid or vehicle (n = 4–6 sections of 2–3 mice/group). Data are presented as mean ± SEM. *p < 0.05 and ***p < 0.001. ns, not significant. Scale bar = 100 μm
Astrocyte-specific Myd88 KO did not affect metabolic phenotypes in the normal diet condition
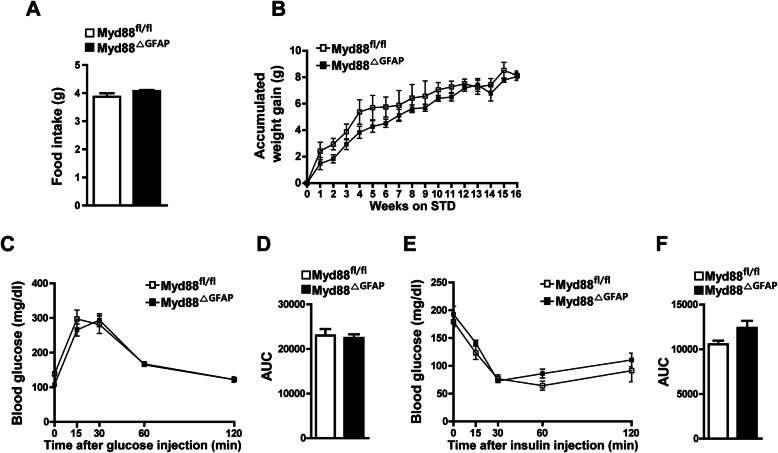
Before we determined whether HFD-induced metabolic disorder could be ameliorated by specific Myd88 KO in the astrocytes, we investigated the effect of astrocyte-specific Myd88 KO on energy metabolism under the STD feeding condition. We examined alterations in metabolic parameters (food intake, body weight, and glucose metabolism) between the conditional KO (Myd88ΔGFAP) mice and control mice. The Myd88ΔGFAP mice did not show any difference in food intake or body weight compared with control mice (Fig. 4a, b). Furthermore, the Myd88ΔGFAP mice displayed normal glucose metabolism in the GTTs and ITTs (Fig. 4c–f). These results indicate that astrocyte-specific Myd88 KO did not cause metabolic abnormalities in the STD condition.
Fig. 4.
Astrocyte-specific Myd88 KO does not change energy metabolism of mice under a standard diet (STD) feeding condition. Metabolic parameters of astrocyte-specific Myd88 KO (Myd88△GFAP) mice and control Myd88fl/fl mice were determined after STD feeding for 16 weeks. No difference was observed between the experimental groups in average daily food intake (a), body weight (b), glucose tolerance test (c, d), or insulin tolerance test (e, f) (n = 4–5/group). STD, standard food. AUC, area under curve. Data are presented as mean ± SEM
Astrocyte-specific ablation of Myd88 alleviated HFD-induced metabolic aggravation
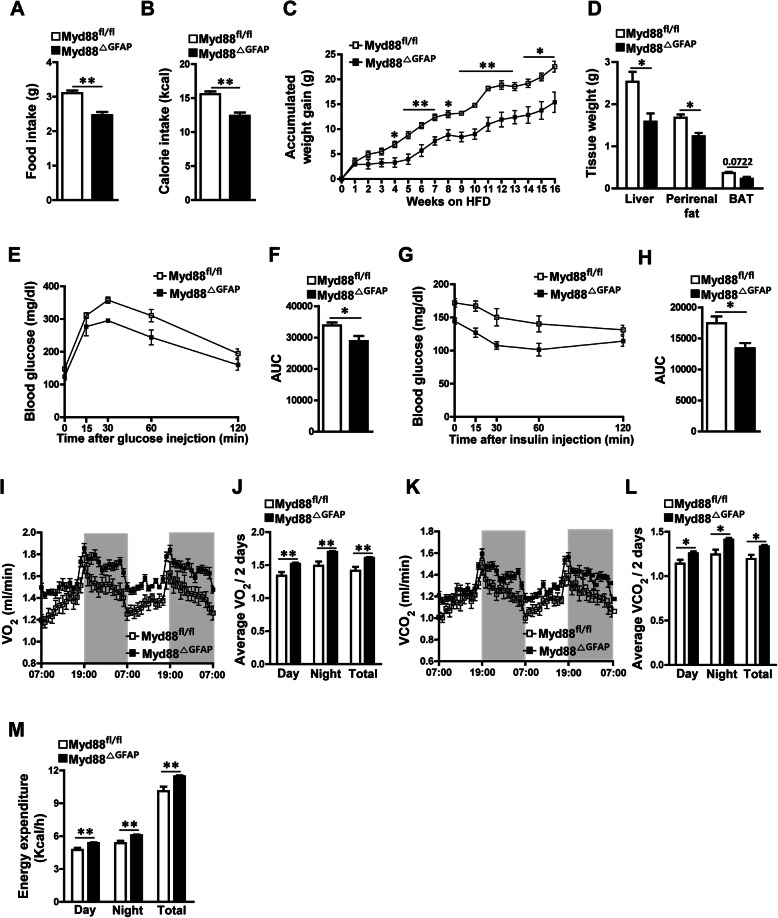
To identify the pathological relevance of the reduced hypothalamic gliosis seen in the HFD-treated Myd88ΔGFAP mice, both control and Myd88ΔGFAP mice were fed an HFD for 16 weeks, and then their metabolic parameters were measured. The Myd88ΔGFAP mice fed an HFD revealed a significant decrease in food intake and calorie intake compared with the HFD-fed control mice (Fig. 5a, b). Additionally, HFD-induced weight gain was significantly alleviated in the Myd88ΔGFAP mice compared with control mice during the observation period (Fig. 5c). Along with the difference in body weight gain during HFD feeding, the peripheral metabolic organs, such as the liver and perirenal fat, of the Myd88ΔGFAP mice weighed less than those of the control mice (Fig. 5d). Accordingly, the Myd88ΔGFAP mice displayed improved glucose metabolism, as shown in GTTs and ITTs, after long-term exposure to HFD (Fig. 5e–h). To further investigate the effect of astrocyte-specific Myd88 KO on energy expenditure, we measured multiple metabolic parameters using indirect calorimetry. The Myd88ΔGFAP mice showed significant elevations of VO2, VCO2, and energy expenditure after long-term exposure to HFD, compared with control mice (Fig. 5i–m). Collectively, these observations demonstrate that selective ablation of the Myd88 gene in astrocytes ameliorates diet-induced obesity (DIO) and impaired glucose metabolism by affecting food intake and energy expenditure.
Fig. 5.
Astrocyte-specific Myd88 KO affects HFD-induced obesity. Astrocyte-specific Myd88 KO (Myd88△GFAP) mice and control Myd88fl/fl mice were fed an HFD for 16 weeks, and then their metabolic parameters were measured. a, b Average daily food intake (a) and average daily calorie intake (b) were measured for a week at 23–24 weeks of age during HFD feeding (n = 4/group). c The accumulated weight gain of the mice was observed every week during HFD feeding (n = 4/group). HFD, high-fat diet. d The weight of adipose tissues was measured at 24 weeks of age (n = 4/group). BAT, brown adipose tissue. e–h Glucose tolerance testing (e, f) and insulin tolerance testing (g, h) were carried out in mice after HFD feeding for 16 weeks (n = 5–9/group). AUC, area under curve. i–m Indirect calorimetry measurements were performed in metabolic cages to determine changes in the oxygen consumption (VO2) (i, j), carbon dioxide generation (VCO2) (k, l), and energy expenditure (m) of mice after HFD feeding for 16 weeks (n = 8/group). Data are presented as mean ± SEM. *p < 0.05, and **p < 0.01
Astrocyte-specific deletion of the Myd88 gene ameliorated long-term HFD feeding–induced leptin resistance
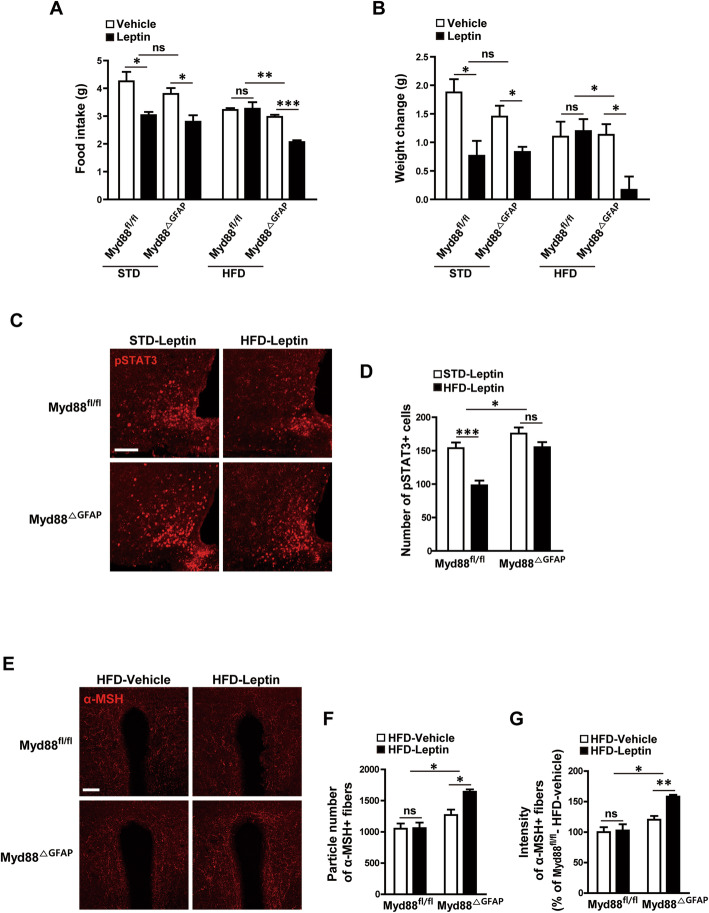
It has been well established that hypothalamic inflammation is a primary cause of leptin resistance, which is deeply associated with obesity pathogenesis. Therefore, we next investigated the responsiveness of the Myd88ΔGFAP mice to leptin after 16 weeks of HFD feeding. An ip administration of leptin (2 mg/kg body weight) effectively reduced food intake and body weight in both control and Myd88ΔGFAP mice fed a STD diet, indicating that astrocyte-specific Myd88 KO did not affect leptin responsiveness under non-obesity conditions. Leptin-induced reduction in food intake and body weight disappeared completely in control (Myd88fl/fl) mice fed an HFD for 16 weeks, indicating a condition of leptin resistance. However, Myd88ΔGFAP mice fed an HFD for 16 weeks continued to display a decrease in food intake and body weight in response to leptin, indicating ameliorated leptin resistance (Fig. 6a, b).
Fig. 6.
Astrocyte-specific Myd88 KO enhances leptin responsiveness after long-term HFD feeding. a, b To identify the effects of astrocyte-specific Myd88 KO (Myd88△GFAP) on HFD-induced leptin resistance, mice were fed an HFD for 16 weeks, and their food intake (a) and body weight (b) were measured for 24 h after an intraperitoneal injection of leptin (2 mg/kg) or vehicle (n = 3–4/group). c, d Representative immunohistochemical images (c) and calculated graphs (d) show that leptin-induced pSTAT3 in the arcuate nucleus had deteriorated in the control Myd88fl/fl mice after 16 weeks of HFD feeding. However, the Myd88△GFAP mice fed the HFD for 16 weeks showed a leptin-induced pSTAT3 level similar to those with STD feeding. e–g Representative images (e) and calculated data (f, g) reveal that leptin induced an increase in α-MSH immuno-positive signals in the paraventricular nucleus of the Myd88△GFAP mice but not the control Myd88fl/fl mice after 16 weeks of HFD feeding (n = 3 sections of 3 mice/group). Data are presented as mean ± SEM. *p < 0.05, **p<0.01 and ***p < 0.001. ns, not significant. Scale bar = 100 μm
Because phosphorylation of signal transducer and activator of transcription 3 (STAT3) is a cellular event that reflects the activation of leptin signaling, we determined leptin-induced STAT3 phosphorylation (pSTAT3) in the Myd88ΔGFAP mice. The Myd88ΔGFAP mice preserved leptin-induced pSTAT3 in the hypothalamic ARC after 16 weeks of HFD feeding, whereas the control mice lost the normalcy of leptin-induced pSTAT3 after long-term HFD feeding (Fig. 6c, d). Furthermore, leptin induced an increase in the number and intensity of α-MSH fibers in the paraventricular nucleus (PVN) of the Myd88ΔGFAP mice after HFD feeding for 16 weeks, whereas it failed to induce a significant change in the α-MSH fibers of the PVN of the control Myd88fl/fl mice (Fig. 6e–g). Collectively, these observations suggest that astrocyte MyD88 signaling is closely correlated with leptin responsiveness during long-term over-nutrition.
Discussion
In the present study, we found that Myd88 expression in hypothalamic astrocytes was increased by long-term HFD feeding and that astrocyte-specific ablation of Myd88 ameliorated the obesity-related metabolic phenotype induced by HFD consumption. The current observations demonstrate that MyD88 signaling in astrocytes is a critical contributor to the hypothalamic inflammation–induced pathogenesis of obesity.
In this study, we focused on the role of MyD88 signaling in astrocytes as a mediator of hypothalamic inflammation during HFD feeding. Interestingly, we found that HFD-induced reactive gliosis was decreased in hypothalamic ARC of mutant mice lacking Myd88 expression in astrocytes compared to control mice. Moreover, a direct icv infusion of sFFAs induced reactive gliosis in hypothalamic ARC of control mice, but not in astrocyte-specific Myd88 KO mice. These results suggested that MyD88 signaling in astrocytes plays a critical role in the hypothalamic reactive gliosis induced by HFD and sFFAs. In animals chronically fed an HFD, reactive astrocytes in the hypothalamus induced neuronal damage by secreting proinflammatory cytokines [14, 26]. In this DIO model, the proinflammatory cytokines released by hypothalamic astrocytes and microglia activated cytokine receptors on the hypothalamic proopiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons [12, 20, 30, 31]. In the current study, astrocyte-specific Myd88 KO resulted in a decrease in activation of microglia in the ARC and expression of proinflammatory cytokines in the hypothalamus during HFD feeding. These changes might be due to that astrocyte-specific deletion of Myd88 gene caused a decrease in HFD-induced intracellular signaling for the activation of proinflammatory cytokine expression in the hypothalamic astrocytes. Since the ARC of the hypothalamus is considered as a major site for the actions of POMC and AgRP neurons that dynamically participate in the central control of energy homeostasis, we focused on the action of astrocytes in the hypothalamic ARC. However, we could not exclude a possible contribution of astrocytes in other sites of the hypothalamus including the median eminence and third ventricular linings to the development of inflammatory processes. Therefore, further studies are required to identify subpopulations of hypothalamic astrocytes that are important in the initiation of hypothalamic inflammation during a long-term exposure to over-nutrition.
HFD-induced hypothalamic reactive gliosis and inflammatory responses are important factors in leptin sensitivity during HFD consumption [14, 20]. Despite high circulating leptin levels, over-nutrition-induced obese mice show a reduced responsiveness to the appetite- and weight gain–suppressing effects of leptin, which is generally called leptin resistance and is a critical element in the development of obesity. It has been proposed that development of leptin resistance is coupled to multiple cellular events including abnormalities of leptin signaling, leptin transportation, and leptin receptor trafficking [32]. Particularly, it is currently accepted that the hypothalamic gliosis accompanied by enhanced inflammation responses is regarded as a crucial pathological element during the over-nutrition period [12, 14, 20]. In line with these notions, we observed that the Myd88ΔGFAP mice displayed anti-obesity phenotype in association with improved responsiveness to leptin after long-term HFD feeding. In accordance with the behavioral observations, we confirmed the leptin-triggered induction of pSTAT3, a general molecular marker for the leptin responsiveness, in the hypothalamic ARC. Given that leptin signaling operates the activity of POMC neurons that govern hypothalamic cells expressing melanocortin receptors by releasing α-MSH, we further validated improved responsiveness of the Myd88 KO mice to leptin by identifying the enhanced innervation of α-MSH in the hypothalamic PVN, even after long-term HFD feeding. These observations strengthen the underappreciated role of astrocytic TLR-MyD88 signaling in the regulation of over-nutrition-induced hypothalamic inflammation and metabolic abnormalities. Notably, previous literatures have suggested that leptin receptors are present in hypothalamic astrocytes and participate in the central control of energy metabolism [33–35]. However, active role of the leptin receptors in hypothalamic astrocytes is still controversial. Thus, this study raised an important question whether MyD88 is connected to the leptin receptor signaling in hypothalamic astrocytes. It is well-known that over-nutrition-induced inflammatory factors activate NF-κB signaling, which upregulates negative regulators of leptin signaling, such as suppressor of cytokine signaling 3, and thus inhibits pSTAT3 signaling [20, 36, 37]. In addition, elevated activity of TLRs and MyD88 coupling leads to the activation of NF-κB signaling in hypothalamic neurons [20, 21]. Therefore, these evidences and current findings together suggest that MyD88 signaling in astrocytes is important in leptin resistance caused by HFD-induced inflammation and obesity pathogenesis.
Although studies to understand the mechanisms that underlie leptin resistance during HFD consumption have focused on the role of astrocytes and microglia, the cooperative actions and relationships between them during HFD-induced neuroinflammation remain largely unknown. Several reports have revealed that crosstalk occurs between astrocytes and microglia through the release of signaling factors (such as cytokines and chemokines) that contribute to the pathogenesis of neuroinflammation and neurodegeneration [31, 38]. A recent in vitro study showed that the accumulation of lipid droplets in hypothalamic astrocytes could be induced by sFFA treatment and led to the activation of microglia through inflammatory cytokines [39]. In our study, astrocyte-specific Myd88 KO blocked HFD-induced microglial activation. In line with previous reports, this result might indicate that astrocyte-specific Myd88 KO decreased HFD-induced expression of cytokines in astrocytes, which further indicates crosstalk between astrocytes and microglia and the importance of astrocyte MyD88 signaling in HFD-induced obesity.
Conclusions
The present study reports that MyD88 signaling in astrocytes is a key mediator of obesity pathogenesis and highlights its contribution to over-nutrition-induced reactive gliosis and leptin resistance in the hypothalamus. These observations suggest that MyD88 signaling in hypothalamic astrocytes could be an important novel target for the treatment of metabolic disorders such as leptin resistance and obesity.
Acknowledgements
Not applicable.
Abbreviations
- KO
Knockout
- HFD
High-fat diet
- MyD88
Myeloid differentiation primary response 88
- TLR
Toll-like receptor
- sFFA
Saturated free fatty acid
- STD
Standard diet
- GFAP
Glial fibrillary acidic protein
- HA
Hemagglutinin
- BSA
Bovine serum albumin
- BrdU
Bromodeoxyuridine
- PB
Phosphate buffer
- ARC
Arcuate nucleus
- α-MSH
α-Melanocyte stimulating hormone
- GTTs
Glucose tolerance tests
- ITTs
Insulin tolerance tests
- VO2
Oxygen consumption
- VCO2
Carbon dioxide generation
- Iba1
Ionized calcium-binding adaptor molecule 1
- NeuN
Neuronal nuclei
- TNF-α
Tumor necrosis factor-α
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- DIO
Diet-induced obesity
- STAT3
Signal transducer and activator of transcription 3
- PVN
Paraventricular nucleus
- NF-κB
Nuclear factor kappa B
- POMC
Proopiomelanocortin
- AgRP
Agouti-related peptide
Authors’ contributions
SJ, KKK, JGK, and BJL designed the experiments, interpreted results, and wrote the manuscript. SJ and KKK performed and analyzed most of the experiments. BSP performed the indirect calorimetry analysis. DHK, BJ, and DK performed Ribo-Tag system processes and the real-time PCR analysis. THL performed histological analyses. JWP provided intellectual input. The authors read and approved the final manuscript.
Funding
This research was supported by a Korea Science and Engineering Foundation (KOSEF) grant (NRF-2015M3A9E7029177) and the Priority Research Centers Program (NRF-2014R1A6A1030318) through the National Research Foundation of Korea. JGK was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1600).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee of the University of Ulsan (permission numbers: BJL-15-010, BJL-18-010, and BJL-19-010).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sungho Jin and Kwang Kon Kim contributed equally to this work.
Contributor Information
Jae Geun Kim, Email: jgkim@inu.ac.kr.
Byung Ju Lee, Email: bjlee@ulsan.ac.kr.
References
- 1.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 2.Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 3.Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Horvath TL. Synaptic plasticity in energy balance regulation. Obesity. 2006;14(Suppl 5):228S–233S. doi: 10.1038/oby.2006.314. [DOI] [PubMed] [Google Scholar]
- 6.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 7.Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22:962–970. doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalin S, Heppner FL, Bechmann I, Prinz M, Tschop MH, Yi CX. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol. 2015;11:339–351. doi: 10.1038/nrendo.2015.48. [DOI] [PubMed] [Google Scholar]
- 12.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2013;521:1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Caceres C, Yi CX, Tschop MH. Hypothalamic astrocytes in obesity. Endocrinol Metab Clin North Am. 2013;42:57–66. doi: 10.1016/j.ecl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Argente-Arizon P, Freire-Regatillo A, Argente J, Chowen JA. Role of non-neuronal cells in body weight and appetite control. Front Endocrinol. 2015;6:42. doi: 10.3389/fendo.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 18.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Santamarina AB, Jamar G, Mennitti LV, de Rosso VV, Cesar HC, Oyama LM, Pisani LP. The Use of Jucara (Euterpe edulis Mart.) Supplementation for suppression of NF-kappaB pathway in the hypothalamus after high-fat diet in Wistar rats. Molecules. 2018:23. [DOI] [PMC free article] [PubMed]
- 20.de Git KC, Adan RA. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes Rev. 2015;16:207–224. doi: 10.1111/obr.12243. [DOI] [PubMed] [Google Scholar]
- 21.Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang SS, Ebbert MTW, Baker KE, Cook C, Wang X, Sens JP, Kocher JP, Petrucelli L, Fryer JD. Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J Exp Med. 2018;215:2235–2245. doi: 10.1084/jem.20180653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JD, Yoon NA, Jin S, Diano S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metab. 2019;30:952–962. doi: 10.1016/j.cmet.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Caceres C, Fuente-Martin E, Argente J, Chowen JA. Emerging role of glial cells in the control of body weight. Mol Metab. 2012;1:37–46. doi: 10.1016/j.molmet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S, Kim JG, Park JW, Koch M, Horvath TL, Lee BJ. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci Rep. 2016;6:29424. doi: 10.1038/srep29424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman MH, Kim MS, Lee IK, Yu R, Suk K. Interglial crosstalk in obesity-induced hypothalamic inflammation. Front Neurosci. 2018;12:939. doi: 10.3389/fnins.2018.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdearcos M, Myers MG, Koliwad SK. Hypothalamic microglia as potential regulators of metabolic physiology. Nat Metab. 2019;7:731–742. doi: 10.1038/s42255-019-0040-0. [DOI] [PubMed] [Google Scholar]
- 34.Tsunekawa T, Banno R, Mizoguchi A, Sugiyama M, Tominaga T, Onoue T, Hagiwara D, Ito Y, Iwama S, Goto M, et al. Deficiency of PTP1B attenuates hypothalamic inflammation via activation of the JAK2-STAT3 pathway in microglia. EBioMedicine. 2017;16:172–183. doi: 10.1016/j.ebiom.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang PG, Namkoong C, Kang GM, Hur MW, Kim SW, Kim GH, Kang Y, Jeon MJ, Kim EH, Lee MS, et al. NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia. J Biol Chem. 2010;285:9706–9715. doi: 10.1074/jbc.M109.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi X, Wang X, Li Q, Su M, Chew E, Wong ET, Lacza Z, Radda GK, Tergaonkar V, Han W. Nuclear factor kappaB (NF-kappaB) suppresses food intake and energy expenditure in mice by directly activating the Pomc promoter. Diabetologia. 2013;56:925–936. doi: 10.1007/s00125-013-2831-2. [DOI] [PubMed] [Google Scholar]
- 38.Jha MK, Jo M, Kim JH, Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25:227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 39.Kwon YH, Kim J, Kim CS, Tu TH, Kim MS, Suk K, Kim DH, Lee BJ, Choi HS, Park T, et al. Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett. 2017;591:1742–1751. doi: 10.1002/1873-3468.12691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.