Abstract
Background
Using probiotics have become popular. They are considered an alternative to Antibiotic Growth Promoters (AGP). Probiotics are supplemented into animal feed for improving growth performance along with preventing and controlling enteric pathogens. The aim of this work was to study the impact of dietary supplementation of Mannan-oligosaccharide and β-Glucan (Agrimos®) on broiler challenged with Escherichia coli O78 (E. coli O78 - marked with an antibiotic (320 μg ciprofloxacin/ml broth) on growth performance, serum biochemistry, immune organs-histopathology, E-coli colonization, and hepatic transcripts of Tumor necrosis factor-alpha (TNF-α) and Nuclear factor-kappa B (NF-ϰB). A total of 125 one-day-old chicks were used for conducting the experiment. Five one-day-old chicks were slaughtered for measuring the initial weight of the lymphoid tissue. The remaining chicks (120) were allotted into four groups according to Mannan-oligosaccharide and β-Glucan supplementation, and E. coli infection. The data were analyzed using SPSS version 16.
Results
Results indicated significant alteration of growth performance, serum biochemistry, and selected liver gene expression with pathological lesions, especially in the lymphoid organs due to E. coli infection. These alterations were mitigated by Mannan-oligosaccharide and β-Glucan supplementation.
Conclusion
It could be concluded, Mannan-oligosaccharide and β-Glucan supplementation in broiler’s diet improved the immune response of broilers and mitigated pathological lesion resulted from E. coli infection.
Keywords: Antioxidant, Gene expression, Growth performance, Histopathology, Immunostimulant, Agrimos
Background
The increasing demand for animal protein worldwide led to a gap, thus searching for growth promoters to compensate for this gap. There are many growth promoters as antibiotic growth promoters (AGPs). AGPs are widely used to prevent poultry disease through prevention of pathogens and improve growth performance. But the use of AGPs in the diet of the poultry can cause serious problems such as antibiotic-resistant pathogens and drug residues [1]. Also, Tayeri et al. [2] reported routine use of an antibiotic (flavomycin) increase Enterococci. Thus, searching for alternatives to antibiotics is very urgent. Prebiotics are used as one of the alternatives to antibiotic growth promoters [3]. Prebiotics are non-digestible components of feed derived from sugars, including raffinose, galactooligosaccharides, and β-glucans [4]. They prevent enteric diseases and improve performance in poultry. Prebiotics have been shown to alter the immune system, modify gastrointestinal microflora, and reduce invasion of the pathogen such as Salmonella spp. and E. coli [5]. The major function of prebiotics is to activate the metabolism of some groups of beneficial bacteria in the intestinal tract and/or stimulate their growth. Pelicano et al. [6], Spring et al. [7], and Xu et al. [8] have shown that the addition of prebiotics to broilers’ diet results in the improvement of the gut microflora and growth as well. The gut microflora composition plays an important role in digestion, which can be performed in a positive, negative, or neutral manner. Gastrointestinal microflora modifications reduce attachment of the pathogen and may have a beneficial effect on the nutrients digestibility [9]. Administration of agrimos® (Mannan-oligosaccharides (MOS), and β-Glucans), which obtained from a specific strain of Saccharomyces cerevisiae cell wall was found to improve the productive performance and immune functions in broiler chickens [10]. Also, Wang et al. [1] and Dawood et al. [11] reported the antioxidant effect of Mannan-oligosaccharides in broilers and red sea bream, respectively.
Hence, this study was conducted to examined agrimos® (MOS and β-Glucans) effect on growth, immunity, serum biochemistry, histopathology, selected liver gene expressions, and colonization of E. coli in broilers.
Results
Clinical signs and postmortem (PM) lesions of E. coli infection
Experimental infection with E. coli revealed suggestive clinical signs and PM lesions after 48 h. post-infection in the form of depression with whitish diarrhea.
PM lesions revealed liver enlarged and congested and distended gallbladder and cecum. Such changes were less prominent in the agrimos-infected group (see Table 1).
Table 1.
The mortality rate of broiler chicken infected with E. coli and fed on agrimos® at 35 days (n = 30)
| Groups Parameter |
Control noninfected | Control infected | Agrimos® noninfected | Agrimos® infected |
|---|---|---|---|---|
| Total No. | 30 | 30 | 30 | 30 |
| Dead No. | 1 | 2 | 0 | 1 |
| Survival % | 96.67 | 93.33 | 100 | 96.67 |
| Mortality % | 3.33 | 6.67 | 0 | 3.33 |
Growth performance
The feed supplemented with agrimos® in the third and fourth groups significantly (P ≤ 0.05) increased the body weight (BW) and total gain along with the whole experiment, and improved feed conversion ratio (FCR) compared to the other groups (see Table 2). On the other hand, the above-mentioned parameters were decreased significantly (P ≤ 0.05) in the control-infected group as compared to the other groups.
Table 2.
Growth performance of broiler chicken infected with E. coli and fed on agrimos® at 35 days (n = 30)
| Groups Parameters |
Control noninfected | Control infected | Agrimos® noninfected | Agrimos® infected |
|---|---|---|---|---|
| Initial body weight (g/chick) | 45.33 ± 0.88a | 45 ± 1.00a | 45.67 ± 0.88a | 45.67 ± 1.20a |
| Final body weight (g/bird) | 1544.33 ± 1.76b | 1358.67 ± 2.03c | 1736.33 ± 2.33a | 1547.00 ± 1.73b |
| Total Weight gain (g/bird) | 1499 ± 1.53b | 1313.67 ± 2.19c | 1690.67 ± 1.45a | 1501.33 ± 0.67b |
| FCR value | 1.67 ± 0.005b | 1.87 ± 0.006a | 1.54 ± 0.003c | 1.66 ± 0.003b |
Values are expressed as mean ± standard errors. Means in the same row (a-c) with different letters significantly differ at (p ≤ 0.05)
Serum parameters related to liver function
In (Table 3), there were significant (P ≤ 0.05) increased in the activities of alanine transaminase (ALT) and aspartate aminotransferase (AST) enzymes in the group of broiler chickens infected with E. coli compared to the control non-infected group. However, serum total protein and albumin were significantly (P ≤ 0.05) decreased. Agrimos® supplementation did not affect serum ALT, AST, and albumin, but the serum total protein and globulin affected significantly (P ≤ 0.05) when compared with the control non-infected group.
Table 3.
Serum liver function of broiler chicken infected with E. coli and fed on agrimos® at 35 days (n = 5)
| Groups Parameters |
Control noninfected | Control infected | Agrimos® noninfected | Agrimos® infected |
|---|---|---|---|---|
| ALT (u/l) | 19.33 ± 0.88b | 26.33 ± 0.88a | 18.67 ± 0.88b | 20.67 ± 0.67b |
| AST (u/l) | 53.33 ± 1.76b | 92.67 ± 0.88a | 51.33 ± 0.88b | 52.67 ± 1.45b |
| Total protein (g/dl) | 2.27 ± 0.08b | 1.9 ± 0.06c | 2.9 ± 0.12a | 2.7 ± 0.11a |
| Albumin (g/dl) | 1.20 ± 0.02a | 0.47 ± 0.05b | 1.24 ± 0.03a | 1.21 ± 0.01a |
| Globulin (g/dl) | 1.06 ± 0.09b | 1.43 ± 0.01a | 1.66 ± 0.12a | 1.49 ± 0.11a |
Values are expressed as mean ± standard errors. Means in the same row (a-c) with different letters significantly differ at (p ≤ 0.05)
Serum antioxidant enzymes activity
Serum MDA level was significantly (P ≤ 0.05) increased in broiler chickens infected with E. coli (see Table 4). However, serum catalase (CAT) and superoxide dismutase (SOD) activities were significantly (P ≤ 0.05) reduced compared to the other groups. Agrimos® supplementation significantly (P ≤ 0.05) decreased serum lipid peroxidation (MDA) with significant (P ≤ 0.05) increased serum CAT and SOD when compared to the control-infected group.
Table 4.
Serum antioxidant of broiler chicken infected with E. coli and fed on agrimos® at 35 days (n = 5)
| Groups Parameters |
Control noninfected | Control infected | Agrimos® noninfected | Agrimos® infected |
|---|---|---|---|---|
| MDA (n.mol/l) | 0.65 ± 0.02c | 1.17 ± 0.08a | 0.65 ± 0.01c | 0.8 ± 0.03b |
| CAT (u/ml) | 12.7 ± 0.01b | 9.73 ± 0.15c | 15.36 ± 0.13a | 13.03 ± 0.09b |
| SOD (u/ml) | 12.18 ± 0.0c | 9.37 ± 0.0d | 17.2 ± 0.89a | 15.0 ± 0.0b |
Values are expressed as mean ± standard errors. Means in the same row (a-c) with different letters significantly differ at (p ≤ 0.05)
Immune response against Newcastle disease (ND)
Immune response to vaccination of ND, which evaluated by Hemagglutination Inhibition (HI) titer, revealed a difference in log2 of GM. There was a significant difference in HI titer in the second week after infection between each of the E. coli infected group and the control negative group, and the agrimos infected group. In addition, the agrimos group significantly differs about the other three groups.
After vaccination in the second and third week, this pattern was observed where no difference was recorded between each of the E. coli infected group, control non-infected, and agrimos infected group. Besides, the agrimos group significantly differs about the other three groups (see Table 5).
Table 5.
HI titer for ND at different periods (n = 5)
| Groups Age |
Control infected | Control noninfected | Agrimos® infected | Agrimos® noninfected |
|---|---|---|---|---|
| 0 day |
4.6 ± 0.07b C.V = 11.6% |
4.8 ± 0.06b C.V = 9.03% |
4.8 ± 0.06b CV = 9.3% |
5.2 ± 0.06a CV = 8.3% |
| 1st weeks p. v |
5.2 ± 0.06c C.V = 8.6% |
5.6 ± 0.7b CV = 9.5% |
5.6 ± 0.07b CV = 9.5% |
6.2 ± 0.06a CV = 7.2% |
| 2nd weeks P. v |
6 ± 0.09b C.V = 11.5 |
6.2 ± 0.11b CV = 13.4 |
6.2 ± 0.06b CV = 6.9% |
7 ± 0.09a CV = 10% |
| 3rd weeks P. v |
4.95 ± 0.09b C.V = 14.5% |
4.6 ± 0.24b CV = 11.6% |
4.8 ± 0.06b CV = 9% |
6 ± 0.09a CV = 11.7% |
| Average of log2GM ± SEM | 5.19 ± 0.3b | 5.3 ± 0.37b | 5.35 ± 0.34b | 6.1 ± 0.37a |
No difference between item carries the same letter in the same row
GM Geometric mean, CV Coefficient of variation, p. v Post vaccination
Colonization of E. coli
The colonization of E. coli in the control infected group in different organs showed several rates of 67, 44, 22, 44, and 53% in the respiratory tract, liver, gallbladder, spleen, and fecal swab, respectively (see Table 6). However, in the agrimos infected group, the colonization of E. coli in different organs showed reduced rates of 22, 33, 22, 11, and 33% in the respiratory tract, liver, gallbladder, spleen, and fecal swab, respectively.
Table 6.
Colonization of E. coli and rate of shedding as judged by intestinal colonization (n = 5)
| R. T. swab | Liver | G. bladder | Spleen | Fecal swab | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | T | % | + | T | % | + | T | % | + | T | % | + | T | % | |
| E. coli | 6 | 9 | 67 | 4 | 9 | 44 | 2 | 9 | 22 | 4 | 9 | 44 | 8 | 15 | 53 |
| E. coli + Aa | 2 | 9 | 22 | 3 | 9 | 33 | 2 | 9 | 22 | 1 | 9 | 11 | 5 | 15 | 33 |
R. T. swab Respiratory tract swab aE. coli + A = E. coli + Agrimos®
The weight of immune organs
At zero, 15, and 35 days of age, the weight of the immune organ versus body weight was evaluated (see Table 7 and Fig. 1). On days 15 and 35, the results of thymus weight showed a significant increase (P ≤ 0.05) in the agrimos non-infected and infected groups in comparison with the other groups. However, there was a significant decrease (P ≤ 0.05) in the control infected group compared to the control non-infected group.
Table 7.
The weight of Immune organs of broilers at 35 days (n = 5)
| Organs | Treatment and age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C- | C+ | A- | A+ | |||||||||
| Zero- day | At 15 days | At 35 days | Zero-day | At 15 days | At 35 days | Zero- day | At 15 days | At 35 days | Zero- day | At 15 days | At 35 days | |
| T/B.W. |
0.287 ± 0.033Y |
0.59 ± 0.012 bX |
0.134 ± 0.008 BZ |
0.287 ± 0.033Y |
0.377 ± 0.013 cX |
0.069 ± 0.006 CZ |
0.287 ± 0.033Y |
0.65 ± 0.006aX |
0.282 ± 0.012AY |
0.287 ± 0.033Y |
0.627 ± 0.009 aX |
0.276 ± 0.011 AY |
| S/B.W. |
0.036 ± 0.007Y |
0.046 ± 0.003 dY |
0.132 ± 0.011 BX |
0.036 ± 0.007Z |
0.094 ± 0.004 cX |
0.056 ± 0.006 CY |
0.036 ± 0.007Z |
0.11 ± 0.006 bY |
0.229 ± 0.009 AX |
0.036 ± 0.007Z |
0.127 ± 0.003 aY |
0.159 ± 0.005 BX |
| B/B.W. |
0.157 ± 0.024Y |
0.329 ± 0.003 bX |
0.121 ± 0.004 CY |
0.157 ± 0.024Y |
0.259 ± 0.01 cX |
0.064 ± 0.006 DZ |
0.157 ± 0.024Y |
0.394 ± 0.003 aX |
0.355 ± 0.014 AX |
0.157 ± 0.024Y |
0.338 ± 0.017 bX |
0.317 ± 0.013 BX |
Values are expressed as mean ± standard errors. Means in the same row (a-d), (A-C) and (X-Z) with different letters significantly differ at (p ≤ 0.05)
T Thymus B. W. Body weight, S Spleen, B Bursa
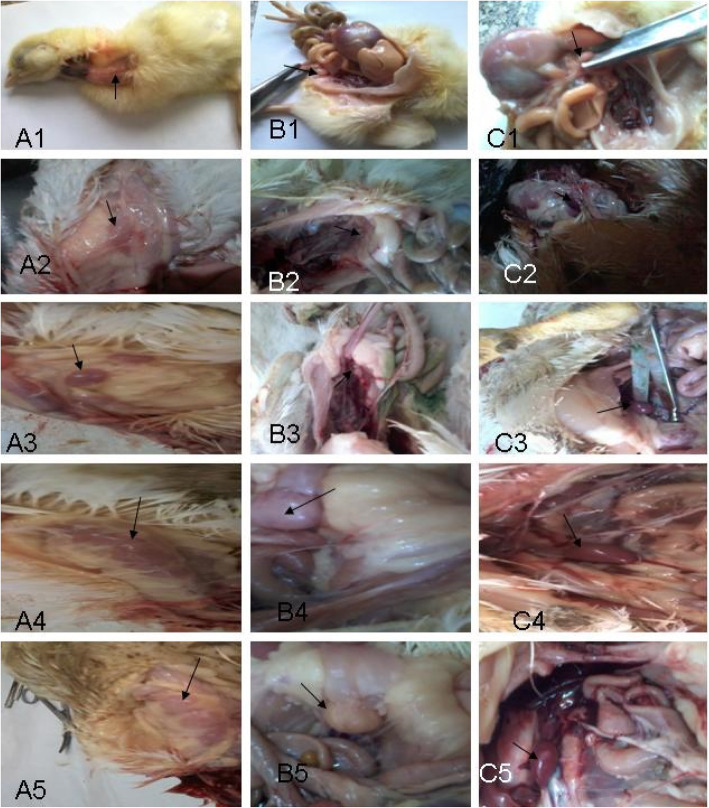
Fig. 1.
Thymus of one-day-old chicken (A1). Thymus of control non-infected group at 35 days (A2). Thymus of control infected group at 35 days (A3). Thymus of agrimos non-infected group at 35 days (A4). Thymus of agrimos infected group at 35 days (A5). Bursa of one-day-old chicken (B1). Bursa of control non-infected group at 35 days (B2). Bursa of control infected group at 35 days (B3). Bursa of agrimos non-infected group at 35 days (B4). Bursa of agrimos infected group at 35 days (B5). Spleen of one-day-old chicken (C 1). Spleen of control non-infected group at 35 days (C 2). Spleen of control infected group at 35 days (C 3). Spleen of agrimos non-infected group at 35 days (C 4). Spleen of agrimos infected group at 35 days (C 5)
The results of spleen on day 15 showed that there was a significant increase (P ≤ 0.05) in the agrimos infected group compared to the other groups. Furthermore, there was a significant increase (P ≤ 0.05) in the agrimos non-infected group compared to the control non-infected and infected groups. However, on day 35, the agrimos non-infected group witnessed a significant increase in the spleen’s weight (P ≤ 0.05) compared to the other groups. As well, the control infected group had a significant increase (P ≤ 0.05) compared to the control non-infected group on day 15 but a significant decrease (P ≤ 0.05) on day 35.
The bursa’s weight was significantly increased (P ≤ 0.05) in the agrimos non-infected group compared to the other groups, but it was significantly decreased (P ≤ 0.05) in the control infected group compared to the other groups on days 15 and 35.
The thymus’ and bursa’s weight in the control non-infected group were significantly (P ≤ 0.05) decreased, but the spleen’s weight was significantly (P ≤ 0.05) increased. The control-infected group showed a significant (P ≤ 0.05) increase in the weights of the thymus, spleen, and bursa on day 15, and then showed a significant (P ≤ 0.05) decrease on day 35. The agrimos non-infected and infected groups showed a significant (P ≤ 0.05) increase in the weights of the spleen and bursa on days 15 and 35 but showed a significant (P ≤ 0.05) increase in the weight of the thymus on day 15 only, which returned to its normal size on day 35.
The pathological findings of broiler chickens
The histopathological examination of the spleen, bursa, thymus, liver, and duodenum of the chicks of the control non-infected group (on days 15 and 35 after infection), showed no obvious histopathological alterations (see Fig. 2a and b). Concerning the control infected group (on day 15), the bursa showed variable degrees of epithelial hyperplasia, degeneration, and ulceration in some cases. These changes were associated with subcortical fibrous tissue proliferation in some cases (see Fig. 2c, d, and e). The thymus of this group showed a narrowing in the cortical width and an increase in the medulla in most cases. Some other cases had the appearance of clear areas or holes that contained small dark nuclei (a defining characteristic of the apoptosis in the lymphoid organ), (see Fig. 2f). No microscopical changes were detected on the spleen of this group. Regarding the control infected group (on day 35), similar changes were seen on day 15, but more holes were detected in the thymus. Also, the focal area of round cells aggregation was seen in the liver of this group (see Fig. 3a). The duodenum of this group showed hyperplasia on the epithelial cells lining the intestinal villi accompanied by vacuolation (see Fig. 3b).
Fig. 2.
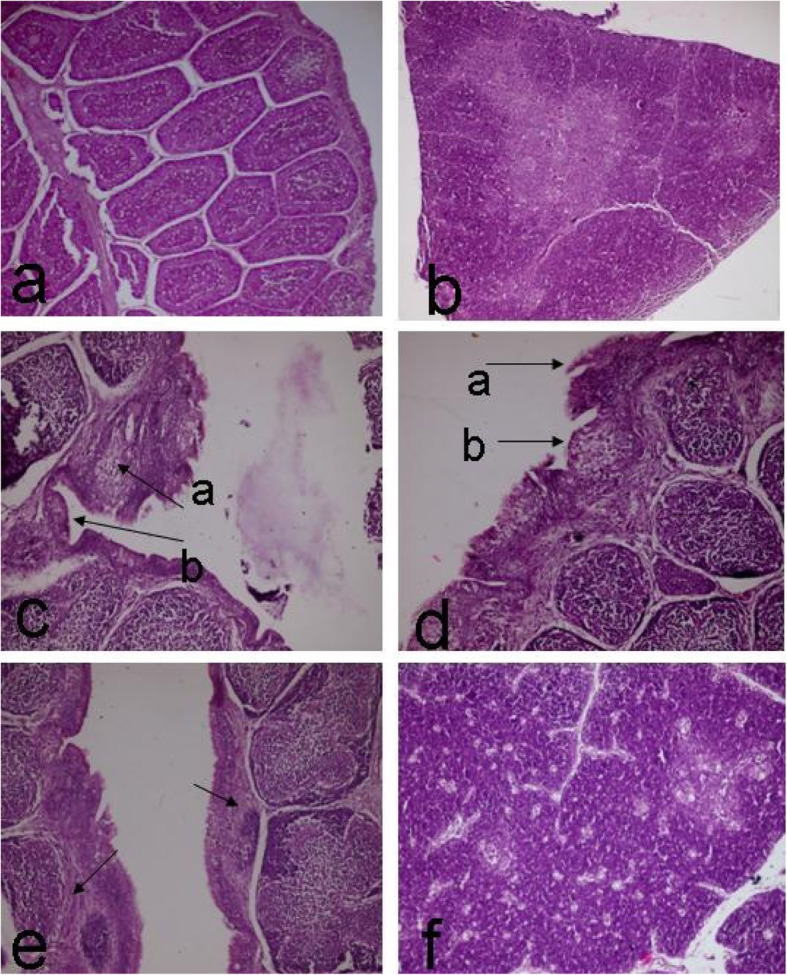
a. showing normal bursa of Fabricius. Control negative one-day-old chick. b. showing normal thymus. Control negative one-day-old chick. C. bursa of Fabricius showing variable degree of epithelial hyperplasia (arrow a) and ulceration (arrow b). Control infected group at 15 days post infection. d. Bursa of Fabricius showing variable degree of epithelial hyperplasia (arrow a) associated with degeneration and ulceration (arrow b). Control infected group at 15 days post infection. e. Bursa of Fabricius showing variable degree of epithelial hyperplasia associated with degeneration and sub cortical fibrous tissues proliferation. Control infected group at 15 days post infection. f. Thymus showing numerous holes on the cortex and medulla. Control infected group at 15 days post infection. H&E. a and b X 100 other Figs. X 200
Fig. 3.
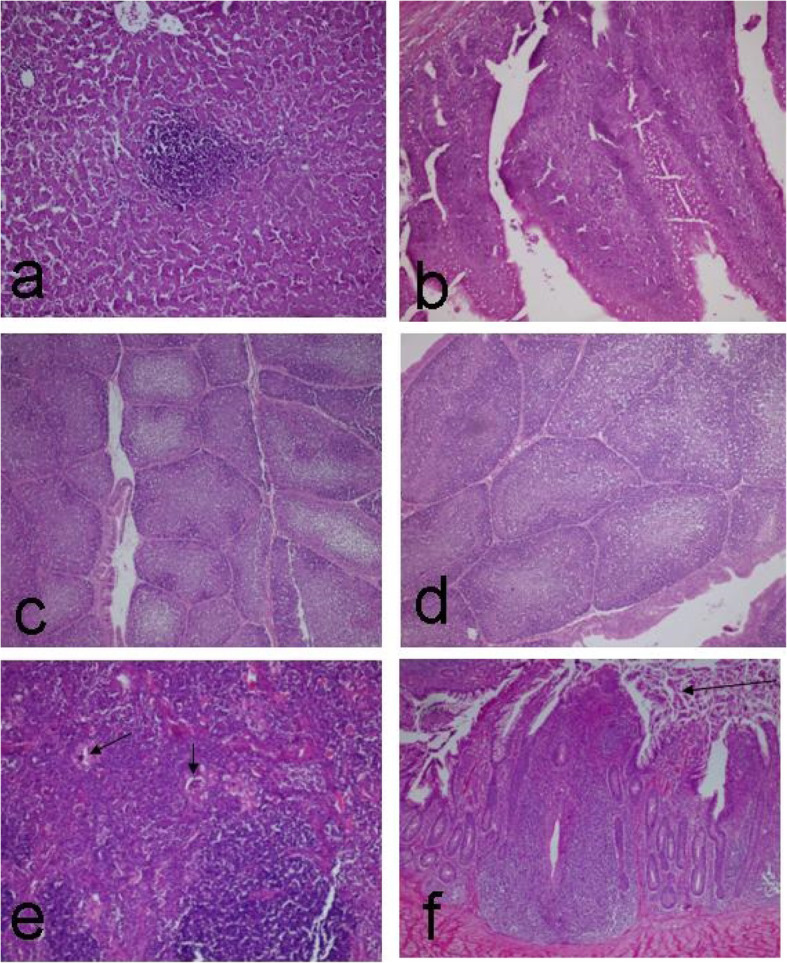
a. Liver showing focal area of round cells aggregation. Control infected group at 35 days post infection. b. Intestine (duodenum) showing hyperplasia of the epithelial lining the intestinal villi associated with vaculation. Control infected group at 35 days. C. bursa of Fabricius showing normal atypical fold. Agrimos® non-infected group at 15 days. d. Bursa of Fabricius showing pores within the cortex. Agrimos® non-infected group at 35 days. e. Thymus showing thrombus formation (arrow). Agrimos® non-infected group at 15–35 days. f. Caecal tonsil showing epithelial sloughing (arrow). Agrimos® non-infected group at 15–35 days. H&E. X 200 for all figures except c X 100
The histopathological examination of the bursa of Fabricius in most cases in the agrimos non-infected group on day 15 (4 out of 5), showed normal a typical fold (plica) (see Fig. 3c). Some of these cases showed a highly dilated germinal center and narrow cortex, associated with a mild degree of epithelial lobulation. One case showed follicular lymphocytic depletion. Similar histopathological changes were seen on the bursa of Fabricius in the agrimos non-infected group on day 35. One case showed pores within the cortex of many plicae (see Fig. 3d), and this was associated with interfollicular edema. Regarding the thymus gland in both times (days 15 and 35), no obvious histopathological alterations were seen on the thymic lobe except in few cases (2 out of 10), which showed thrombus formation within the thymic vasculature (see Fig. 3e). Concerning the caecal tonsil of the agrimos non-infected group on days 15 and 35, just epithelial sloughing was seen in some cases (see Fig. 3f), and no obvious histopathological alterations were viewed on the lymphoid nodules (lymphoid aggregation), (see Fig. 4a). Two cases (out of 10) showed a necrotic cyst (see Fig. 4b). No obvious microscopical changes were seen on the spleen of this group (see Fig. 4c). The microscopical examination of the liver of this group showed mild focal areas of round cell aggregation (see Fig. 4d) in two cases (out of 10).
Fig. 4.
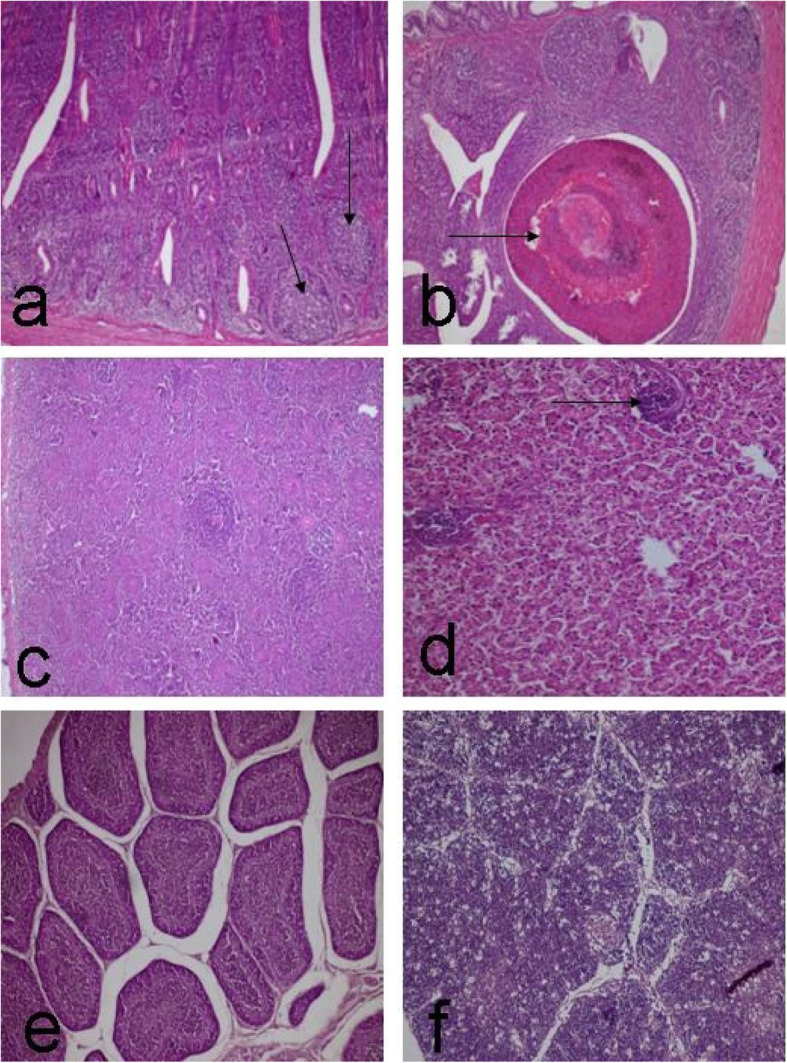
a. Caecal tonsil showing normal lymphoid aggregation (arrow). Agrimos® non-infected group at 15–35 days. b. Caecal tonsil showing necrotic cyst (arrow). Agrimos® non-infected group at 15–35 days. c. Spleen showing normal appearance. Agrimos® non-infected group at 15–35 days. d. Liver showing mild focal areas of round cells aggregation (arrow). Agrimos® non-infected group at 15–35 days. e. Bursa of Fabricius showing inter follicular edema. Agrimos® infected group at 15 days post infection. f. Thymus showing numerous cortical holes in cortex and medulla (these holes containing apoptotic bodies). Agrimos® infected group at 15 days post infection. H&E. X 200 for all figures except e X 100
The histopathological examination of the bursa of Fabricius in most cases in the agrimos infected group on days 15 and 35 after infection (2 out of 4), showed normal morphological appearances but 2 cases showed interfollicular edema (see Fig. 4e) and epithelial hyperplasia and folding associated with mucous (goblet) cell activation. Regarding the thymus gland in both times (days 15 and 35), no obvious histopathological alterations were seen on the thymic lobe except in few cases (1 out of 4), which showed focal areas of necrosis and hyalinization (see Fig. 4f). No obvious microscopical changes were seen on the spleen of this group (see Tables 8 and 9).
Table 8.
Histopathological assessment of dietary agrimos® supplementation on E. coli challenged broiler chicken ((n = 10)
| Lesions | Infected group | Agrimos non-infected group | Agrimos infected group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At 15 days | At 35 days | At 15 days | At 35 days | At 15 days | At 35 days | |||||||||||||
| B | T | S | B | T | S | B | T | S | B | T | S | B | T | S | B | T | S | |
| Sub capsular heterophilic aggregation | – | Normal | – | Normal | Normal | Normal | Normal | Normal | Normal | Normal | – | Normal | Normal | – | Normal | |||
| Sub and interstitial edema | – | – | + | + | ||||||||||||||
| Interstitial hemorrhages | – | – | – | + | ||||||||||||||
| Interstitial connective tissue proliferation | – | – | – | – | ||||||||||||||
| Epithelial folding and lobulation | + | – | – | – | ||||||||||||||
| Epithelial hyperplasia | + | + | + | + | ||||||||||||||
| Epithelial degeneration and/or necrosis | + | + | – | + | ||||||||||||||
| Reduction in size and numbers of follicles | – | – | – | – | ||||||||||||||
| Follicular atrophy or cortical atrophy | – | – | + | – | + | + | ||||||||||||
| Follicular cyst and necrosis | – | – | – | + | ||||||||||||||
| Lymphocytic depletion | – | – | – | + | ||||||||||||||
| Appearance of holes in medulla | – | + | – | ++ | – | – | ||||||||||||
| Reticular cells proliferation | – | – | – | – | ||||||||||||||
| Vasculitis | – | – | – | – | ||||||||||||||
| Cortical necrosis | – | – | – | – | ||||||||||||||
| Apoptotic bodies and/or karyorhexis | – | + | – | – | – | |||||||||||||
| Hyalinization and thrombosis of blood vessels | – | – | – | – | ||||||||||||||
+++ Sever ++ Moderate + Mild B = Bursa T = Thymus S=Spleen
Table 9.
Incidence of pathological changes in organs at different periods (n = 10)
| Groups | Infected group | Agrimos non-infected group | Agrimos infected group | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Periods | At 15 days | At 35 days | At 15 days | At 35 days | At 15 days | At 35 days | ||||||||||||||||||
| Parameter | N | A | T | % | N | A | T | % | N | A | T | % | N | A | T | % | N | A | T | % | N | A | T | % |
| B | 2 | 2 | 4 | 50 | 1 | 3 | 4 | 75 | 4 | 1 | 5 | 20 | 4 | 1 | 5 | 20 | 2 | 2 | 4 | 50 | 2 | 2 | 4 | 50 |
| T | 4 | 0 | 4 | 0 | 2 | 2 | 4 | 50 | 3 | 2 | 5 | 40 | 4 | 1 | 5 | 20 | 3 | 1 | 4 | 25 | 3 | 1 | 4 | 25 |
| S | 3 | 1 | 4 | 25 | 4 | 0 | 4 | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 |
B Bursa T Thymus S Spleen, N Normal A Abnormal T Total % = Ratio
Gene expression related to E. coli infection and Agrimos supplementation
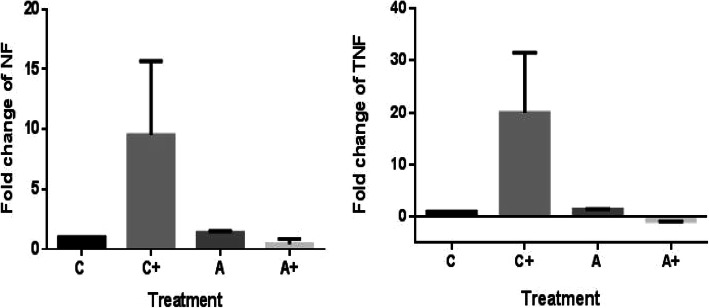
The results of Q-PCR revealed that the expression levels of nuclear factor-kappa (NF) and tumor necrosis factor-alpha (TNF) genes of the liver tissues were significantly (P ≥ 0.05) increased in the group infected by E. coli (Fig. 5) compared to the negative control group.
Fig. 5.
Changes in the gene expression of NF (right) and TNF-α (left) among experimental groups. C (negative control), C+ (positive control), A (agrimos® treated without infection), A+ (agrimos® treated group with E. coli infection)
In the agrimos non-infected group, the gene expression of NF and TNF in liver tissues did not show significant differences in comparison with the control non-infected group. The levels of such genes were similar to those of the control non-infected group.
It was found that the NF and TNF expression levels were significantly (P ≥ 0.05) reduced due to agrimos® supplementation when compared to those of the infected group without supplementation. This shows the protective effect of agrimos® supplementation on the expression of the above-mentioned genes of the infected broiler chicks.
Discussion
In the present study, the control infected group with E. coli showed varied clinical signs according to their age, an organ involved, and concurrent disease conditions. Results of clinical signs in the E. coli infected groups (even in the treated one) similar to the results of Remus et al. [12] who recorded severe liver damage occurs in cases of E. coli infection. However, dietary supplementation of agrimos® decreased the clinical signs and PM lesions. This result is consistent with Sohail et al. [13]. On the other hand, the results of the growth performance in the control infected group showed significant retardation. This retardation may be attributed to the E. coli infection. This result is compatible with Manafi et al. [14]. Meanwhile, agrimos® dietary supplementation significantly improved growth performance, which may be ascribed to the increased digestibility and feed intake as well. Jahanian and Ashnagar [15] reported that dietary MOS supplementation improves digestibility coefficients of dry matter and crude protein. These results in harmony with the results of El-Far et al. [16] and Mousa et al. [17] in broilers and Japanese quail, respectively.
Results of biochemical parameters showed a significant increase in serum AST and ALT activities, and globulin concentration, meanwhile, serum total protein and albumin were significantly decreased in the control infected group. These results agree with Manafi et al. [14], who reported the same results when birds were challenged with E. coli. These results may be attributed to E. coli infection. Pathogenic E. coli infection in broilers leads to local or systemic infection (colibacillosis). Colibacillosis is an infectious disease characterized by acute fatal septicemia or sub-acute fibrinous pericarditis, airsacculitis, salpingitis, and peritonitis [18]. In the present study, E. coli infection occurred colibacillosis infection, which indicated by results of E. coli colonization and increased serum biochemical parameters. In addition, Sharma et al. [19] reported that the increase in serum activities of ALT is indicative of cellular injury to hepatocytes and AST in cardiac muscles and hepatocytes as well. On the other hand, hypoproteinemia may be due to hepatocyte damage, which results in failure in the synthesis of plasma protein, where the liver is a site for albumin synthesis. Also, hypoproteinemia may be due to kidney disease, which leads to protein loss and congestive heart failure [19, 20]. At the same time, hyperglobulinemia is associated with liver cirrhosis, hepatitis, and Kuffer cell proliferation [19]. However, dietary supplementation of agrimos® not affected serum AST and ALT activity and albumin concentration but significantly increased total protein and globulin of the serum. The above results are consonant with those of Mousa et al. [17]. Meanwhile, Yalçinkaya et al. [21] found a significant decrease in the activities of AST and ALT. The results of the present study are incompatible with Rokade et al. [22], who found a significant increase in the serum activities of AST and ALT, and protein concentration in broilers dietary supplemented with MOS. These results may be attributed to the antioxidant properties of the MOS, which was reported by Dawood et al. [11]. On the other hand, results of antioxidant enzymes and MDA in harmony with the results of Zhou et al. [23] found a significant decrease of antioxidant enzymes in birds infected with necrotic enteritis. These results may be attributed to infection, which was previously reported by Mishra B, Jha [24]. On the other hand, Abudabos et al. [25] reported that Salmonella typhimurium had no impact on total antioxidant capacity or oxidative stress in challenged broilers. However, agrimos® supplementation significantly decreased serum MDA and increased CAT. This result is compatible with that of El-Kader et al. [26] in broilers.
The reduction of HI titer in the E. coli infected groups may be attributed to the stress of infection and diarrhea, which may change the acid-base balance [27]. In addition, the increase in HI titer due to the nutrition of agrimos® appeared to be insignificant. This may be attributed to the fact that agrimos® tend to initiate non-specific immunity rather than humoral immunity [28]. The dietary MOS stimulates humoral immune responses against infectious bursal disease (IBDV) and Newcastle disease (NDV) vaccine viruses [29]. Regarding results of E. coli colonization, the higher colonization rates of E. coli in different organs of control infected group were in the respiratory tract, fecal swab, liver, and spleen, where avian pathogenic E. coli cause severe respiratory and systemic diseases in poultry [30]. This result is in harmony with that of Manafi et al. [14]. However, this colonization was decreased by agrimos® dietary supplementation, which was supported by the results of Sohail et al. [13].
Cloacae bursa plays a crucial role in the poultry immune system. In addition, the bursal weight reflects the anatomical response to immune status in broilers [14]. In the present investigation, there were significantly decreased in thymus and bursa’s weights versus body weight in the control infected group, which was supported by Gottardo et al. [31]. However, these results are incompatible with those obtained by Manafi et al. [14]. Also, the spleen’s weight versus body weight in the control infected group significantly increased on day 15 then decreased on day 35. These results may be attributable to the infection, which was supported by the results of Huff et al. [32] in turkeys. On the other hand, there were significantly increased in thymus’, spleen, and bursa’s weights versus body weight in the agrimos® groups. These results are in harmony with that of Sohail et al. [13].
Malfunctions of lymphoid organs reduce resistance to bacterial, viral, parasitic, and fungal infections. Thus birds become more susceptible to other infections, leading to high economic losses due to morbidity and mortality [33]. Therefore, the rational assessment of the immunosuppression in the poultry warrants rapid and accurate diagnostic approaches. Thus, the present study focused on the pathology of lymphoid organs.
The pathological changes in the control infected group were apoptosis in the lymphoid organ and focal area of round cell aggregation in the liver. These results are in harmony with that of Kumar et al. [34]. Meanwhile, spleen revealed depletion of lymphocytes, areas of congestion, and necrosis of lymphocytes from the white pulp. Also, bursa of Fabricius showed lymphoid depletion in follicles, and thymus showed some lesions like medullary congestion and mild cortical depletion [33]. Remus et al. [12] reported E. coli caused severe liver and epithelial damage. However, agrimos® supplementation to broilers revealed no pathological changes. This result is compatible with that of Awaad et al. [10]. As well, in the infected group, the supplementation of agrimos® mitigated the pathological changes caused by infection. This result may be due to the composition of agrimos®, which contains β-Glucans and MOS.
Pro-inflammatory cytokines such as nuclear factor (NF) and tumor necrosis factor (TNF) produced during the acute phase response can induce the production of several acute-phase proteins (APPs) by hepatocytes [35]. In the present study, it was found that agrimos® had no clear effect on liver NF or TNF expression levels of the uninfected broiler chicks. However, the reduction in liver enzyme activities and the down-regulation of NF and TNF gene expressions indicated the protective effects and anti-inflammatory properties of agrimos® in broiler chicks with E. coli infection. These results are consistent with that obtained by Markazi [36] in Salmonella challenge broilers.
Most of the previous research has been performed on healthy chicks without infection conditions or has lacked to clear some gene expressions at the site of infection. E. coli infection is considered a common disease in poultry production, which can induce different oxidative and inflammatory stress. Thus, in the present study, E. coli-inoculated chickens were used to investigate the effects of agrimos® on healthy chickens and under such conditions.
Together with TNF or NF data, agrimos® can apparently regulate the cytokine responses of broiler chicks with E. coli infection, and its immunomodulatory effects seem to be protective by repressing the ongoing inflammation. In brief, the decreased TNF and NF expressions of liver tissues in the agrimos® fed broiler chicks with E. coli infection may be an indication of slowdown inflammation and gradual restoration of the infected broiler chicks’ health status.
Socio-economic importance
Veterinary medicine plays a crucial role in public health. The scope of veterinary public health includes food hygiene practices development and supervision in addition to zoonotic disease control and eradication [37]. In addition, antibiotic usage in animals plays a major role in the emerging public health crisis of antibiotic resistance [38]. Thus, nowadays, using probiotics or prebiotics have become popular. They are considered an alternative to Antibiotic Growth Promoters (AGP). Probiotics are supplemented into animal feed for improving growth performance along with preventing and controlling enteric pathogens [39].
Attention should be given to the enteric pathogens in the poultry field due to their economic effects (death, loss of weight, and poor conversion ratio). Besides, the spread of E. coli and the high usage of antibiotics in the poultry industry can result in bacterial resistance to such antibiotics. Instead, it is recommended to use prebiotics.
Conclusion
In the present study, the control infected group showed retardation in the growth performance parameters with abnormal serum biochemical parameters. These retardation and abnormalities were mitigated by agrimos® supplementation, which improved growth performance. Also, agrimos® improved the non-specific immunity, which in turn led to an improvement in the specific immunity against ND, which was reported in the discussion above. On the other hand, agrimos® reduced oxidative stress and slowed down inflammation that occurs as a result of E. coli infection through decreased TNF and NF expressions in liver tissue. Consequently, there was no need to used antibiotics. So, Agrimos® proved to be a good alternative to antibiotics for controlling and reducing E. coli infection and colonization.
Methods
Agrimos®
The combination of Manno-oligosaccharides (MOS), and Beta-Glucans, which was extracted from Saccharomyces cerevisiae with a specific dedicated manufacturing process produced agrimos®. Agrimos® modulates the non-specific immune response and favors the development of the specific response. It was made in Lallemand Company (a Canadian company) and imported by Egavet (Egyptian Group for Trade and veterinary Services) Company, Egypt.
Experimental design, feeding program, and management
125 mixed-sex chicks (one-day-old), were used (Cobb-505 broiler strain). The chicks were obtained from a private farm at Kafrelsheikh Governorate, where five chicks were anesthetized (intraperitoneal injection of sodium pentobarbital (50 mg/kg) to minimize suffering during slaughtering) and slaughtered after weighting at the beginning of the experiment. Their bursa, spleen, and thymus gland were weighed. The remaining broiler chicks (120) were kept in a clean room, which was furnished with sawdust with good ventilation at the Animal Health Research Institute, Kafrelsheikh branch, where the experiment was done. Chicks were supplemented with feed and water ad libitum, and kept under good hygienic management and sanitation with light/dark 23/1 h./day. Chicks (average body weight = 40–50 g/chick), after 3 days acclimatization period were equally allotted into (ranking method), 4 groups each group contains 3 replicates (10 chicks/replicate), where the experiment was started at 4 days old. The negative control group (first group) was fed on a diet [40] without any additives (basal) or infection (Table 10). The positive control (second group) was fed on a diet without any additives but infected with E. coli. The third group was fed on agrimos® (2 kg/ton) supplemented diet but without infection. The fourth group was fed on agrimos® (2 kg/ton) supplemented diet and infected. All chicks groups were vaccinated against ND and IBD at 7 & 12 days, respectively. The chemical analysis of feed was determined according to AOAC [41]. Individual bird body weight was recorded at the starting of the experiment. All performance parameters (BW, weight gain (WG), and FCR) were calculated according to Vohra and Roudybush [42], Castell and Tiews [43], and Tacon [44], respectively. The feed intake for each pen was recorded weekly with the observation of chicks for symptoms, PM, and mortality along the experimental period.
Table 10.
Experimental feeding program
| Physical composition | Basal diet (0–3) weeks | Basal diet (3–6) weeks |
|---|---|---|
| Yellow corn | 55 | 61.35 |
| Soybean meal 48% | 32 | 30.6 |
| Corn gluten 62% | 4.42 | 0 |
| Sunflower oil | 4.4 | 4.4 |
| Methionine | 0.16 | 0.08 |
| Dicalcium phosphate | 1.85 | 1.4 |
| Lime stone | 1.25 | 1.3 |
| Choline 60% | 0.22 | 0.17 |
| Common salt | 0.4 | 0.4 |
| Premixa | 0.3 | 0.3 |
| Chemical composition % | ||
| ME Kcal/kg | 3218.5 | 3229.7 |
| Crude protein | 23.02 | 20.03 |
| Calcium | 1 | .9 |
| Available phosphorus | 0.45 | 0.35 |
| Lysine | 1.1 | 1 |
| Methionine + cysteine | 0.9 | .72 |
| Choline | 1300 mg/1 kg | 1000 mg/1 kg |
a The used premix (Multivita Co.) composed of retinol 12,000,000 IU, cholecalciferol 2,200,000 IU, tocopherol 10,000 mg, menadione 2000 mg, thiamin 1000 mg, riboflavin 5000 mg, pyridoxal 1500 mg, cobalamin 10 mg, Niacin 30,000 mg, Biotin 50 mg, Folic acid 1000 mg, Pantothenic acid 10,000 mg, Iron 30,000 mg, Manganese 60,000 mg, Copper 4000 mg, Zinc 50,000 mg, Iodine 1000 mg, Cobalt 100 mg, Selenium 100 mg, calcium carbonate (CaCO3) carrier to 3000 g
The sacrificed birds (Five birds from each group were anaesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg) to minimize suffering during slaughtering) from the experimental groups were used for sample collection. The remaining live birds were suffocated in strong bags by CO2 suffocation. All birds (125), dead birds and slaughtered ones, as well as remnants of samples and bedding material, were buried in the strict hygienically controlled properly constructed burial pit.
Experimental infection
At the starting of the experiment, all chicks in the two experimentally infected groups were infected with E. coli O78 via the oral route (1 ml containing 3 × 108). This strain was supplied from the Animal Health Research Institute, Kafrelsheikh Regional Lab, Poultry, and Rabbit Disease Department). E. coli O78 was isolated from poultry and contained the virulence genes, which are a defining characteristic of Avian Pathogenic E. coli O78 (APEC). E. coli O78 was marked with an antibiotic (320 μg ciprofloxacin/ml broth) and prepared according to McFarland [45]. All groups (30 birds/group) were vaccinated against ND and IBD at days 7 and 12, respectively.
Sample collection and measurements of the serum parameters
On day 35, blood (5 ml blood/bird) samples were collected from 5 birds randomly selected/group after slaughtering (the birds were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg) to minimize suffering during slaughtering). The selection was not based on any pre-specified effect. After coagulation of the blood, serum was separated (centrifugation at 3000 rpm for 15 min.) and kept in a freezer (−20o C) until analysis. The investigators were not blinded during data collection. Blinding was used during analysis. Computational analysis was not performed blinded. Serum activities of CAT, SOD, ALT, and AST and concentration of MDA, total protein, and albumin were measured by using commercial kits obtained from BIODIAGNOSTIC Company.
Immune response against Newcastle disease (ND)
HI, titer was performed against ND [46]. The Newcastle disease virus and positive control serum were kindly provided by the Animal Health Research Institute, Kafrelsheikh.
Colonization of E. coli
Shedding of the E. coil in the fecal matter and its colonization in organs was recorded and confirmed by the re-isolation of E. coli from infected birds.
The weight of immune organs
Five chicks on zero-day of age and five chicks from each group were weighted then slaughtered on 15 and 35 days of age. Each organ including thymus, spleen, and bursa of Fabricius was isolated and weighed. The weight of the targeted immune organs was calculated as described by Keil et al. [47] following the equation below:
Immune Organ Weight/Body Weight = Organ Weight (gm)/Body Weight (gm) × 100.
Necropsy and histopathology
Sections of liver, intestine, bursa, thymus gland, and spleen were taken after necropsy and fixed immediately in 10% buffered formalin and processed for histopathological evaluation, using routine paraffin embedding section. Sections of 3 μm thickness were cut and stained using H&E according to Bancroft and Gamble [48].
Q-PCR (real-time polymerase chain reaction)
Collection of the sample
On day 35, samples of the liver were collected on liquid nitrogen from 4 birds randomly selected/group into clean Eppendorf tubes and stored at − 80 °C until use.
Q-PCR (real-time polymerase chain reaction)
The liver samples were collected (35 days) on liquid nitrogen from 4 birds/group into clean Eppendorf tubes and stored at − 80 °C until use.
Total RNA from the liver samples was extracted using easy RED total RNA extraction kits (iNtRON Biotechnology, Inc.) according to the manufacturer’s instructions. The RNA integrity was verified by using agarose gel electrophoresis. The first-strand cDNA was synthesized with total RNA using Intron-Power cDNA synthesis kit (Cat. no. 25011).
The specific primers were used to amplify the selected genes of the chicken (Gallus gallus) with GAPDH as housekeeping (internal standard) gene-primer sequence (Table 11). The qRT-PCR assay was carried out using a Stratagene MX300P Q-PCR system (Agilent Technologies), using Real MODTM Green FAST qPCR master mix (S) following the manufacturer’s recommendations. MxPro QPCR Software was used for data collection.
Table 11.
Primers used for qPCR analysis
| Primer | Sequence | Reference |
|---|---|---|
| TNF-α |
for: 5′-GAGCTGTGGGGAGAACAAAAGGA-3′ Rev.: 5′-TTGGCCCTTGAAGAGGACCTG-3′ |
Tabatabaei et al. [49] |
| NF-ϰB |
for: 5′-CAAGGCAGCAAATAGACGAG-3′ Rev.: 5′-GTTGAGAGTTAGCAGTGAGGCA-3’ |
|
| GAPDH |
For- 5’CCTCTCTGGCAAAGTCCAAG3′ Rev- 5’CAACATCAAATGGGCAGATG3’ |
TNF-α: Tumor necrosis factor-alpha, NF-ϰB: Nuclear factor-kappa B, GAPDH: Glyceraldehyde-3 phosphate dehydrogenase
The relative gene expression levels were evaluated using the 2 − ΔΔct method [50].
Statistical analysis
The obtained results were statistically analyzed by one-way ANOVA using SPSS version 16.
Acknowledgments
We would like to thanks Prof. Rawhya Emran, Dep. of Pathology, Molecular Pathology Unit, Animal Health Research Institute for her valuable support.
Abbreviations
- AGP
Antibiotic Growth Promoters
- ALT
Alanine transaminase
- APPs
Acute-phase proteins
- AST
Aspartate aminotransferase
- BW
Body weight
- CAT
Catalase
- DNA
Deoxyribonucleic acid
- E. coli
Escherichia coli
- FCR
Feed conversion ratio
- HI
Hemagglutination Inhibition
- IBDV
Infectious bursal disease
- Q-PCR
Real-Time Polymerase Chain Reaction
- MDA
Lipid peroxidation
- MOS
Mannan oligosaccharide
- ND
Newcastle Disease
- NDV
Newcastle disease virus
- NF
Nuclear factor
- PM
Postmortem
- RNA
Ribonucleic acid
- SOD
Superoxide dismutase
- TNF
Tumor necrosis factor
Authors’ contributions
All authors contributed equally to this work whereas they designed, conducted the experiment and wrote the manuscript. All authors have read and approved the manuscript. SF and AS measured serum biochemistry. GE followed up isolation of E. coli, colonization and reisolation of E. coli. OS designed diet formulation and measured growth performance parameters. AA examined pathological changes. AP measured Q-PCR for the collected samples. HAM followed up immune response against Newcastle disease, clinical signs and mortalities. SF made interpretation of the results.
Funding
This research did not receive any specific financial support from funding agencies in public, commercial, or non-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.
Ethics approval and consent to participate
The current study was approved by the Ethical Committee for live birds sampling at the Animal Health Research Institute, Egypt (License No. AHRI 42102017), according to local Egyptian laws.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sabreen Ezzat Fadl, Email: nourmallak@yahoo.com.
Ghada Ahmed El-Gammal, Email: gh_ahmed7710@yahoo.com.
Osama Atia Sakr, Email: osamaatia119@yahoo.com.
Aly A. B. S. Salah, Email: alysalah80@yahoo.com
Ayman Ali Atia, Email: aymanatia@yahoo.com.
Abdelbary Mohammed Prince, Email: bioproteomics@yahoo.com.
Abdelhaleem Mohamed Hegazy, Email: mmbarakat2003@yahoo.com.
References
- 1.Wang Y, Dong Z, Song D, Zhou H, Wang W, Miao H, Wang L, Li A. Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food Agric Immunol. 2018;29(1):859–869. doi: 10.1080/09540105.2018.1463972. [DOI] [Google Scholar]
- 2.Tayeri V, Seidavi A, Asadpour L, Phillips CJ. A comparison of the effects of antibiotics, probiotics, synbiotics and prebiotics on the performance and carcass characteristics of broilers. Vet Res Commun. 2018;42(3):195–207. doi: 10.1007/s11259-018-9724-2. [DOI] [PubMed] [Google Scholar]
- 3.Tavaniello S, Maiorano G, Stadnicka K, Mucci R, Bogucka J, Bednarczyk M. Prebiotics offered to broiler chicken exert positive effect on meat quality traits irrespective of delivery route. Poult Sci. 2018;97(8):2979–2987. doi: 10.3382/ps/pey149. [DOI] [PubMed] [Google Scholar]
- 4.Sobolewska A, Elminowska-Wenda G, Bogucka J, Dankowiakowska A, Kułakowska A, Szczerba A, Stadnicka K, Szpinda M, Bednarczyk M. The influence of in ovo injection with the prebiotic DiNovo® on the development of histomorphological parameters of the duodenum, body mass and productivity in large-scale poultry production conditions. J Anim Sci Biotechnol. 2017;8(1):45. doi: 10.1186/s40104-017-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings JH, Macfarlane GT. Gastrointestinal effects of prebiotics. Br J Nutr. 2002;87(Suppl.2):145–151. doi: 10.1079/BJN/2002530. [DOI] [PubMed] [Google Scholar]
- 6.Pelicano ER, Souza PA, Souza HB, Figueiredo DF, Boiago MM, Carvalho SR, Bordon VF. Intestinal mucosa development in broiler chickens fed natural growth promoters. Braz J Poultry Sci. 2005;7(4):221–229. doi: 10.1590/S1516-635X2005000400005. [DOI] [Google Scholar]
- 7.Spring P, Wenk C, Dawson KA, Newman KE. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult Sci. 2000;79(2):205–211. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- 8.Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 2003;82(6):1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- 9.Hajati H, Rezaei M. The application of prebiotics in poultry production. Int J Poult Sci. 2010;9(3):298–304. doi: 10.3923/ijps.2010.298.304. [DOI] [Google Scholar]
- 10.Awaad MH, Atta AM, El-Ghany WA, Elmenawey M, Ahmed K, Hassan AA, Nada AA, Abdelaleem GA. Effect of a specific combination of mannan-oligosaccharides and β-glucans extracted from yeast cell wall on the health status and growth performance of ochratoxicated broiler chicken. J Am Sci. 2011;7(3):82–96. [Google Scholar]
- 11.Dawood MA, Koshio S, Fadl SE, Ahmed HA, El Asely A, Abdel-Daim MM, Alkahtani S. The modulatory effect of mannanoligosaccharide on oxidative status, selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major) Aquacult Rep. 2020;16:100278. doi: 10.1016/j.aqrep.2020.100278. [DOI] [Google Scholar]
- 12.Remus A, Hauschild L, Andretta I, Kipper M, Lehnen CR, Sakomura NK. A meta-analysis of the feed intake and growth performance of broiler chickens challenged by bacteria. Poult Sci. 2014;93(5):1149–1158. doi: 10.3382/ps.2013-03540. [DOI] [PubMed] [Google Scholar]
- 13.Sohail MU, Ijaz A, Younus M, Shabbir MZ, Kamran Z, Ahmad S, Anwar H, Yousaf MS, Ashraf K, Shahzad AH, Rehman H. Effect of supplementation of mannan oligosaccharide and probiotic on growth performance, relative weights of viscera, and population of selected intestinal bacteria in cyclic heat-stressed broilers. J Appl Poult Res. 2013;22(3):485–491. doi: 10.3382/japr.2012-00682. [DOI] [Google Scholar]
- 14.Manafi M, Hedayati M, Khalaji S, Kamely M. Assessment of a natural, non-antibiotic blend on performance, blood biochemistry, intestinal microflora, and morphology of broilers challenged with Escherichia coli. Rev Bras Zootec. 2016;45(12):745–754. doi: 10.1590/s1806-92902016001200003. [DOI] [Google Scholar]
- 15.Jahanian R, Ashnagar M. Effect of dietary supplementation of mannan-oligosaccharides on performance, blood metabolites, ileal nutrient digestibility, and gut microflora in Escherichia coli-challenged laying hens. Poult Sci. 2015;94(9):2165–2172. doi: 10.3382/ps/pev180. [DOI] [PubMed] [Google Scholar]
- 16.El-Far AH, Ahmed HA, Shaheen HM. Dietary supplementation of phoenix dactylifera seeds enhances performance, immune response, and antioxidant status in broilers. Oxidative Med Cell Longev. 2016;2016. 10.1155/2016/5454963. [DOI] [PMC free article] [PubMed]
- 17.Mousa SM, Soliman MM, Bahakaim AS. Effect of mannan oligosaccharides and β-glucans on productive performance and some physiological and immunological parameters of growing Japanese quail chicks. Egypt Poult Sci J. 2014;34(2):2019. [Google Scholar]
- 18.Kabir SM. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Public Health. 2010;7(1):89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma V, Jakhar KK, Nehra V, Kumar S. Biochemical studies in experimentally Escherichia coli infected broiler chicken supplemented with neem (Azadirachta indica) leaf extract. Vet World. 2015;8(11):1340. doi: 10.14202/vetworld.2015.1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blood DC, Radostits OM, Gay CC, Arundel CH, Ikede BO, Mckenzie RA, Trembley RRM, Henderson JA. Vet Med. 8. Eastbourne: The English Language Book Society and Bailliere Tindall; 1994. [Google Scholar]
- 21.Yalçinkaya İ, Güngör T, Başalan M, Erdem E. Mannan oligosaccharides (MOS) from Saccharomyces cerevisiae in broilers: Effects on performance and blood biochemistry. Turk J Vet Anim Sci. 2008;32(1):43–48. [Google Scholar]
- 22.Rokade JJ, Kagate M, Bhanja SK, Mehra M, Goel A, Vispute M, Mandal AB. Effect of mannan-oligosaccharides (MOS) supplementation on performance, immunity and HSP70 gene expression in broiler chicken during hot-dry summer. Indian J Anim Res. 2018;52(6):868–874. doi: 10.18805/ijar. [DOI] [Google Scholar]
- 23.Zhou M, Zeng D, Ni X, Tu T, Yin Z, Pan K, Jing B. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids Health Dis. 2016;15(1):48. doi: 10.1186/s12944-016-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra B, Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front Vet Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abudabos AM, Alyemni AH, Zakaria HA. Effect of two strains of probiotics on the antioxidant capacity, oxidative stress, and immune responses of Salmonella-challenged broilers. Braz J Poult Sci. 2016;18(1):175–180. doi: 10.1590/18069061-2015-0052. [DOI] [Google Scholar]
- 26.El-Kader HA, Alam SS, Nafeaa AA, Mahrous KF. Influence of dietary supplementation of mannanoligosaccharide (bio-Mos®) prebiotic on the genotoxic and antioxidant status in Japanese quail. Int J Pharm Sci Rev Res. 2014;27:289–295. [Google Scholar]
- 27.Sil GC, Das PM, Islam MR, Rahman MM. Management and disease problems of cockrels in some farms of Mymensingh, Bangladesh. Int J Poult Sci. 2002;1(4):102–105. doi: 10.3923/ijps.2002.102.105. [DOI] [Google Scholar]
- 28.Eddie Ip WK, Takahashi K, Alan Ezekowitz R, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230(1):9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira MC, Figueiredo-Lima DF, Faria Filho DE, Marques RH, Moraes VM. Effect of mannanoligosaccharides and/or enzymes on antibody titers against infectious bursal and Newcastle disease viruses. Arq Bras Med Vet Zoo. 2009;61(1):6–11. doi: 10.1590/S0102-09352009000100002. [DOI] [Google Scholar]
- 30.Sadeyen JR, Kaiser P, Stevens MP, Dziva F. Analysis of immune responses induced by avian pathogenic Escherichia coli infection in turkeys and their association with resistance to homologous re-challenge. Vet Res. 2014;45(1):19. doi: 10.1186/1297-9716-45-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottardo ET, Burin Junior ÁM, Lemke BV, Silva AM, Pasa B, Cassiano L, Muller Fernandes JI. Immune response in Eimeria sp. and E. coli challenged broilers supplemented with amino acids. Austral J Vet Sci. 2017;49(3):175–184. doi: 10.4067/S0719-81322017000300175. [DOI] [Google Scholar]
- 32.Huff GR, Huff WE, Balog JM, Rath NC. The effects of dexamethasone immunosuppression on Turkey osteomyelitis complex in an experimental Escherichia coli respiratory infection. Poult Sci. 1998;77(5):654–661. doi: 10.1093/ps/77.5.654. [DOI] [PubMed] [Google Scholar]
- 33.Wani BM, Darzi MM. Masood Saleem Mir SA, Shakeel I. pathological and Pharmacochemical evaluation of broiler chicken affected naturally with Colibacillosis in Kashmir Valley. Int J Pharmacol. 2017;13(4):388–395. doi: 10.3923/ijp.2017.388.395. [DOI] [Google Scholar]
- 34.Kumar A, Jindal N, Shukla CL, Asrani RK, Ledoux DR, Rottinghaus GE. Pathological changes in broiler chickens fed ochratoxin a and inoculated with Escherichia coli. Avian Pathol. 2004;33(4):413–417. doi: 10.1080/03079450410001724021. [DOI] [PubMed] [Google Scholar]
- 35.Petersen HH, Nielsen JP, Heegaard PM. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35(2):163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 36.Markazi A. Effects of Whole Yeast Cell Product Supplementation in Chickens Post-coccidial and Post-Salmonella Challenge (doctoral dissertation, the Ohio State University) 2015. [Google Scholar]
- 37.Lathers CM. Role of veterinary medicine in public health: antibiotic use in food animals and humans and the effect on evolution of antibacterial resistance. J Clin Pharmacol. 2001;41(6):595–599. doi: 10.1177/00912700122010474. [DOI] [PubMed] [Google Scholar]
- 38.Landers TF, Cohen B, Wittum TE, Larson EL. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127(1):4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajagai YS, Klieve AV, Dart PJ, Bryden WL. Probiotics in animal nutrition: production, impact and regulation. FAO. 2016. [Google Scholar]
- 40.NRC . Nutrient requirements of poul. 9. Washington: National Academy of Sci; 1994. [Google Scholar]
- 41.AOAC . In: Official methods of analysis. 13. Horwitz w W, editor. Washington: Academic Press; 2000. p. 925.09. [Google Scholar]
- 42.Vohra P, Roudybush T. The effect of various levels of dietary protein on the growth and egg production of Coturnix coturnix japonica. Poult Sci. 1971;50(4):1081–1084. doi: 10.3382/ps.0501081. [DOI] [Google Scholar]
- 43.Castell JD, Tiews K. Report of the EIFAC. IUNS and ICES working group on the standraization of methodology in fish nutrition research. Hamburg, Fedral Republic of Germany, EIFAC. Technology. 1980;36:24. [Google Scholar]
- 44.Tacon A. The nutrition and feeding of farmed fish and shrimp a training manual. V61. 1. The essential nutrients FAO. 1987. pp. 117–130. [Google Scholar]
- 45.McFarland J. Nephelometer: an instrument for media used for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc. 1907;14:1176–1178. doi: 10.1001/jama.1907.25320140022001f. [DOI] [Google Scholar]
- 46.King DJ, Seal BS. Biological and molecular characterization of ND. Virus field isolates with comparison to reference NDV strain and pathogenicity after chicken or embryo passage of selected isolates. Avian Dis. 1998;42:507–516. doi: 10.2307/1592677. [DOI] [PubMed] [Google Scholar]
- 47.Keil DE, Mehlmann T, Butterworth L, Peden-Adams MM. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci. 2008;103(1):77–85. doi: 10.1093/toxsci/kfn015. [DOI] [PubMed] [Google Scholar]
- 48.Bancroft JD, Gamble M. Theory and practice of histological techniques. Elsevier health sciences. 2008. [Google Scholar]
- 49.Tabatabaei SM, Badalzadeh R, Mohammadnezhad GR, Reza BR. Effects of Cinnamon extract on biochemical enzymes, TNF-α and NF-κB gene expression levels in liver of broiler chickens inoculated with Escherichia coli. Pesqui Vet Bras. 2015;35(9):781–787. doi: 10.1590/S0100-736X2015000900003. [DOI] [Google Scholar]
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.