Abstract
Background
Leukemias are a group of life-threatening malignant disorders of the blood and bone marrow. The incidence of leukemia varies by pathological types and among different populations.
Methods
We retrieved the incidence data for leukemia by sex, age, location, calendar year, and type from the Global Burden of Disease online database. The estimated average percentage change (EAPC) was used to quantify the trends of the age-standardized incidence rate (ASIR) of leukemia from 1990 to 2017.
Results
Globally, while the number of newly diagnosed leukemia cases increased from 354.5 thousand in 1990 to 518.5 thousand in 2017, the ASIR decreased by 0.43% per year. The number of acute lymphoblastic leukemia (ALL) cases worldwide increased from 49.1 thousand in 1990 to 64.2 thousand in 2017, whereas the ASIR experienced a decrease (EAPC = − 0.08, 95% CI − 0.15, − 0.02). Between 1990 and 2017, there were 55, 29, and 111 countries or territories that experienced a significant increase, remained stable, and experienced a significant decrease in ASIR of ALL, respectively. The case of chronic lymphocytic leukemia (CLL) has increased more than twice between 1990 and 2017. The ASIR of CLL increased by 0.46% per year from 1990 to 2017. More than 85% of all countries saw an increase in ASIR of CLL. In 1990, acute myeloid leukemia (AML) accounted for 18.0% of the total leukemia cases worldwide. This proportion increased to 23.1% in 2017. The ASIR of AML increased from 1.35/100,000 to 1.54/100,000, with an EAPC of 0.56 (95% CI 0.49, 0.62). A total of 127 countries or territories experienced a significant increase in the ASIR of AML. The number of chronic myeloid leukemia (CML) cases increased from 31.8 thousand in 1990 to 34.2 thousand in 2017. The ASIR of CML decreased from 0.75/100,000 to 0.43/100,000. A total of 141 countries or territories saw a decrease in ASIR of CML.
Conclusions
A significant decrease in leukemia incidence was observed between 1990 and 2017. However, in the same period, the incidence rates of AML and CLL significantly increased in most countries, suggesting that both types of leukemia might become a major global public health concern.
Keywords: Leukemia, ALL, CLL, AML, CML, Incidence, Global
Background
Leukemias are a group of malignant disorders that present with increased numbers of leucocytes in the blood and/or bone marrow. In 2018, it is estimated there were a total of 437.0 thousand new cases of and 309.0 thousand cancer deaths from leukemia worldwide [1]. Leukemia types vary in pathogenesis, origin, incidence, and prognosis. The dominantly presenting leukemia cells can be mature cells, such as in chronic lymphocytic leukemia (CLL); precursor cells of various lineage, such as in the acute leukemias; or both precursor and mature cells, such as in chronic myeloid leukemia (CML) [2, 3]. Among acute leukemia types, acute lymphoblastic leukemia (ALL) is frequently diagnosed in children and young adults, with incidence peaks between 2 and 5 years of age [4], whereas acute myeloid leukemia (AML) is the most common acute type in adults, accounting for 1.3% of new cancer cases in the USA [5]. In addition, a set of rarely diagnosed leukemia types (e.g., atypical chronic myeloid leukemia) differs from the four main types in many aspects [3, 6, 7].
The incidence of leukemia varies among people of different ages, sexes, and races [8]. Such disparities are mainly associated with the levels of exposure to environmental and genetic risk factors. For example, about 10% of individuals who develop CLL have a family history of the disease [9], while ionizing radiation is an established causal exposure for childhood ALL, as evidenced by the modest but significantly elevated risk caused by X-ray pelvimetry during pregnancy [10]. Owing to the previous efforts to combat leukemia, the epidemiology of leukemia might change over time and vary from country to country. Knowing the updated epidemiological data for leukemia and analyzing the temporal trends of leukemia incidence are vital for learning the disease burden of leukemia and assessing the effectiveness of previous prevention strategies. To fill this gap, we used data from the Global Burden of Disease (GBD) 2017 study to analyze the incidence trends of leukemia by sex, location, and type at the global, regional, and national levels.
Methods
Data sources
The GBD study provides a tool to quantify health loss from hundreds of diseases, injuries, and risk factors so that health systems can be improved and disparities eliminated [11]. Data from the GBD study have also been widely used to learn the disease burden of cancer [12–16]. In the GBD study framework, the cancer incidence data were retrieved from individual cancer registries or aggregated databases of cancer registry data, such as “Cancer Incidence in Five Continents” (CI5), EUREG, or NORDCAN. Data were excluded if they were not representative of the coverage population (e.g., hospital-based registries), if they did not cover all malignant neoplasms as defined in ICD-9 (140–208) or ICD-10 (C00-C96) (e.g., specialty cancer registry), if they did not include data for both sexes and all age groups, if the data were limited to years prior to 1980, or if the source did not provide details on the population covered. Preference was given to registries with national coverage over those with only local coverage, except for countries where the GBD study provides subnational estimates [17]. The leukemia cases were identified using ICD-10 codes (C91–C93.7, C93.9–C95.2, C95.7–C95.92, Z80.6, Z85.6) and ICD-9 codes (204–208.92, V10.59–V10.69, V16.6). The ALL, CLL, AML, and CML cases were identified using the ICD-10 codes of “C91.0–C91.02,” “C91.1–C91.12,” “C92.0–C92.02, C92.3–C92.62, C93.0–C93.02, C94.0–C94.02, C94.2–C94.22, C94.4–C94.5,” and “C92.1–C92.12″ and the ICD-9 codes of “204.0–204.02,” “204.1–204.12,” “205.0–205.02, 205.3–205.32, 206.0–206.02, 207.0,” and “205.1–205.12, 206.1–206.12, 207.1,” respectively. Leukemias outside the four main types were defined as “other leukemias” and identified using the ICD-10 codes of “C91.2-C91.9, C92.2, C92.7–C92.9, C93.1–C93.9, C94.1, C94.3, C94.6–C95.9″ and the ICD-9 codes of “204.2–204.9, 205.2, 205.8–205.9, 206.2–207, 207.2–208.9.” In the current study, we retrieved the incidence data of leukemia by sex, calendar year, location, and age from the GBD online database [18]. A total of 5 Socio-demographic Index (SDI) regions (e.g., high SDI region), 21 GBD geographic regions (e.g., East Asia), and 195 countries or territories were included. The national SDI values were also retrieved from the GBD online database. SDI provides a composite average of rankings for the incomes per capita, average educational attainment, and fertility rates of all areas in the GBD study.
Statistical analysis
We used the estimated average percentage change (EAPC) to quantify the trends of the age-standardized incidence rate (ASIR) of leukemia from 1990 to 2017. As described previously, EAPC can be calculated by a regression line, which was fitted to the natural logarithm of the rates, i.e., y = α + βx + ɛ, where y = ln(rate) and x = calendar year [19]. If the EAPC is statistically significant but the uncertainty intervals of the GBD estimates overlap, the ASIR is still considered to be stable [20]. The Pearson correlation tests were applied to assess the correlations between SDI values and changing leukemia incidence trends. All statistical analyses were performed using the R program (Version 3.5.3, R core team, Vienna, Austria). A P-value less than 0.05 was considered statistically significant.
Results
Leukemia incidence trends at the global level
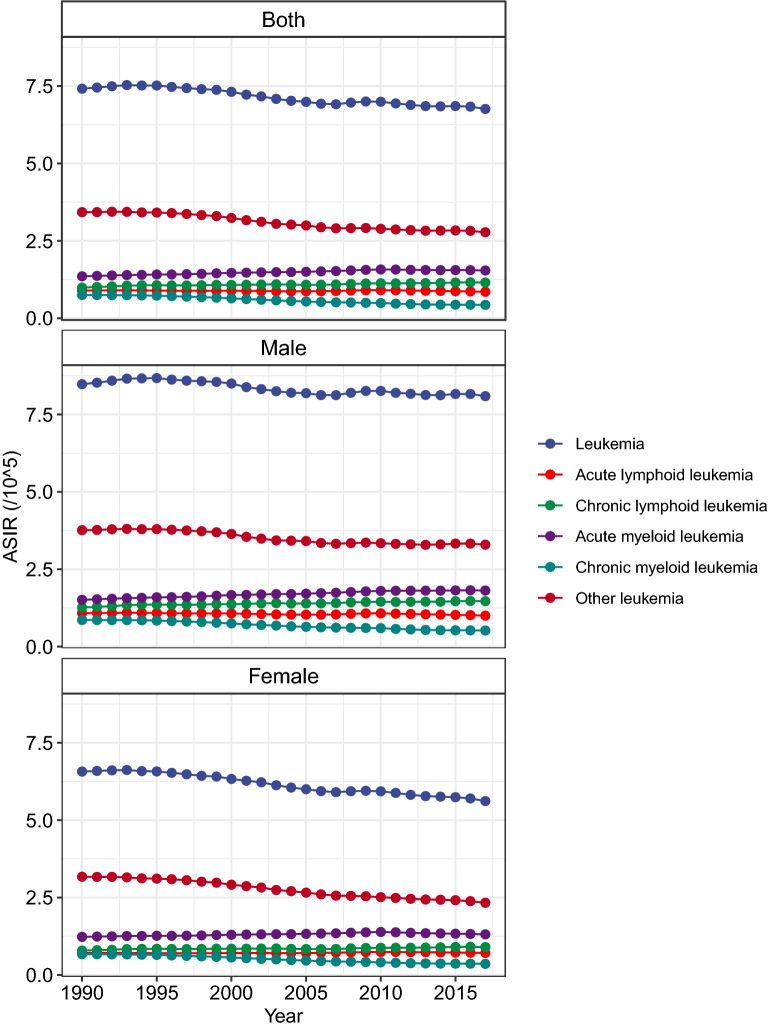
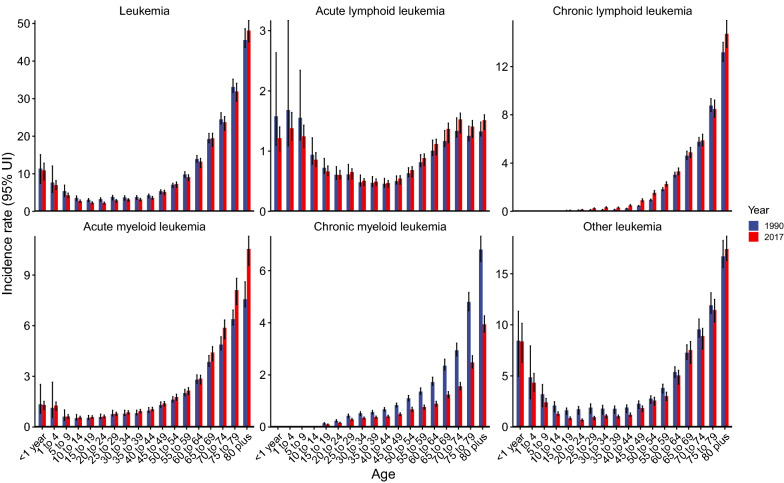
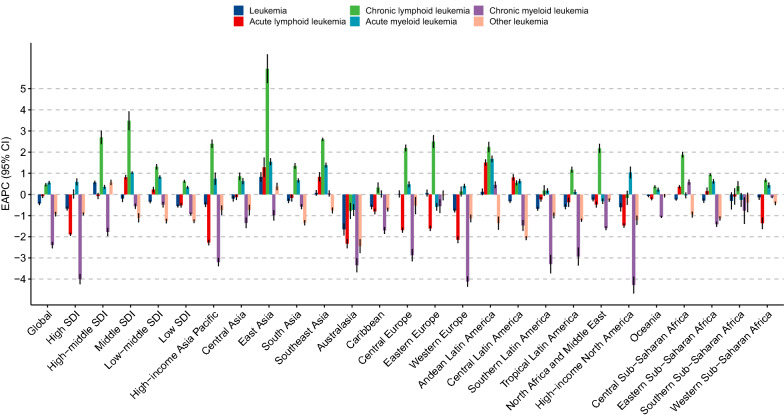
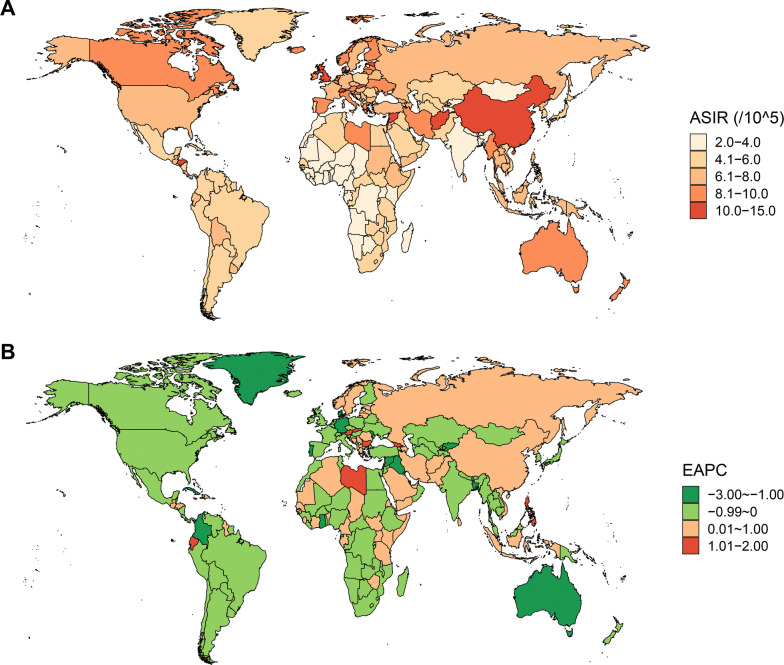
Globally, while the number of newly diagnosed leukemia cases increased from 354.5 thousand in 1990 to 518.5 thousand in 2017, the ASIR deceased by 0.43% per year during the same period (Table 1; Fig. 1). The leukemia ASIR was higher in males than in females, whereas females experienced a more pronounced decrease in ASIR during the study period when compared to males (Table 1). Figure 2 displays a J-shaped age distribution of leukemia incidence in both 1990 and 2017. We integrated the 18 age groups into five age groups and found that the leukemia incidence only increased among people aged ≥ 70 years (Table 1). The greatest decrease was observed in people aged 5–14 years. More than half of the total cancer cases occurred in high and high-middle SDI regions. In 1990, the highest incidence occurred in high SDI regions (9.10/100,000), whereas in 2017, the highest incidence occurred in high-middle SDI regions (8.68/100,000). Only high-middle regions experienced a significant increase in leukemia ASIR during the study period (Table 1; Fig. 3). In 1990, the high leukemia ASIR was found in regions with advanced economies, such as Western Europe and Australasia. The ASIR experienced a remarkable decrease in these regions between 1990 and 2017. In 2017, the highest ASIR was seen in East Asia, where the ASIR increased by 0.83% per year (Table 1; Fig. 3). At the national level, the leukemia ASIR was highest in Syria (14.83/100,000), followed by the UK, Denmark, and Lebanon in 2017 (Fig. 4a). Between 1990 and 2017, a total of 58, 31, and 106 countries or territories experienced a significant increase, remained stable, and experienced a significant decrease in leukemia ASIR, respectively. The greatest increase was found in Slovakia (EAPC = 1.80, 95% CI 1.59, 2.00), followed by Jamaica and Ecuador (Fig. 4b; Additional file 1: Table S1). The most pronounced decrease was found in Bahrain (EAPC = − 2.60, 95% CI − 3.04, − 2.16), followed by Iraq and Australia (Fig. 4b; Additional file 1: Table S1).
Table 1.
The incidence and its trends of leukemia, by sex, age, type, and region, between 1990 and 2017
| 1990 | 2017 | 1990-2017 | |||
|---|---|---|---|---|---|
| Case number () | ASIR (/105) | Case number () | ASIR (/105) | EAPC (95% CI) | |
| Overall | 354.5 | 7.42 | 518.5 | 6.76 | − 0.43 (− 0.48, − 0.38) |
| Sex | |||||
| Male | 191.3 | 8.48 | 295.4 | 8.09 | − 0.26 (− 0.32, − 0.21) |
| Female | 163.1 | 6.57 | 223.1 | 5.62 | − 0.65 (− 0.70, − 0.60) |
| Age (years)a | |||||
| Under 5 | 54.4 | 8.44 | 52.9 | 7.78 | − 0.12 (− 0.20, − 0.05) |
| 5–14 | 50.6 | 4.51 | 45.5 | 3.51 | − 1.18 (− 1.38, − 0.98) |
| 15–49 | 102.4 | 3.74 | 123.1 | 3.15 | − 0.89 (− 1.00, − 0.77) |
| 50–69 | 80.0 | 11.64 | 149.7 | 11.36 | − 0.24 (− 0.33, − 0.15) |
| 70+ | 67.0 | 32.96 | 147.3 | 34.03 | 0.11 (0.08, 0.14) |
| Types | |||||
| ALL | 49.1 | 0.89 | 64.2 | 0.85 | − 0.08 (− 0.15, − 0.02) |
| CLL | 38.0 | 0.99 | 90.6 | 1.15 | 0.46 (0.40, 0.52) |
| AML | 63.8 | 1.35 | 119.6 | 1.54 | 0.56 (0.49, 0.62) |
| CML | 31.8 | 0.75 | 34.2 | 0.43 | − 2.40 (− 2.53, − 2.26) |
| Others | 171.8 | 3.43 | 209.9 | 2.78 | − 0.93 (− 1.02, − 0.84) |
| SDI region | |||||
| High | 106.6 | 9.10 | 150.8 | 7.73 | − 0.67 (− 0.74, − 0.60) |
| High-middle | 80.4 | 7.59 | 123.7 | 8.68 | 0.56 (0.50, 0.63) |
| Middle | 93.2 | 6.57 | 131.9 | 6.47 | − 0.19 (− 0.34, − 0.04) |
| Low-middle | 44.4 | 4.87 | 66.8 | 4.54 | − 0.34 (− 0.40, − 0.28) |
| Low | 27.9 | 4.68 | 42.6 | 4.14 | − 0.55 (− 0.59, − 0.50) |
| GBD region | |||||
| Andean Latin America | 2.1 | 6.00 | 3.5 | 5.97 | 0.12 (− 0.01, 0.26) |
| Australasia | 3.0 | 13.38 | 4.2 | 9.62 | − 1.65 (− 1.92, − 1.38) |
| Caribbean | 2.1 | 6.72 | 2.8 | 5.82 | − 0.59 (− 0.68, − 0.50) |
| Central Asia | 3.5 | 5.17 | 4.0 | 4.74 | − 0.20 (− 0.31, − 0.08) |
| Central Europe | 9.1 | 6.71 | 11.1 | 6.37 | 0.02 (− 0.12, 0.17) |
| Central Latin America | 9.1 | 6.39 | 14.5 | 5.89 | − 0.32 (− 0.38, − 0.27) |
| Central sub-Saharan Africa | 1.8 | 4.07 | 3.4 | 3.87 | − 0.24 (− 0.27, − 0.20) |
| East Asia | 102.3 | 8.49 | 147.7 | 10.54 | 0.83 (0.63, 1.02) |
| Eastern Europe | 17.1 | 7.00 | 19.7 | 6.91 | 0.06 (− 0.08, 0.21) |
| Eastern sub-Saharan Africa | 7.2 | 4.66 | 13.3 | 4.48 | − 0.29 (− 0.38, − 0.20) |
| High-income Asia Pacific | 11.3 | 6.22 | 17.8 | 5.27 | − 0.47 (− 0.57, − 0.38) |
| High-income North America | 27.4 | 8.17 | 39.6 | 7.17 | − 0.61 (− 0.78, − 0.43) |
| North Africa and Middle East | 19.3 | 7.08 | 32.3 | 6.37 | − 0.24 (− 0.31, − 0.17) |
| Oceania | 0.3 | 6.61 | 0.6 | 6.34 | − 0.07 (− 0.11, − 0.03) |
| South Asia | 40.7 | 4.24 | 62.0 | 3.99 | − 0.30 (− 0.39, − 0.22) |
| Southeast Asia | 24.2 | 6.26 | 38.9 | 6.37 | 0.07 (− 0.04, 0.19) |
| Southern Latin America | 3.0 | 6.25 | 3.9 | 5.29 | − 0.68 (− 0.75, − 0.61) |
| Southern sub-Saharan Africa | 1.6 | 4.36 | 2.5 | 4.10 | − 0.30 (− 0.69, 0.08) |
| Tropical Latin America | 7.2 | 5.50 | 10.2 | 4.65 | − 0.58 (− 0.67, − 0.49) |
| Western Europe | 56.8 | 10.62 | 75.7 | 8.86 | − 0.76 (− 0.83, − 0.69) |
| Western sub-Saharan Africa | 5.3 | 3.44 | 10.8 | 3.40 | − 0.14 (− 0.23, − 0.05) |
ASIR age-standardized incidence rate, EAPC estimated average percentage change, SDI socio-demographic index, ALL acute lymphoblastic leukemia, CLL chronic lymphoblastic leukemia, AML acute myeloid leukemia, CML chronic myeloid leukemia
aThe incidence rates for each age group were crude incidence rather than the age-standardized incidence
Fig. 1.
The temporal trends for the age-standardized incidence rate (ASIR) of leukemia by type and sex between 1990 and 2017
Fig. 2.
The incidence rate of leukemia by type and year among people of different ages
Fig. 3.
The estimated average percentage change (EAPC) and its 95% confidence interval (CI) of leukemia by type and location between 1990 and 2017
Fig. 4.
The age-standardized incidence rate (ASIR) of leukemia at the national level in 2017 (a) and the estimated average percentage change (EAPC) of leukemia ASIR between 1990 and 2017 (b)
ALL incidence trends at the global, regional, and national level
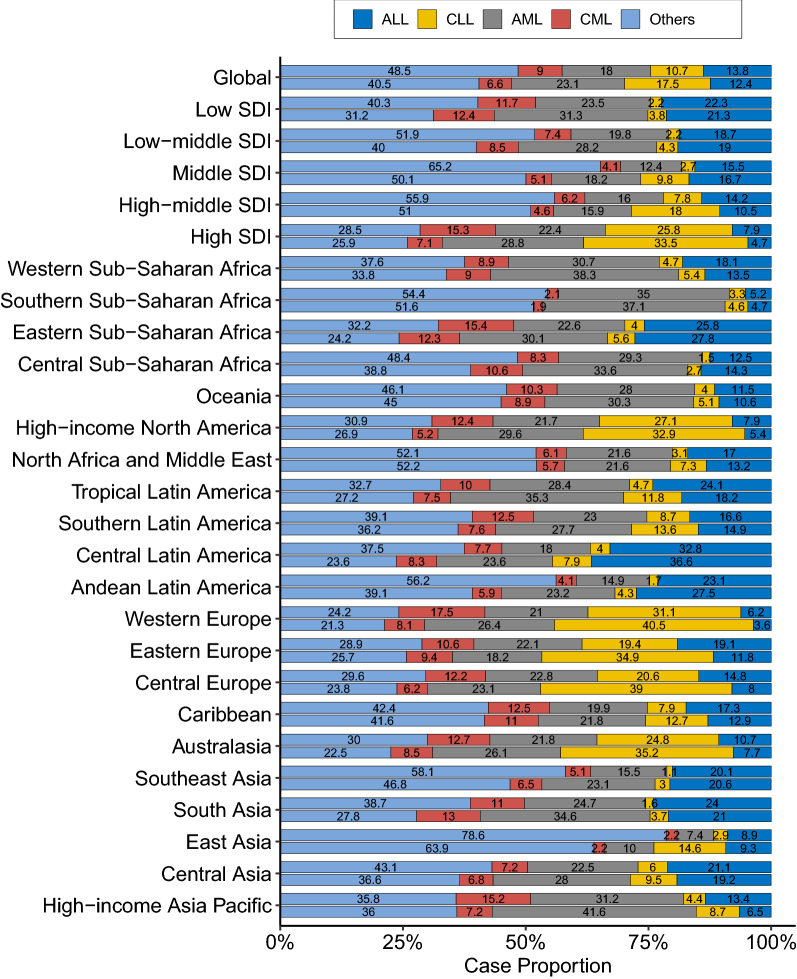
The global number of ALL cases increased from 49.1 thousand in 1990 to 64.2 thousand in 2017, whereas the ASIR experienced a mild decrease during this period (EAPC = − 0.08, 95% CI − 0.15, − 0.02; Fig. 1; Table 1). The proportion of ALL cases decreased from 13.8% to 12.4% between 1990 and 2017 (Fig. 5). ALL was more frequently diagnosed among children and young adults and in developing regions (Figs. 2 and 5). For example, in low-SDI regions, ALL cases accounted for > 20% of total leukemia cases in both 1990 and 2017. In Central Latin America, these proportions were > 30% (Fig. 5). Only five GBD regions experienced a significant increase in ASIR of ALL, with the greatest increase being observed in Andean Latin America, followed by East Asia and Southeast Asia (Fig. 3; Additional file 1: Table S1). As shown in Additional file 2: Figure S1, the ALL incidence was high in most developing countries. The highest incidence was found in Honduras (3.83/100,000), followed by Mexico and Dominica. Between 1990 and 2017, there were 55, 29, and 111 countries or territories that experienced a significant increase, remained stable, and experienced a significant decrease in ASIR of ALL, respectively (Additional file 1: Table S1, Additional file 2: Figure S1). The most pronounced increase was seen in El Salvador (EAPC = 5.20, 95% CI 4.34, 6.07; Additional file 1: Table S1, Additional file 2: Figure S1), followed by Guatemala and Ecuador. The greatest decrease was detected in Ghana (EAPC = − 5.18, 95% CI − 6.23, − 4.1), followed by Czech Republic and Guam (Additional file 1: Table S1, Additional file 2: Figure S1).
Fig. 5.
Proportions of leukemia cases by type and location in 1990 (top bar) and 2017 (bottom bar)
CLL incidence trends at the global, regional, and national level
The proportion of CLL cases more than doubled between 1990 and 2017, increasing from 10.7 to 17.5% during this period (Table 1; Fig. 5). The ASIR of CLL increased by 0.46% per year from 1990 to 2017 (Table 1; Fig. 1). CLL was rarely diagnosed among young people, and the highest incidence was found in people aged ≥ 80 years (Fig. 2). Between 1990 and 2017, most regions experienced a significant increase in the ASIR of CLL. The greatest increase was detected in East Asia (EAPC = 6.26, 95% CI 5.64 6.88), followed by Southeast Asia and Eastern Europe (Fig. 3; Additional file 1: Table S1). As shown in Additional file 2: Figure S2, the CLL incidence was high in developed countries, with the highest incidence being observed in the UK (5.27/100,000), followed by Denmark and Slovakia in 2017. At the national level, more than 85% of all countries experienced an increase in the ASIR of CLL between 1990 and 2017 (Additional file 1: Table S1, Additional file 2: Figure S1). The greatest increase was found in Jamaica (EAPC = 7.02, 95% CI 6.18, 7.86), followed by China and South Korea. Only 12 countries or territories experienced a significant decrease in the ASIR of CLL. The most pronounced decrease was observed in the Netherlands (EAPC = − 2.98, 95% CI − 4.12, − 1.82), followed by Bahrain and Kyrgyzstan (Additional file 1: Table S1, Additional file 2: Figure S1).
AML incidence trends at the global, regional, and national level
In 1990, AML accounted for 18.0% of total leukemia cases worldwide. This proportion increased to 23.1% in 2017 (Table 1; Fig. 5). The ASIR of AML increased from 1.35/100,000 to 1.54/100,000, with an EAPC of 0.56 (95% CI 0.49, 0.62) during this period (Table 1; Fig. 1). The incidence of AML showed a J-shaped age-pattern. In both 1990 and 2017, the lowest incidence was found among people aged 10–14 years (Fig. 2). The ASIR of AML increased in all five SDI regions and in a total of 13 GBD regions (Fig. 3; Additional file 1: Table S1). The greatest increase was found in Andean Latin America (EAPC = 1.68, 95% CI 1.55, 1.82), followed by East Asia and Southeast Asia. At the national level, the high incidence of AML was mostly observed in Europe. The highest incidence was found in the UK (4.05/100,000), followed by Brunei and Slovakia in 2017 (Additional file 2: Figure S3). A total of 127 countries or territories experienced a significant increase in the ASIR of AML between 1990 and 2017 (Additional file 1: Table S1; Additional file 2: Figure S3), with the highest increase being found in Ecuador (EAPC = 3.31, 95% CI 2.94, 3.69). Forty countries or territories experienced a significant decrease in the ASIR of AML during the same period. Most of these countries were located in the Middle East and Europe. The greatest decrease was found in Bahrain (EAPC = − 3.09, 95% CI − 3.57, − 2.60), followed by Qatar and Guyana (Additional file 1: Table S1; Additional file 2: Figure S3).
CML incidence trends at the global, regional, and national level
CML is diagnosed relatively less often compared to other types of leukemia (Table 1; Fig. 5). Globally, the number of CML cases increased from 31.8 thousand in 1990 to 34.2 thousand in 2017. The ASIR of CML decreased from 0.75/100,000 to 0.43/100,000 during the same period (Table 1; Fig. 1). CML is rarely diagnosed among children and young adults. The incidence of CML decreased among all age groups between 1990 and 2017 (Fig. 2). The ASIR of CML decreased in all SDI and GBD regions, with the exception of Andean Latin America (EAPC = 0.46, 95% CI 0.32, 0.60) and Central sub-Saharan Africa (EAPC = 0.58, 95% CI 0.47, 0.69) (Fig. 3). At the national level, the highest incidence of CML was found in Ethiopia (1.98/100,000), followed by Brunei and Honduras in 2017 (Additional file 2: Figure S4). Only 34 countries or territories experienced a significant increase in the ASIR of CML between 1990 and 2017. The greatest increase was found in Jamaica (EAPC = 2.87, 95% CI 2.55, 3.20), followed by El Salvador and Ecuador (Additional file 1: Table S1; Additional file 2: Figure S4). Conversely, 141 countries or territories saw a decrease in the ASIR of CML. The most pronounced decrease was found in Germany (EAPC = − 5.23, 95% CI − 5.52, − 4.95), followed by the UK and Hungary (Additional file 1: Table S1; Additional file 2: Figure S4).
Incidence trends of other leukemias at the global, regional, and national level
Worldwide, the number of cases for other leukemias increased from 171.8 thousand in 1990 to 209.9 thousand in 2017 (Table 1). The ASIR decreased by 0.93% per year during the study period (Table 1; Fig. 1). As shown in Fig. 2, the incidence of other leukemias showed a J-shaped age pattern. Approximately all SDI and GBD regions experienced a decreasing trend in the ASIR of other leukemias (Fig. 3). Exceptions were found in high-middle SDI regions and East Asia. In 2017, the highest ASIR was found in Syria (9.42/100,000), followed by Lebanon and China (Additional file 2: Figure S5). Between 1990 and 2017, 31 countries or territories experienced a significant increase in the ASIR of other leukemias. The greatest increase was observed in Slovakia (EAPC = 5.04, 95% CI 4.34, 5.75), followed by the Netherlands and Estonia (Additional file 1: Table S1; Additional file 2: Figure S5). In contrast, 139 countries or territories experienced a significant decrease in the ASIR of other leukemias. The most remarkable decrease was found in Equatorial Guinea (EAPC = − 3.5, 95% CI − 3.79, − 3.21), followed by Kyrgyzstan and Portugal (Additional file 1: Table S1; Additional file 2: Figure S5).
Discussion
In this study, we comprehensively analyzed the secular leukemia incidence trends at the global, regional, and national level. We found that the overall ASIRs of leukemia, ALL, CML, and other leukemias significantly decreased over the last three decades. However, the ASIRs of AML and CLL increased at different magnitudes. The incidence of leukemia was heterogeneous among people in different age groups and from country to country. ALL was commonly diagnosed among children and young adults, whereas CLL, AML, and CML more frequently occurred among older people. From a geographical perspective, ALL was more commonly diagnosed in developing countries located in Latin America, the Middle East, and Southeast Asia. In contrast, CLL and AML were more commonly diagnosed in developed countries located in Europe and North America. Globally, CML was rarely diagnosed compared to the other three main types of leukemia. The changing incidence trends for the four major types varied worldwide. For example, the incidence of ALL decreased in most countries, whereas the incidence increased in several Asian and African countries. Conversely, the incidence of CLL increased in most countries. The decreasing trends were only seen in a few developed countries (e.g., the USA and Australia). The international variations in leukemia incidence and its changing trends not only mirror the effectiveness of previous prevention strategies but also indicate that updated and tailored prevention strategies have been established.
Although the risk factors contributing to leukemia have been extensively investigated, the current understanding of leukemia tumorigenesis remains limited. Previous studies have reported that exposure to ionizing radiation, herbicides and pesticides, and radon is associated with an increased risk of leukemia [21–23]. Moreover, the development of leukemia has been partly ascribed to genetic risk factors. For example, the initiating chromosomal aberrations comprise the deletion of chromosome 13q (del[13q]) in about 55% of CLL cases and the acquisition of chromosome 12 (trisomy 12) in 10–20% of CLL cases [24]. Epigenetic mutations of DNMT3A, TET2, and ASXL1 have been identified in preleukaemic haemopoietic stem cells decades before the development of AML, suggesting that these are early founder events that precede leukemogenic transformation [25]. ALL in infants (< 12 months) is usually associated with MLL gene rearrangement. In contrast, non-MLL-rearranged B-ALL has a peak incidence between 2 and 5 years and a concordance rate of 10–15%, suggesting that, although initiation in utero is common, other “promotional” exposures are probably required for the later emergence of disease [4, 26]. CML is a clonal haemopoietic stem cell disorder characterized by a reciprocal translocation between the long arms of chromosomes 9 and 22 [27]. Despite the prior efforts, the 5-year survival rates of leukemia are not high, especially among high-risk patients and in developing countries [28–30]. Indeed, the discovery of the activity of interferon α in 1983, and the later addition of homoharringtonine (now known as omacetaxine and approved by the US Food and Drug Administration for the treatment of CML in 2012) and low-dose cytarabine resulted in a complete cytogenetic response rate (0% Ph-positive metaphases) of 20–30% and an improvement of the median survival to 6–7 years [31]. In standard medical and oncology textbooks, CLL is considered incurable [32]. In childhood ALL, the optimization of chemotherapy regimens over four decades, using mostly the same agents in improved combinations, has resulted in CR rates of 90–100% and potential cure rates of 80% or more. However, in adult ALL, the pediatric regimens have been modified over time to resemble the adult AML treatments, with a shorter duration of maintenance chemotherapy (long maintenance likely is responsible for an increased cure rate of 10–15%) and truncated classical postinduction consolidation in favor of early autologous and allogeneic stem cell transplantation [31]. These strategies have proven to be less successful.
In our study, we observed a significant decrease in leukemia incidence over the past three decades, mainly in developed countries. For example, the incidence of all types of leukemia decreased in Australia. Likewise, in Western Europe, only the incidence of AML showed an increasing trend. The decreases may have been at least partly driven by the following: (1) reducing the exposure to environmental risk factors, such as chemicals, particularly among children and pregnant women; (2) abstaining from high-risk parental behaviors, such as cigarette smoking; (3) increasing the intake of folate and vitamin supplementation during the preconception period or pregnancy; and (4) expanding the genetic screening for high-risk germline mutations. However, we also observed a significant increase in leukemia incidence in some developing countries. Such increases might be mainly explained by the continuous improvement of healthcare facilities and the quality of cancer surveillance systems in these countries. More cancer cases were diagnosed and recorded. On the other hand, the increases also suggest that leukemia is a hard-to-ignore public health concern in the relevant countries. More interventions are therefore needed. In addition, we also observed a significant decrease in the incidence of other leukemias, especially in developing regions. We assessed that this decrease could be ascribed to the development of diagnostic approaches that subsequently allowed for more leukemia cases to be precisely classified.
Surprisingly, we found that the incidence of both AML and CLL increased in most countries. The rise of AML incidence was partly due to an increasing prevalence of therapy for AML as more patients treated with cytotoxic chemotherapy are cured of their primary malignancy [33]. For CLL, previous studies reported a strong birth-cohort effect underlying this increasing trend and suggested that lifestyles and environmental factors may play a role in the development of CLL [34, 35]. Since AML and CLL accounted for nearly 40% of the total leukemia cases worldwide, the rising incidence indicates a further expansion of leukemia cases and the increase of the leukemia disease burden. The currently available toxicologic and observational epidemiological studies have provided strong evidentiary basis for the presence of casual associations (of small to moderate sizes) between several environmental exposures and leukemia. Awaiting a more complete evidentiary basis for decision-making, though ideal, will result in significant delays. However, educating clinicians and the public on primary prevention—actions that individuals can take to reduce their own family’s exposures to chemical risk factors for childhood leukemia and other disorders—is something that can occur now. Ultimately, regulatory actions based on the evolving science are needed to shift the burden from the individual to producers [36].
The limitations of our study should also be noted. First, all data were derived from mathematical models based on surveillance data rather than the surveillance data itself. Second, owing to the conventional limitations of cancer surveillance systems (e.g., changes between coding systems can lead to artificial differences in disease estimates), the disease burden of leukemia might be underestimated in some developing countries. Third, the diagnostic and classification criteria for leukemia types varied from country to country, which might introduce biases to learn the changing trends of certain types of leukemia.
Conclusion
In sum, in the current study, we analyzed the incidence trends of leukemia types at the global, regional, and national level. We reported a significant decrease in leukemia incidence between 1990 and 2017. However, the incidence of both AML and CLL significantly increased in most countries during the study period, suggesting that these types of leukemia might become a major global public health concern. More importantly, the incidence of leukemia and its changing trends were highly heterogeneous across the world. Therefore, more prevention strategies tailored to each country are needed.
Supplementary information
Additional file 1. Supplementary Table S1.
Additional file 2. Supplementary Figures S1–S5.
Acknowledgements
We highly appreciate the works by the Global Burden of Disease Study 2017 collaborators.
Authors’ contributions
Conceptualization: YL and QW. Data curation: QZ and YD; Formal analysis: OS and YD. Funding acquisition: QW. Methodology: YD and OS. Supervision: YL and WW. Visualization: YD and XL. Roles/writing-original draft: all authors. All authors read and approved the final manuscript.
Funding
This Project is supported by High-level Hospital Construction Research Project of Maoming People’s Hospital
This Project is supported by the National Natural Science Foundation of China (Grant NO.: 81960587); Guizhou Provincial Science and Technology Planning Project (Grant NO.: LH [2015]7171); and Guizhou provincial local Standardization Planning Project (Grant NO.: 2019-89)
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Dong and Oumin Shi contribute equally to the manuscript as co-first author
Contributor Information
Yong Li, Email: liyong7229771@163.com.
Qi Wang, Email: qwang5545@sina.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40164-020-00170-6.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, O’Brien S, Gribben J, Rai K. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096. doi: 10.1038/nrdp.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Hough R. Leukemia. Progress in tumor research. 2016;43:87–100. doi: 10.1159/000447076. [DOI] [PubMed] [Google Scholar]
- 4.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz LC, Mascarenhas J. Current and evolving understanding of atypical chronic myeloid leukemia. Blood Rev. 2019;33:74–81. doi: 10.1016/j.blre.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Li B, Gale RP, Xiao Z. Molecular genetics of chronic neutrophilic leukemia, chronic myelomonocytic leukemia and atypical chronic myeloid leukemia. J Hematol Oncol. 2014;7:93. doi: 10.1186/s13045-014-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim JY, Bhatia S, Robison LL, Yang JJ. Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer. 2014;120(7):955–962. doi: 10.1002/cncr.28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerhan JR, Slager SL. Familial predisposition and genetic risk factors for lymphoma. Blood. 2015;126(20):2265–2273. doi: 10.1182/blood-2015-04-537498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed]
- 13.Ren ZH, Hu CY, He HR, Li YJ, Lyu J. Global and regional burdens of oral cancer from 1990 to 2017: results from the global burden of disease study. Cancer Commun (London, England) 2020;40(2–3):81–92. doi: 10.1002/cac2.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Liu W, Mi L, Zeng X, Cai C, Ma J, Wang L. Incidence and mortality of multiple myeloma in China, 2006-2016: an analysis of the Global Burden of Disease Study 2016. J Hematol Oncol. 2019;12(1):136. doi: 10.1186/s13045-019-0807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Deng Y, Zhou L, Tian T, Yang S, Wu Y, Zheng Y, Zhai Z, Hao Q, Song D, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):140. doi: 10.1186/s13045-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Liu J, Song Y, Zeng X, Wang X, Mi L, Cai C, Wang L, Ma J, Zhu J. Burden of lymphoma in China, 2006–2016: an analysis of the Global Burden of Disease Study 2016. J Hematol Oncol. 2019;12(1):115. doi: 10.1186/s13045-019-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA oncology. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triantos C, Konstantakis C, Tselekouni P, Kalafateli M, Aggeletopoulou I, Manolakopoulos S. Epidemiology of hepatitis C in Greece. World J Gastroenterol. 2016;22(36):8094–8102. doi: 10.3748/wjg.v22.i36.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S, Yang WS, Bray F, Va P, Zhang W, Gao J, Xiang YB. Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976–2005. Eur J Epidemiol. 2012;27(1):39–46. doi: 10.1007/s10654-011-9636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Deng Y, Li N, Zheng Y, Tian T, Zhai Z, Yang S, Hao Q, Wu Y, Song D, et al. Global, regional, and national burden of Hodgkin lymphoma from 1990 to 2017: estimates from the 2017 Global Burden of Disease study. J Hematol Oncol. 2019;12(1):107. doi: 10.1186/s13045-019-0799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikkilä A, Erme S, Arvela H, Holmgren O, Raitanen J, Lohi O, Auvinen A. Background radiation and childhood leukemia: a nationwide register-based case-control study. Int J Cancer. 2016;139(9):1975–1982. doi: 10.1002/ijc.30264. [DOI] [PubMed] [Google Scholar]
- 22.Andreotti G, Koutros S, Hofmann JN, Sandler DP, Lubin JH, Lynch CF, Lerro CC, De Roos AJ, Parks CG, Alavanja MC, et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J Natl Cancer Inst. 2018;110(5):509–516. doi: 10.1093/jnci/djx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong J, Qin L, Cao Y, Li J, Zhang J, Nie J, An Y. Environmental radon exposure and childhood leukemia. J Toxicol Environ Health Part B. 2012;15(5):332–347. doi: 10.1080/10937404.2012.689555. [DOI] [PubMed] [Google Scholar]
- 24.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–1537. doi: 10.1016/S0140-6736(18)30422-7. [DOI] [PubMed] [Google Scholar]
- 25.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506(7488):328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6(3):193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 27.Apperley JF. Chronic myeloid leukaemia. Lancet. 2015;385(9976):1447–1459. doi: 10.1016/S0140-6736(13)62120-0. [DOI] [PubMed] [Google Scholar]
- 28.Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi X, Pulsipher MA, Chang BH, Mullighan C, Iacobucci I, Silverman LB, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with philadelphia chromosome-positive acute lymphoblastic leukemia: results of children’s oncology group trial AALL0622. J Clin Oncol. 2018;36(22):2306–2314. doi: 10.1200/JCO.2017.76.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, Lange E, Köppler H, Kiehl M, Sökler M, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 30.Veisani Y, Khazaei S, Delpisheh A. 5-year survival rates based on the type of leukemia in Iran, a Meta-analysis. Caspian J Intern Med. 2018;9(4):316–324. doi: 10.22088/cjim.9.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantarjian HM, Keating MJ, Freireich EJ. Toward the potential cure of leukemias in the next decade. Cancer. 2018;124(22):4301–4313. doi: 10.1002/cncr.31669. [DOI] [PubMed] [Google Scholar]
- 32.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17(9):513–527. doi: 10.1038/nrc.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu SJ, Huang SY, Lin CT, Lin YJ, Chang CJ, Tien HF. The incidence of chronic lymphocytic leukemia in Taiwan, 1986–2005: a distinct increasing trend with birth-cohort effect. Blood. 2010;116(22):4430–4435. doi: 10.1182/blood-2010-05-285221. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Tien HF, Park HJ, Kim JA, Lee DS. Gradual increase of chronic lymphocytic leukemia incidence in Korea, 1999-2010: comparison to plasma cell myeloma. Leukemia & lymphoma. 2016;57(3):585–589. doi: 10.3109/10428194.2015.1068307. [DOI] [PubMed] [Google Scholar]
- 36.Whitehead TP, Metayer C, Wiemels JL, Singer AW, Miller MD. Childhood Leukemia and Primary Prevention. Curr Probl Pediat Adolesc Health Care. 2016;46(10):317–352. doi: 10.1016/j.cppeds.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Table S1.
Additional file 2. Supplementary Figures S1–S5.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.